Diagnostic appraisal of grade 12 students' understanding of reaction kinetics
Yaw Kai
Yan
and
R.
Subramaniam
*
Nanyang Technological University, National Institute of Education, 1 Nanyang Walk, Singapore 637616. E-mail: subramaniam.r@nie.edu.sg
First published on 15th September 2016
Abstract
The study explored grade 12 students' understanding of reaction kinetics, a topic which has not been extensively explored in the chemistry education literature at this level. A 3-tier diagnostic instrument with 11 questions was developed – this format is of very recent origin and has been the subject of only a handful of studies. The findings reveal that reaction kinetics is not an easy topic for the students to understand. A total of 23 alternative conceptions (ACs) as well as some indication of their strengths and other confidence-related measures have been documented for the students (N = 137) – most of these ACs have not been reported before. When the overall scores of the students in the diagnostic test are ranked, it was found that there are a number of differences in the ACs held by students in the upper and lower 25% of the sample. While most of the ACs held by these groups are common to the overall sample, a number of these are not found in the overall sample. The findings further underscore the diagnostic utility of the 3-tier format. Some implications of the findings are discussed.
Introduction
The present study focuses on Advanced level (A-level) students' understanding of reaction kinetics, an important topic in the Chemistry syllabus. (A-level is equivalent to grades 11–12.)Students have a general introduction to chemical reactions at secondary level in the topic of stoichiometry, where they are exposed to calculations in relation to relative atomic mass, moles, Avogadro constant, gas molar volume, and other aspects. However, the dynamic aspects of chemical reactions are usually encountered when they are taught reaction kinetics and chemical equilibria at advanced level. We focus more on reaction kinetics as this is the subject of our study. In this topic, students are taught concepts such as rate of reaction; order of reaction; effect of variables such as particle size, pressure, temperature and catalyst on rate of reaction; and rate constant. Some of these concepts are quantitative in nature, hence students need to have a good grasp of mathematics to understand them well. Solving computational problems does not necessarily lead to better understanding of the concepts. In fact, we note that many students are successful at solving such problems without really understanding the concepts. As the topic entails understanding at the conceptual and computational level, it creates some obstacles for students to achieve good functional understanding. Justi (2003) and Van Driel (2002) have also noted that reaction kinetics is a difficult topic for school students to understand.
Since the majority of kinetics questions at A-level examinations involve computation, teachers tend to focus more on teaching their students the mechanics of solving typical kinetics problems, and less on the qualitative concepts which form the conceptual basis of the topic of kinetics. As a result of this drill and practice, students' ability to score well for numerical kinetics problems does not necessarily reflect a good grasp of kinetics concepts.
The predominance of numerical kinetics problems in A-level examinations also means that teachers are more used to setting numerical problems rather than conceptual problems for this topic. Although it is necessary to deal with the computational aspects of kinetics, this also makes it difficult to discern genuine ACs in kinetics from weaknesses in mathematics when students perform poorly in such tasks. This might be an obstacle to the development of diagnostic instruments for kinetics. In the development of the instrument reported herein, we have made it a point to avoid as far as possible items that predominantly test mathematical skills.
A scan of the literature shows that the number of ACs documented on reaction kinetics at the grade 12 level is rather limited, considering that it is a topic with significant content diversity. There is thus a need for further work.
Literature review
Reaction kinetics commonly require understanding of concepts such as rate of reaction, order of reaction, temperature dependence of rate of reaction, activation energy, catalyst and rate determining step. All these concepts have been the subject of studies, which have surfaced ACs in students. An excellent review on educational studies in reaction kinetics has recently appeared (Bain and Towns, 2016), and this builds on an earlier review by Justi (2003).A number of studies have reported that students have varying understandings, and hence ACs, of the term ‘reaction rate’. Some of the common ACs related to reaction rate include: it increases to a maximum value, remains constant at that value and then decreases to zero (Cakmakci et al., 2006; Aydin et al., 2009; Tastan-Kırık and Boz, 2012); it decreases to a minimum value and then stays constant at that value (Kolomuç and Tekin, 2011); it is equal to the product of concentrations of reactants and products; an increase in surface area of a solid reactant increases the probability of collision as well as kinetic energy of the particles (Yalçınkaya et al., 2012); it is dependent on the concentration of reactants and products (Yalçınkaya et al., 2012); an increase in concentration of reactants increases reaction rate (Cakmakci et al., 2006; Turanyi and Toth, 2013); it increases or decreases as the reaction proceeds (Hackling and Garnett, 1985; Cakmakci et al., 2006; Cakmakci, 2010; Bektasli and Çakmakci, 2011; Kolomuç and Tekin, 2011); it is the time required for reactants to form products (Akkus et al., 2003; Cakmakci, 2010; Çalik et al., 2010; Kolomuç and Tekin, 2011; Tastan-Kırık and Boz, 2012; Yalçınkaya et al., 2012); it is constant for a reaction (Cakmakci et al., 2006; Bektasli and Çakmakci, 2011; Kolomuç and Tekin, 2011); and it is the amount of substance transforming into products per unit time at a constant temperature and concentration (Bektasli and Çakmakci, 2011).
The order of a reaction is also a concept that is prone to misunderstanding and hence producing ACs in students. Some of these include: for a zero order reaction, increasing the initial concentration of reactants would increase/decrease the rate of reaction (Cakmakci, 2010); and in the rate equation, the concentration terms have exponents equal to the corresponding stoichiometric ratios in the balanced chemical equation (Cakmakei et al., 2006; Cakmakei and Aydogdu, 2011; Turanyi and Toth, 2013).
The temperature dependence of reaction rate is also an aspect that is fraught with ACs. Some of these include: raising the temperature increases the time necessary for a reaction to occur (Van Driel, 2002; Tastan-Kırık and Boz, 2012); when the temperature of an exothermic reaction is increased, the rate of the forward reaction is increased (Yalçınkaya et al., 2012); when temperature is raised, rate of an endothermic reaction increases while the rate of an exothermic reaction decreases (Hackling and Garnett, 1985; Akkus et al., 2003; Aydin et al., 2009; Cakmakci, 2010; Sozbilir et al., 2010; Çakmakci and Aydogdu, 2011; Kurt and Ayas, 2012; Tastan-Kırık and Boz, 2012); increase in temperature has no effect on the rate of exothermic reactions (Cakmakci, 2010; Yalçınkaya et al., 2012); at the same temperature, the rates of an exothermic reaction and endothermic reaction are equal (Kolomuç and Tekin, 2011); exothermic reactions occur more rapidly than endothermic reactions (Cakmakci, 2010; Sozbilir et al., 2010; Çakmakci and Aydogdu, 2011; Kolomuç and Tekin, 2011; Tastan-Kırık and Boz, 2012; Yalçınkaya et al., 2012); and endothermic reactions occur more rapidly than exothermic reactions (Cakmakci, 2010; Sozbilir et al., 2010; Kolomuç and Tekin, 2011; Yalçınkaya et al., 2012).
Concepts related to activation energy are also not easy to understand for students. Some of the ACs reported include: it is the energy released in a reaction (Cakmakci, 2010); it is the kinetic energy possessed by the reactant molecules (Cakmakci, 2010); for a particular value of activation energy, the reaction rate is dependent on whether the species are single atoms or multi-atoms (Kolomuç and Tekin, 2011); increasing the temperature increases (Yalçınkaya et al., 2012) or decreases (Tastan-Kırık and Boz, 2012) the activation energy of the reaction; activation energies are lower for exothermic reactions than for endothermic reactions (Tastan-Kırık and Boz, 2012; Yalçınkaya et al., 2012); and in reactions with high activation energy, the probability of collisions occurring between molecules is less (Kolomuç and Tekin, 2011).
A number of ACs have also been identified in relation to students' understanding of the term ‘catalyst’. Some of these include: a catalyst is necessary to initiate a reaction (Kıngır and Geban, 2012); it increases the yield of products (Cakmakci, 2010); it increases the average speed of the molecules (Kurt and Ayas, 2012; Tastan-Kırık and Boz, 2012); it does not affect the mechanism of a reaction (Cakmakci, 2010; Çakmakci and Aydogdu, 2011; Kurt and Ayas, 2012; Tastan-Kırık and Boz, 2012; Yalçınkaya et al., 2012); it does not react with the reactants or products (Yalçınkaya et al., 2012); it increases the rate of reaction by decreasing the kinetic energy of the molecules (Yalçınkaya et al., 2012); it speeds up only the forward reaction (Hackling and Garnett, 1985; Voska and Heikkinen, 2000; Akkus et al., 2003; Bilgin and Geban, 2006; Kıngır and Geban, 2012; Yalçınkaya et al., 2012); and it increases the activation energy of the reaction (Tastan-Kırık and Boz, 2012; Kaya and Geban, 2012).
The rate determining step is another concept encountered in reaction kinetics. ACs documented include: if more than one product is formed in a chemical reaction, the rate determining step is the same for each in all cases; and a chain reaction does not have a rate determining step (Laidler, 1988).
Approaches used to document ACs on reaction kinetics
In documenting ACs on reaction kinetics, some of the approaches used in the literature include multiple choice questions (Kungr and Geban, 2012), two-tier MCQs (Supasorn and Promarak, 2015); open-ended questions (Camakci, 2010), and sharing of instructor experiences (Laidler, 1988).Gaps in the literature
Examining the literature review on ACs on reaction kinetics, it can be seen that the grade 12 level has hardly been explored. Even the nearest level (grade 11) has not been the subject of studies relating to identification of ACs on reaction kinetics though there have been studies on promoting understanding of this topic at this level (for example, Çalik et al., 2010; Kurt and Ayas, 2012; Tastan-Kirik and Boz, 2012; Yalçınkaya et al., 2012; Supasorn and Premark, 2015) by leveraging on common ACs. Also, several of the ACs documented at the various levels are those which can generally be regarded as related to the key concepts. There is a need for documenting ACs in more advanced contexts for the benefit of instructors.Open-ended questions have been used in a number of these studies. While these are useful in probing nuances of students' understanding at the qualitative level, procedurally these are time consuming to administer and analyze. Also, the strengths of the ACs are difficult to be known from such questions. It is essential to have some sense of the strengths of the ACs so that interventions can then focus more on those ACs that are strongly entrenched in students' minds and which can interfere with their learning. Multiple choice questions have been used in a number of studies but there is an element of chance in answering questions in such instruments. A wrong answer in an MCQ does not necessarily indicate an AC – it could also be due to students' lack of knowledge. A two-tier conceptual test on reaction rates has been reported (Supasorn and Promarak, 2015) for use in an intervention to promote understanding via inquiry and analogies but it is in the Thai language. All the studies focused on selected aspects of reaction kinetics.
Multi-tier diagnostic instruments come in various forms. The two-tier MCQ is the most popular form and has been the basis of numerous studies in the literature to document ACs. It comes in two variants. In one form, both the answer and reason tiers are in MCQ format. In another form, the answer tier is in MCQ format while the reason tier is in open-ended format. Compared to MCQs, two-tier MCQs are more robust in documenting misconceptions for a number of reasons – the distracters are ACs, either derived from the literature or from prior field work; the incorporation of a second tier for students to justify their answer in the first tier mandates them to draw on their explanatory frameworks for reasoning; and the probability of getting it correct is less than that for a MCQ. In the case of two-tier questions where the answer tier is in MCQ format and the reason tier is in open-ended format, while the robustness of the question is enhanced further, the issue is often coming up with coding schemes to analyse the open-ended responses. Compared to the version in which both tiers are in MCQ format, the two tier instrument where the answer tier is in MCQ format and the reason tier is in open-ended format has been the basis of relatively fewer studies in the literature.
There are a few drawbacks of MCQs and two-tier MCQs. For example, it cannot distinguish between incorrect responses on the basis of whether these are due to guesswork or lack of understanding (Caleon and Subramaniam, 2010a). Likewise, it cannot distinguish correct responses on the basis of whether these are due to guesswork or content mastery. These limitations can be addressed significantly with the use of 4-tier (Caleon and Subramaniam, 2010a) or 3-tier diagnostic questions (Caleon and Subramaniam, 2010b). In these instruments, a confidence rating – typically on a scale from just guessing (1) to absolutely confident (6), is added. Where it is appended to both tiers, it becomes a 4-tier MCQ item, and where a mean rating is required in respect of both tiers, it becomes a 3-tier MCQ. Three- and 4-tier instruments are of more recent origins (Caleon and Subramaniam, 2010b; Cetin-Dindar and Geban, 2011; Arslanab et al., 2012; McClary and Bretz, 2012; Sreenivasulu and Subramaniam, 2013, 2014). The aspect related to confidence measures is something that has been given minimal attention in the misconceptions literature in the sciences in general and chemistry in particular. There is thus a need for further work to assess its efficacy in chemistry education research.
In a 4-tier instrument, students provide different confidence ratings for their responses to the answer and reason tiers. This is because both tiers may have different difficulty levels and it is reasonable to assume that students would have different levels of confidence for both tiers. However, it is also clear that as the answer and reason are linked, it may not be easy for students to disambiguate their confidence levels for the reason tier without being influenced by that for the answer tier. So, in this study, we have chosen to use a composite confidence rating for each question. In other words, we would be focusing on the 3-tier format in this study.
Looking at the literature, it is clear that most studies on reaction kinetics have focused at the secondary and tertiary level, a point also apparent in the recent comprehensive review by Bain and Towns (2016). The grade 12 level (upper high school) does not seem to have been given sufficient attention by researchers. For example, Seçken and Seyhan (2015) explored the relationship between grade 11 students' academic achievement and anxiety about rate of reaction problems set as graphs. However, they did not document any ACs on rate of reaction. A two-tier conceptual test on reaction rates has been reported (Supasorn and Promarak, 2015) for use in an intervention to promote understanding via inquiry and analogies but the Thai language in which it is set restricts its wider use by others. It featured questions where the second tier is either in MCQ format or open-ended format as well as where workings for calculations have to be shown. Also the instrument is more conceptual rather than diagnostic in nature. Moreover, the instrument focuses only on reaction rates. It is to be noted that the grade 12 level is the transition point between high school and university, and if ACs are not addressed at this level, students may carry these forward to university if they study chemistry. Identification of ACs at this level is thus important as it can offer useful pointers for instructors to keep in mind during lesson delivery.
The principal objective of this study is to assess grade 12 students' understanding of reaction kinetics through the development of a 3-tier diagnostic instrument. The specific research questions which we seek to better understand are:
(a) What are the ACs held by A-level students on the topic of reaction kinetics?
(b) What do the confidence measures related to the ACs convey?
(c) What is the diagnostic utility of the 3-tier format for AC studies based on this work?
Methodology
Procedure
The procedure for developing a diagnostic instrument on reaction kinetics basically parallels the approach used by Treagust (1988) for 2-tier instruments but with some modifications. The content boundaries of the topic were demarcated and focus was directed only on certain aspects of the topic which, based on our teaching experience, were fraught with learning difficulties or misconceptions – rate of reaction, temperature dependence, reaction order, rate constant, activation energy, and catalysts. To come up with MCQs on these aspects, the following sources were consulted: literature on ACs on reaction kinetics, past year examination questions on reaction kinetics, assessment books on chemistry, and teaching experiences of the authors. In this way, 15 MCQs were drafted for the preliminary version of the instrument, each with about 2–4 options and a blank space for students to write their reason for the choice of answer for each question. This was administered to a sample of A-level students (N = 97). Based on the open-ended responses given by the students, the second tier of the questions was framed. Where students' responses did not unequivocally permit a response to be framed for the reason tier, the authors used their own teaching experience to frame the distracters. In this way, 11 two-tier MCQs were framed. This was sent for validation to two experienced chemistry teachers who have taught this topic at A-level. They were in broad agreement regarding the questions used in the instrument. No changes were recommended. Even though the validators found the instrument acceptable, we took the opportunity to revise the instrument slightly in respect of phrasings. A confidence tier was then added to each question. The confidence ratings span the continuum from Just guessing (1) to Absolutely confident (6), similar to that used by Caleon and Subramaniam (2010a).There was no blank space given for students to write their own answer/reason if they disagreed with any of the given combinations. There are two reasons for this. Observation of studies on two-tier diagnostic instruments in the literature indicate that the blank option is minimally exercised. So, it was felt that it was not necessary to incorporate this in our instrument. Even the recent studies by Sreenivasulu and Subramaniam (2013, 2014) on 4-tier instruments did not feature the blank options. Also, we felt that the given response options were adequate.
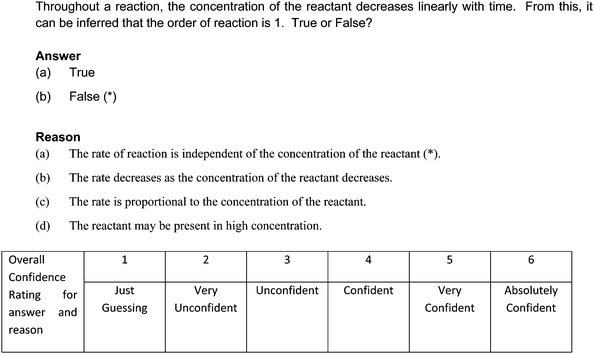
A sample question appears in Fig. 1.
The revised instrument was administered to a sample of 137 students from two junior colleges (grades 11–12 equivalent). These students were different from those that participated in the preliminary study, and have earlier completed a course on reaction kinetics. All students in this study offered Chemistry as one of the subjects in their A-level examinations. They sat for the diagnostic test after having been taught the topic through lectures and tutorials at least two months earlier. The students were reminded a week in advance to revise the topic of reaction kinetics prior to sitting for the test. A copy of the instrument can be obtained from the authors.
The revised instrument was found to have good diagnostic utility in terms of distracter selection and difficulty level. Out of 152 distracters in total (that is, answer and reason tiers combined) for the 11 questions, there were zero selections for only 12 distracters, which means that over 92% of the distracters were functioning in terms of non-zero selections. This was considered adequate for this study. It was therefore considered not necessary to work on refining the instrument further. The data for this instrument is thus reported in this study.
It would be useful if students can also be interviewed to probe their thinking behind the response combinations leading to the ACs. However, this was not pursued in this study for a few reasons. Firstly, interviews are time consuming, and transcripts need to be generated for analyses. Secondly, owing to the large number of ACs identified in this study, it is labor-intensive to probe the understandings of selected students for each of the ACs for better generality. Thirdly, owing to the teaching background of the authors with undergraduates and trainee teachers, they can also comment on the ACs based on their experiences, and this is something which we wished to pursue in this study to the extent possible in place of interviews. This aspect of leveraging on the instructors’ experiences with several cohorts of students and trainee teachers can also shed useful insights on the ACs and should not be discounted – it is an aspect that has not been given adequate attention in the literature on ACs.
Treatment of data
For each question the following data for each student was keyed into an Excel file: response selected for answer tier, response selected for reason tier, and confidence rating for responses. Two rubrics were used for marking: (a) one mark for answer correct, and (b) one mark for both answer and reason correct. The cognitive scores for all students were summed up and averaged for each question as well as for the overall test – these are reported as proportions.Identification of ACs from the answer/reason combinations followed the approach of Tan et al. (2002), where ≥10% selection of the distracters by the sample was classified as such.
To study if there are any differences in the ACs held by the students with high and low overall scores in the test, students' scores in the test were ranked and divided into the upper ∼25% and lower ∼25% in terms of scores. The number of students in these ranges were 39 (those who scored 4–7 marks) and 44 (those who scored 0 and 1 mark) respectively. The ACs in these students were then determined by using the same approach.
Treatment of confidence data follows the approach of Caleon and Subramaniam (2010a). The confidence measures that were determined are as follows:
CF (mean confidence): sum of confidence ratings of students for a question divided by number of students.
CFC (confidence when correct): sum of confidence ratings of students for a question correctly answered divided by number of students who correctly answered.
CFW (confidence when wrong): sum of confidence ratings of students for a question incorrectly answered divided by number of students who incorrectly answered.
CAC: confidence with which an AC is expressed by students, and is equal to the mean confidence linked to the content-reason response selected for a question from which the AC was framed.
CDQ (confidence discrimination quotient): (CFC − CFW)/standard deviation across all confidence ratings. It can be interpreted in terms of the ability of the students to discriminate between what they know and what they do not know (Lundeberg et al., 2000).
CB (confidence bias): (CF − 1)/5 minus the proportion of students who correctly answered in both tiers. It is an index of calibration of students in their confidence. A positive CB indicates that students exhibit over-confidence; a negative CB indicates under-confidence; and if CB is zero, they have perfect calibration, that is, their accuracy matches their confidence exactly (Stankov and Crawford, 1997).
To assess the internal consistency of the data, Cronbach alpha was computed. This was done for the answer tier, reason tier, both tiers and confidence tier.
Ethics approval
Approval to conduct the study was given by the university's Institutional Review Board. Students gave informed consent to participate in this study.Results and discussion
Basic psychometric statistics
Overall, the diagnostic test was on the difficult side (Table 1). The mean score for the test, based on correct responses for both answer and reason tiers, is about 22%. The percentage of correct responses for the answer tier in the questions ranged between 23% and 72%. For the reason tier, it ranged between 7% and 50%. These findings are consistent with other reports in the literature that two tier diagnostic tests are generally difficult for students to score and that they have more difficulties with the reason tier than with the answer tier. If correct responses for both tiers are mandated for scoring, the percentage of correct responses drops even lower: between 6% and 36%.| Question # | Proportion correct | B tier | ||||||
|---|---|---|---|---|---|---|---|---|
| A tier | R tier | B tier | CF | CFC | CFW | CDQ | CB | |
| a Question not considered in framing ACs for reason indicated in text. | ||||||||
| 1 | 0.47 | 0.22 | 0.16 | 3.81 | 4.05 | 3.76 | 0.24 | 0.40 |
| 2 | 0.23 | 0.24 | 0.07 | 2.71 | 2.67 | 2.71 | −0.03 | 0.28 |
| 3a | — | — | — | — | — | — | — | — |
| 4 | 0.72 | 0.14 | 0.34 | 3.46 | 3.61 | 3.39 | 0.22 | 0.16 |
| 5 | 0.29 | 0.07 | 0.06 | 3.92 | 2.88 | 3.98 | −0.89 | 0.53 |
| 6 | 0.31 | 0.24 | 0.20 | 3.45 | 3.63 | 3.41 | 0.17 | 0.29 |
| 7 | 0.56 | 0.47 | 0.36 | 3.18 | 3.76 | 2.85 | 0.65 | 0.07 |
| 8 | 0.23 | 0.25 | 0.20 | 4.15 | 4.07 | 4.17 | −0.08 | 0.43 |
| 9 | 0.47 | 0.48 | 0.36 | 3.27 | 4.14 | 2.79 | 0.92 | 0.09 |
| 10 | 0.24 | 0.14 | 0.09 | 3.12 | 2.75 | 3.16 | −0.31 | 0.34 |
| 11 | 0.46 | 0.50 | 0.33 | 3.55 | 3.87 | 3.40 | 0.35 | 0.18 |
| Mean | 0.40 | 0.28 | 0.22 | 3.46 | 3.54 | 3.36 | 0.12 | 0.28 |
Confidence measures extracted make for interesting reading. Confidence when correct (CFC) ranges between 2.67 and 4.14 out of 6: this is not on the high side and indicates that students are not able to assign the highest possible confidence rating for their correct answer–reason combination. Confidence when wrong (CFW) was between 2.57 and 4.17 out of 6; we would expect students to have low confidence when their response combinations are wrong. This means they have ACs related to the concepts tested.
The CDQ and CB values provide further insights into students' thinking. Four of the questions have negative CDQ values, meaning that students exhibited more confidence when they were wrong in their responses than when they were right. Mean CDQ was 0.12, which indicates that overall, the sample had low discriminating power between what they know and what they do not know. Students ought to have high confidence when they are correct than when they are wrong (Lundeberg et al., 2000). In relation to CB, all questions elicited a positive value, which means that the students are overconfident in the accuracy of their responses. This is generally in line with findings in the educational psychological literature (Lundeberg et al., 2000).
Table 2 presents data related to the internal consistency of the cognitive and confidence measures. Cronbach alpha values for the answer and reason tiers are low. This is due to the criterion-based nature of the test (Popham and Husek, 1969). Low alpha values were also obtained by Caleon and Subramaniam (2010a) and Sreenivasulu and Subramaniam (2013) for their diagnostic instruments based on cognitive scores. This is not necessarily an issue with this study as the aim of the instrument was to surface ACs, and not to grade students like those in high stakes examinations. It has also to be noted that alpha indices are more appropriate for use with a single-construct test and since their value depends also on the number of questions, a diagnostic test with a small number of questions and with the possibility of these constituting more than one construct, can have a low alpha value, In fact, it is acceptable for such a test to have a low alpha value (Adams and Wieman, 2011). As expected, alpha value for the confidence tier is higher, and this is in keeping with the findings reported in other studies (Caleon and Subramaniam, 2010a; Sreenivasulu and Subramaniam, 2013).
| Answer tier | Reason tier | Both tiers | Confidence tier | |
|---|---|---|---|---|
| Note: Alpha values reported refer to those when Q3 is disregarded. See text for explanation. | ||||
| Cronbach alpha | 0.36 | 0.17 | 0.36 | 0.84 |
Table 3 presents a list of 23 ACs based on the administration of the diagnostic instrument to students. Each question in the instrument was able to elicit at least one AC. Some questions, for example, Q1 and Q2 were able to elicit four ACs. These observations further underscore the analytical utility of the instrument for diagnostic purposes. The confidence associated with the ACs (CAC) range from 2.30 to 4.11. Given that these values are >1 with reference to the confidence scale used, the ACs are unlikely to be due to pure guessing or total lack of knowledge. Students do have some knowledge of the content tested but their understanding has not reached a stage where they can assign a higher confidence rating for the correct answer/reason combination. It is to be noted that the higher the CAC, the more firmly is the AC likely to be held by the student. Caleon and Subramaniam (2010a) have further classified ACs by the magnitude of the CAC values: spurious ACs (CAC < 3.5), moderate ACs (4.0 > CAC ≥ 3.5) and strong ACs (CAC ≥ 4.0). Based on this criteria, it can be seen from Table 3, that nine of the ACs can be considered to be spurious ACs, ten can be considered to be moderate ACs and four are strong ACs. However, careful examination of the nature of the ACs framed suggest that some of the spurious ACs may well be moderate or strong ACs, a point which is further reinforced by our teaching experiences. It is likely that the classification proposed by Caleon and Subramaniam (2010a) is somewhat rigorous.
| S/n | Question (ans/reas) | Alternative conception | % sample with AC | CAC |
|---|---|---|---|---|
| 1 | 1ab | When reactant concentration decreases linearly with time, the reaction is of first order since the rate decreases. | 12.0 | 3.25 |
| 2 | 1ac | When reactant concentration decreases linearly with time, the reaction is of first order since the rate is proportional to concentration. | 33.0 | 3.87 |
| 3 | 1bb | When reactant concentration decreases linearly with time, the reaction is not of first order since the rate decreases. | 17.0 | 3.57 |
| 4 | 1bc | When reactant concentration decreases linearly with time, the reaction is not of first order since the rate is proportional to concentration. | 12.0 | 3.88 |
| 5 | 2aa | When the temperature is increased from T1 to T2, the rate constants of two reactions, whose activation energies are Ea and Ea′ (with Ea > Ea′), vary such that k2′/k1′ = k2/k1 since the rate constants increase by the same proportion. | 25.0 | 2.71 |
| 6 | 2bd | When the temperature is increased from T1 to T2, the rate constants of two reactions, whose activation energies are Ea and Ea′ (with Ea > Ea′), vary such that k2′/k1′ < k2/k1 since the fraction of molecules with energy greater than or equal to the activation energy will increase by a larger proportion for the reaction with lower activation energy. | 23.0 | 2.30 |
| 7 | 2cc | When the temperature is increased from T1 to T2, the rate constants of two reactions, whose activation energies are Ea and Ea′ (with Ea > Ea′), vary such that k2′/k1′ > k2/k1 since the fraction of molecules with energy greater than or equal to the activation energy will increase by a larger proportion for the reaction with higher activation energy. | 14.0 | 2.60 |
| 8 | 2dd | When the temperature is increased from T1 to T2, the rate constants of two reactions, whose activation energies are Ea and Ea′ (with Ea > Ea′), vary such that k2′ − k1′ = k2 − k1 since the fraction of molecules with energy greater than or equal to the activation energy will increase by a larger proportion for the reaction with lower activation energy. | 23.0 | 3.50 |
| 9 | 4ab | The rate of reaction is doubled when temperature is raised by 10 °C because the change in rate depends only on the change in temperature. | 15.0 | 3.38 |
| 10 | 4ca | The rate of reaction may or may not be doubled when temperature is raised by 10 °C because the change in rate depends only on the concentration of the reactants. | 16.0 | 3.60 |
| 11 | 4cd | The rate of reaction may or may not be doubled when the temperature is raised by 10 °C because the change in rate depends on whether the reaction is exothermic or endothermic. | 14.0 | 4.11 |
| 12 | 5aa | To increase the rate of reaction, it is not necessary to increase the concentration of the catalyst since a small amount is adequate to change the energy profile of the reaction. | 21.0 | 4.00 |
| 13 | 6bd | The rate constant of a reaction does not increase when a catalyst is used since its value is affected only by a change in temperature. | 14.0 | 3.55 |
| 14 | 6cc | The rate constant of a reaction changes if the concentration of the reactants is increased since the reaction is faster when there are more reactant molecules per unit volume. | 19.0 | 3.92 |
| 15 | 6cd | The rate constant of a reaction changes if the concentration of the reactants is increased since it is affected only by changes in temperature. | 12.0 | 3.27 |
| 16 | 7ac | In the reaction between NO(g) and O2(g), where the reaction is second order with respect to NO and first order with respect to O2, if the concentrations of both reactants are doubled, the rate of reaction increases by a factor of 8 since this represents the factor by which the overall reactant concentration changes. | 10.0 | 3.57 |
| 17 | 8aa | For an exothermic reaction, an increase in temperature increases the rate of the forward reaction as well as decreases the rate of the reverse reaction since the equilibrium is shifted to the right. | 12.0 | 4.25 |
| 18 | 8db | For an exothermic reaction, an increase in temperature decreases the rate of the forward reaction as well as increases the rate of the reverse reaction since the equilibrium is shifted to the left. | 48.0 | 4.10 |
| 19 | 9dd | For a zero-order reaction with rate constant of 0.025 mol dm−3 s−1 and initial reactant concentration of 0.50 mol dm−3, the reactant concentration after 15 seconds would still be the same since for such a reaction the concentration does not change. | 28.0 | 3.59 |
| 20 | 10aa | For a two-step reaction with elementary steps of different reaction orders, the rate limiting step would be the one with the lower reaction order. | 25.0 | 3.21 |
| 21 | 10ab | For a two-step reaction with elementary steps of different reaction orders, the rate limiting step is the one where a reaction intermediate is consumed. | 10.0 | 2.43 |
| 22 | 10ac | For a two-step reaction with elementary steps of different reaction orders, the rate limiting step is the one where an intermediate is formed. | 36.0 | 3.45 |
| 23 | 11aa | For an enzyme-catalyzed reaction which is carried out at 55 °C, the reaction rate would be doubled if the temperature is raised to 65 °C, but the product yield would not be increased since the enzyme would be denatured. | 14.0 | 3.79 |
Studies on misconceptions in science generally do not seek to ascertain the distribution of ACs in the upper and lower performing students in the sample. This is not surprising as generalizability is stronger with the larger overall sample size. However, when the ACs are categorized on the basis of the approximately top 25% and bottom 25% of the sample, further insights can also be obtained. Tables 4 and 5 are presented for these.
| S/n | Question (ans/reas) | Alternative conception | Prop. of sample with AC | CAC |
|---|---|---|---|---|
| a ACs also found in overall sample. b ACs also found in lower 25% of sample. | ||||
| 1 | 1ab | 0.13 | 3.40 | |
| 2 | 1ac | 0.26 | 3.90 | |
| 3 | 1bb | 0.15 | 4.33 | |
| 4 | 2aa | 0.21 | 2.38 | |
| 5 | 2bd | 0.18 | 2.71 | |
| 6 | 2cc | 0.10 | 4.00 | |
| 7 | 2cd | When the temperature is increased from T1 to T2, the rate constants of two reactions, whose activation energies are Ea and Ea′ (with Ea > Ea′), vary such that k2′/k1′ > k2/k1 since the fraction of molecules with energy greater than or equal to the activation energy will increase by a larger proportion for the reaction with lower activation energy.b | 0.28 | 2.82 |
| 8 | 4cd | 0.10 | 4.75 | |
| 9 | 5aa | 0.26 | 4.00 | |
| 10 | 5ac | Increasing the concentration of a catalyst does not speed up a reaction further as the original amount is not used up.b | 0.10 | 4.75 |
| 11 | 5ba | The rate of reaction is dependent on the concentration of the catalyst since a small amount is sufficient to change the energy profile of the reaction. | 0.13 | 4.40 |
| 12 | 5bc | The rate of reaction is dependent on the concentration of the catalyst since it is not used up in the reaction. | 0.10 | 3.50 |
| 13 | 5cb | The concentration of a catalyst does not change during the course of the reaction since it remains chemically unchanged.b | 0.18 | 4.00 |
| 14 | 6cc | 0.21 | 3.88 | |
| 15 | 7ac | 0.10 | 4.00 | |
| 16 | 8aa | 0.13 | 4.00 | |
| 17 | 8db | 0.41 | 4.63 | |
| 18 | 9dd | 0.18 | 3.57 | |
| 19 | 10aa | 0.18 | 3.57 | |
| 20 | 10ac | 0.23 | 3.56 | |
| S/n | Question (ans/reas) | Alternative conception | Prop. of sample with AC | CAC |
|---|---|---|---|---|
| a ACs also found in overall sample. b ACs also found in upper 25% of sample. | ||||
| 1 | 1ac | 0.41 | 3.61 | |
| 2 | 1bb | 0.18 | 5.00 | |
| 3 | 1bc | 0.16 | 1.00 | |
| 4 | 2aa | 0.30 | 2.46 | |
| 5 | 2cc | 0.30 | 2.08 | |
| 6 | 2cd | (less predominant than in upper 25% of sample) | 0.14 | 2.83 |
| 7 | 4ab | 0.23 | 3.00 | |
| 8 | 4ca | 0.18 | 2.75 | |
| 9 | 4cd | 0.14 | 4.17 | |
| 10 | 5aa | 0.16 | 3.14 | |
| 11 | 5ac | (similar predominance as in upper 25% of sample) | 0.11 | 3.80 |
| 12 | 5cb | (more predominant than in upper 25% of sample) | 0.25 | 4.36 |
| 13 | 6bd | 0.20 | 3.56 | |
| 14 | 6cc | 0.18 | 3.75 | |
| 15 | 6cd | 0.16 | 3.00 | |
| 16 | 7ca | In the reaction between NO(g) and O2(g), where the reaction is second order with respect to NO and first order with respect to O2, if the concentrations of both reactants are doubled, the rate of reaction increases by a factor of only 4 since this is determined by the reactant of higher order. | 0.11 | 2.20 |
| 17 | 8aa | 0.11 | 4.40 | |
| 18 | 8db | 0.59 | 4.15 | |
| 19 | 9dd | 0.34 | 3.50 | |
| 20 | 10aa | 0.30 | 3.08 | |
| 21 | 10ac | 0.48 | 3.00 | |
| 22 | 11aa | 0.16 | 3.71 | |
| 23 | 11bb | For an enzyme-catalyzed reaction which is carried out at 55 °C, the rate of reaction will be doubled at 65 °C since all molecules would move faster. | 0.14 | 2.50 |
We have not chosen to focus on the top 10% and bottom 10% of the sample for this particular analysis. This is because when the sample is bifurcated on this basis, an inordinately large number of ACs are obtained since all it needs is just one selection of any distractor to be classified as an AC since this fulfills the common 10% criterion for classifying a distractor as such. (It is to be noted that there are about 10 students each in the top and bottom 10% of the sample.) Thus, for enhanced statistical power and more generalizability, it is prudent to focus on the upper and lower 25% of the sample for such an analysis. Of course, with a larger sample size, it is possible to focus on the upper and lower 10% of the students.
Commentary on alternative conceptions in overall sample
In this section, we present a commentary on the key ACs derived from this study in the overall sample. Where appropriate, we leverage on our teaching experience as well to shed some insights and perspectives on the origins of the ACs.Question 1 makes a statement that the concentration of a reactant decreases linearly with time throughout the reaction and requires students to decide whether this means the reaction order is unity. It has to be borne in mind that for a first order reaction, the concentration versus time plot is a curve – that is, the concentration decreases but not linearly. If students do not keep this in mind, they are likely to harbor ACs. The four ACs found stem from misunderstandings related to this relationship. Given that the CAC values for the ACs range from 3.25 to 3.88, they are not due to guessing but more at not being able to understand the relationship between the two variables. There could also be a mix-up in students' understanding of the mathematical relationship between concentration and time for reactions with reaction orders from 0 to 3, and this could have also precipitated the ACs in the students' minds. The dependence of rate on concentration for the various reaction orders is relatively easy to grasp, but students may find it hard to see the link between this and the concentration versus time relationship, since different variables are involved and the conversion requires integration (∫).
Question 2 explores students' understanding of the relationship between the rate constants of two reactions with different activation energies upon a change in temperature. The activation energy represents the energy barrier that needs to be overcome before reaction can occur. Also, the rate constant for a reaction is a function of temperature – when temperature increases, the value of the rate constant also increases. Intuitively, one would expect the reaction with lower activation energy to benefit more from an increase in temperature. However, when one examines the Boltzmann distribution profiles (Fig. 2) for the two reactions more closely, it would become apparent that although the absolute increase in number of molecules with sufficient energy to overcome the activation barrier would be smaller for the reaction with higher activation energy, the percentage increase in number of molecules with sufficient energy is actually larger for the higher activation energy reaction. This is because the reaction with higher activation energy would have very few molecules with sufficient energy at the lower temperature, so even a small increase in number of molecules with sufficient energy represents a large percentage increase – hence, k2/k1 would be larger than k2′/k1′. This conclusion can also be verified mathematically using the Arrhenius equation. The foregoing needs to be kept in mind when answering this question correctly. The ACs derived from this question are thus likely to be due to inadequate understanding of these factors. Given that the CAC values for the four ACs range from 2.30 to 3.50, it is likely that the ACs originate more from an inability to appreciate the full import of the conceptual underpinnings of the mathematical relationship related to the Boltzmann distribution.
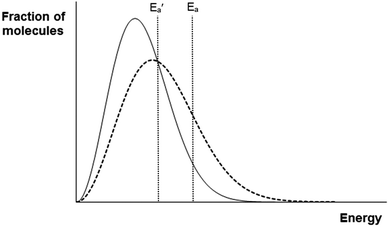 | ||
| Fig. 2 Boltzmann distribution profiles at T1 (solid line) and T2 (dashed line) for a sample of reacting molecules. | ||
Question 3 was originally intended to explore students' understanding of the physical significance of a graph of ln(k) against (1/T) with steeper slope (that the y-variable is strongly dependent on the x-variable), which is foundational to a good understanding of kinetics. However, after the administration of the instrument, we realized that the options in the answer and reason tiers were based on the erroneous assumption that k must be strongly dependent on T if ln(k) is strongly dependent on (1/T). The students' incorrect responses to this question are therefore inadmissible for the identification of ACs. Based on the Arrhenius equation, a steeper slope of the ln(k) vs. (1/T) graph indicates a higher activation energy, which leads to a smaller value of (k2 − k1) when the temperature increases from T1 to T2 (see the discussion of Question 2 above, but realize that this is based on k2/k1 and not k2 − k1). Since (k2 − k1)/(T2 − T1) is small, k is not strongly dependent on T, even though the ln(k) vs. (1/T) graph has a steep slope. This counter-intuitive result highlights the complexity inherent in making deductions about the relationship between k and T based on that between ln(k) and (1/T). It may be unreasonable to expect A-level students to navigate through such a complex deduction process. However, the fact that students selected all possible options in this question and that the question surfaced three ‘ACs’ suggest that students may be fixated on the notion that the correct responses must be among the options given.
Question 4 states that the rate of chemical reactions is doubled for a rise in temperature of 10 °C and students need to assert if this is true, false, or may or may not be. A large number of students asserted that this is true. Perhaps students cannot be faulted for having this idea as standard textbooks commonly mention that reaction rate doubles for every 10 °C rise of temperature. What has to be borne in mind is that this statement is just a rule of thumb for chemical reactions which occur in biological media, for which the activation energies and working temperatures span a range where increasing the temperature by 10 °C roughly doubles the rate of reaction. The actual factor by which the rate changes for a 10 °C rise in temperature depends on the initial temperature and activation energy, and this factor can deviate from 2 by a lot, as may be verified by using the Arrhenius equation. Given that the CAC values for the ACs range from 3.38 to 4.11, it is clear that the ACs are quite firmly entrenched in the students' minds.
Question 5 lists four statements about catalyzed reactions, of which one is not true and students need to identify this. This is a question which was not well answered. Various combinations of answer and reasons were given by students but only one combination reached the 10% criteria for classification as an AC: the rate of reaction does not depend on the concentration of a catalyst as even a small amount of it can change the energy profile of the reaction. A probable source for this AC is the often-made remark by teachers that even a small amount of catalyst can increase the rate of reaction tremendously, implying that adding more catalyst would not make a difference. Teachers should state explicitly that the rate would increase even further if more catalyst were added. The fact that the CAC for this AC is 4.0 means that it could be a firmly entrenched belief.
Question 6 lists four statements about rate constants, of which one is not true. It has to be noted that the rate constant for a reaction at a particular temperature has a singular value – even if the rate of reaction or concentration increases, the rate constant does not increase. Three ACs were found. About 14% of the students thought that the rate constant of a reaction does not increase when a catalyst is used as its value is dependent only on temperature. A possible reason for this AC could be that when students are taught the Arrhenius equation, temperature is explicitly included in the relationship but an explicit variable related to catalyst does not appear in the equation.
k = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−E/RT) exp(−E/RT) |
This may reinforce the point that only temperature affects the rate constant, and could have influenced the students to respond in this manner. However, students should know that the catalyst lowers the activation energy, which is a variable in the equation. Since the catalyzed reaction occurs via a different pathway of lower activation energy, the rate constant will increase, based on the mathematical relationship inherent in the equation. About 19% of the students thought that the rate constant of a reaction changes if the concentration of the reactants is increased since the reaction is faster when there are more reactant molecules per unit volume. While it is true that the reaction is generally faster when the concentration is greater, it has to be borne in mind that the increase is not at the expense of the rate constant, which is constant under the reaction conditions. The possible source for the AC could be the common word ‘rate’ in the terms ‘rate constant’ and ‘rate of reaction’ – students could have thought that as the ‘rate’ of reaction increases with increase in concentration, the ‘rate’ constant also increases. Another possible reason for the occurrence of the AC could be the negative phrasing of the question – students need to disagree with a statement that is false. The high CAC value (3.92) indicates that this could be a strongly held belief among the students. About 12% of the students gave a response that does not seem to make much sense: the rate constant changes if the concentration of the reactants is increased as its value depends only on temperature! This could also be due to the phrasing of the question – students need to disagree with one of the statements in the answer but that statement is phrased in a negative manner: ‘It does not change if the concentration of the reactants is increased’. The cognitive processing required to make sense of such statements is likely to be more compared to statements phrased in a direct manner. Given that the CAC value is about 3.27 for this AC, it can be addressed quite easily.
Question 7 asks by what factor the rate of reaction between nitric oxide and oxygen will change if their concentrations are doubled, given that the order of the reaction is 2 with respect to nitric oxide and 1 with respect to oxygen. This is a question which was generally well answered by the students. Only one AC was found among 10% of the students. These students obtained the correct answer of 8 but their reasoning does not make much sense – the factor by which the rate of reaction changes is equal to the factor by which the overall reactant concentration changes! The term ‘overall reactant concentration’ is absurd, but was included in the distracter to pick out students who have the tendency to use terms imprecisely and hence will not be able to realize that this term is invalid. The CAC value of 3.57 also suggests that it is unlikely to be due to guessing or lack of knowledge but more due to inability to string together the key concepts in an integrated manner to answer the question.
Question 8 probes how an increase in temperature affects the rates of the forward and backward reactions for the case where the reaction is exothermic. One AC that was harbored by nearly half of the students is that the rate of the forward reaction will decrease (CAC = 4.10) – also noted by Sözbilir et al. (2010) and Cakmakci (2010), but the rate of the reverse reaction will increase on account of the equilibrium being shifted to the left. From Le Chatelier's principle, it is clear that since the forward reaction is exothermic, an increase in temperature will facilitate the reverse reaction since it is endothermic and can absorb the increase in heat through shifting of the equilibrium to the left. What students need to remember is that an increase in temperature will increase the rates of both the forward and backward reactions but the reverse reaction is accelerated more in this case for the reason given. The source for the other AC that the rate will increase for the forward reaction and decrease for the reverse reaction owing to the equilibrium shifted toward the right is also due to students not appreciating the nuances in the applications of Le Chatelier's principle – the high CAC value of 4.25 indicates that this is a strong AC.
Question 9 is a simple quantitative problem that probes students' understanding of zero order reactions. It provides the value of the rate constant for a reaction of this type – 0.025 mol dm−3 s−1, and asks students for the reactant concentration after 15 s given that the initial concentration is 0.50 mol dm−3. This is a question that was generally well answered by the students but still 28% of the students held the AC that the concentration remains unchanged after 15 s for a zero order reaction. Students need to keep in mind that for zero order reactions, although the rate is independent of reactant concentration, the reactant is still consumed during the reaction. The AC could also be due to students not appreciating the full import of the rate constant given or the dimensional significance of its units. Since the rate constant is 0.025 mol dm−3 s−1, it means that the reactant concentration would decrease by 0.025 mol dm−3 every second. So, if students can understand the meaning of this unit, they should know that the reactant concentration would have decreased by (0.025 × 15) mol dm−3 after 15 s. Another reason for the AC could be that zero order reactions are not given adequate emphasis during teaching as this type of reactions is rather few as compared to first and second order reactions. Given that the CAC value is above the midpoint (3.59), it suggests that the full import of the dimensional significance of the units of rate constant of zero order reactions has not been properly cognized by the students.
Question 10 tests on students' understanding of the rate-determining step for a reaction (A + 2B + C → W + Z) which is thought to involve two-steps, the first of which has a reaction order of 2 and the second has a reaction order of 3:
Step 1: A + B → W + Q
Step 2: B + C + Q → Z
Very few students were able to get this question correct. It has to be noted that the rate-determining step in a multi-stage reaction is the one which is the slowest to occur. For the reaction in question, it has to be the second step since it entails the need for three different species to come together, a kinetically difficult situation and of probabilistically low occurrence. Whether an intermediate is involved has no bearing on whether a step is rate-determining. The three ACs found can be traced to students' lack of deep understanding of these concepts. A good number of students thought that the first step is rate-determining – it is likely they thought that since an intermediate species is generated in this step, the intermediate species is unstable and thus difficult to form, so it is the rate determining step! The somewhat high CAC value of 3.45 for this AC also suggests some support for this point of view. Some students also thought that the reaction with the lower reaction order is the rate-determining step (CAC = 3.21) – students could have been fixated on some perceived connotation between the terms ‘low and ‘slow’, and linked these together.
Question 11 tests students' understanding of enzyme-catalyzed reactions by asking which of the given four statements is/are incorrect. The only AC (CAC = 3.79) found is due to the students not keeping in mind that enzymes get denatured beyond about 45 °C, and that this affects not just the yield, but also the rate of the reaction. Again, the negative phrasing of the stem of the question could have also contributed to this AC since the cognitive processing is somewhat greater.
Commentary on ACs in upper 25% and lower 25% of sample
When the ACs are considered on the basis of their occurrences in the approximately upper 25% and lower 25% of the sample, it makes for interesting reading. Among the upper 25%, 20 ACs were found, with CACs ranging from 2.38 to 4.75 – 15 of the ACs were similar to those extracted for the overall sample. That means, there are 5 new ACs held by this group which did not manifest in the overall sample. For the lower 25% of the sample, not surprisingly, a slightly higher number of ACs (23) were found, with CACs ranging from 1.00 to 5.00 – of this, 18 of the ACs were similar to those found in the overall sample. That means, there are 5 new ACs found in this group that did not appear in the overall sample. Between the upper and lower 25% of the sample, there are 15 ACs which are common to both groups (including those in the overall sample).For the purpose of commenting on the ACs, we have restricted ourselves only to those found in the overall sample as these have greater generalizability. Furthermore, the majority of the ACs held by the upper and lower 25% of the sample are common to the overall sample. Similar interpretations as those for the overall ACs can be advanced for the ACs found in the upper and lower 25% of the sample. However, we have not commented on these as it would lengthen the manuscript unnecessarily.
Diagnostic utility of 3-tier instrument
The present study is among the very few studies which have explored the use of multi-tier diagnostic instruments for determining ACs harbored by students. A large number of studies have made use of two-tier diagnostic instruments, and these have made possible the documenting of a large number of ACs on several topics, all of which have implications for instruction and conceptual change in science. However, as mentioned earlier, two-tier instruments have a few limitations which can be reasonably addressed with the use of 3-tier or 4-tier instruments. Only a few studies have appeared on the use of 4-tier and 3-tier instruments in the journal literature. More studies are necessary on the use of such diagnostic instruments so as to assess its utility in various settings. The present study is a small contribution in this regard, with the focus on the 3-tier instrument format.The results of the present study attest to the fact that the 3-tier instrument is a viable format for documenting ACs. A total of 23 ACs have been documented in the overall sample. Also, some indication of the strengths of the ACs has been obtained. The strengths of the ACs provide some indication about the robustness with which these are entrenched in students. Obviously, ACs that are firmly entrenched would need more instructional effort for remediation. Except for one of the ACs, the other ACs in this study have not been reported in the literature.
In this instrument, for two of the questions, we used negative phrasing. Even though the cognitive processing is likely to be more, we felt that the junior college students, who represent the 30% of the primary school cohort who go on to study at this level, are intellectually equipped to handle such questions.
Implications
Reaction kinetics at the grade 12 level is a topic that has been the subject of relatively limited diagnostic interest. It is covered in various depths at the secondary, junior college, and university levels, and is generally a content-heavy topic. The current instrument can be used by teachers at advanced level for diagnosing aspects of students' ACs so that remediation of the relevant ACs can be undertaken as a sequel. Non-availability of diagnostic instruments is a key reason why teachers are not able to undertake diagnostic studies on their students on a topic (Morrison and Lederman, 2000).In place of interviews, the authors’ teaching experiences (along with other sources, as mentioned earlier) have been used to come up with not only a preliminary version of the instrument but also to comment on the nature of the ACs identified. Conventionally, student interviews are used in this regard in the literature. Owing to time, cost and scheduling constraints, we have opted instead to draw on our own teaching experiences to comment on the ACs. This type of approach has not been given adequate recognition in the science education literature. Two reasons can be advanced for this – researchers who have done studies on ACs on certain topics could have been primarily educational researchers in academia who use school samples for their study, and they may not have taught the topic. Secondly, there might be a line of thinking that since the ACs are harbored by students, it would be good to probe them for the genesis of these ACs. We have no qualms about this approach. However, we feel that instructor experience can also shed some useful light on the source of the ACs. For example, the first author has taught reaction kinetics at undergraduate level and through his teaching and interactions with students, who were earlier from A-level, is conversant with some of the learning difficulties and ACs students bring to this topic. The second author has experience with trainee teachers in chemistry and is also conversant with these issues among them. As these experiences were gathered over several years, we argue that these can be considered to have more generality than the thoughts of selected interview participants. The findings from the present study thus suggest that instructor experience can also be a useful source for understanding the nature of ACs harbored by students.
As noted by Sreenivasulu and Subramaniam (2014), ‘there is a need to go beyond documenting ACs and looking at other measures which can be used to assess the strength of these ACs as well as study student performance – psychometric measures such as CFC, CFW, CDQ and CB can help to inform on other aspects of learning’. The 3-tier instrument is a viable format for use in AC studies, and the strengths of the ACs provide some indication of the extent to which these are entrenched in students – this can come in useful when devising interventions to address the ACs. There is a need for more studies to be undertaken with this kind of format so that its utility can be assessed more robustly.
Limitations
The findings presented in this study represent the ACs found among a sample of students in two junior colleges in Singapore. As such, it cannot be generalized to the entire cohort or to all A-level students in the country.A diagnostic test, by its very nature, represents a difficult test. Students would generally score lower in such tests. Their performance in this test cannot be used as a proxy to gauge their overall knowledge of reaction kinetics since the test is restricted to limited aspects of the topic.
The test instrument featured questions on selected aspects of reaction kinetics that, from our teaching experience, students are known to have ACs. It cannot thus be regarded as a comprehensive diagnostic instrument on reaction kinetics – that would have lengthened the instrument beyond its intended purpose.
It is assumed that in indicating their confidence levels for each question, the students were conscientious in expressing this truthfully to the extent possible.
Conclusion
Students sat for the diagnostic test after having been taught a course on reaction kinetics, which involves both lectures and tutorials. From a cognitive standpoint, the students' performance in the test is not good – if correct responses for both answer and reason tiers are required to indicate good understanding, the mean score is only about 22%. It is clear that conventional teaching has not managed to address the ACs on reaction kinetics. This is understandable as the de facto approach in such teaching is to complete the syllabus and ensure that students are well prepared for the leaving level examinations, which determines the next trajectory in their life phase. Addressing ACs is thus likely to have been subordinated to the larger interests of the educational dispensation. There is a strong case for teaching for conceptual change. However, this would require teachers to be familiar with ACs on a topic and keeping these in mind while teaching so that these do not take root in students or are addressed. In the process, teaching can be made more effective and students' conceptual understanding can be enhanced further.The 3-tier diagnostic instrument developed in this study has permitted the identification of 23 alternative conceptions on reaction kinetics in the overall sample, including some indication of their strengths. Being among the very few studies that have used the 3-tier diagnostic instrument, it is suggested that the 3-tier format has good utility for diagnostic testing. The instrument on reaction kinetics can be used by teachers to diagnose ACs on some aspects of this topic.
In summary, the contributions of our study to the literature can be summarized as follows:
• A 3-tier diagnostic instrument on reaction kinetics has been developed.
• A total of 23 alternative conceptions on reaction kinetics has been identified – most have not been reported before.
• In previous studies on reaction kinetics, there was no mention of whether the ACs found are due to genuine misconceptions or lack of knowledge – the use of confidence ratings in our study has afforded some insights on segregating among these possibilities in the ACs we found.
• It is shown that the upper 25% and lower 25% of the sample have a number of ACs somewhat different from those present in the overall sample even though the majority are common – this approach is something which has not been given adequate attention in previous studies on misconceptions in science.
• Most studies on reaction kinetics have been done at the secondary and tertiary level – ours is among the very few that have focused at the high school level (grade 12).
Acknowledgements
We thank the reviewers for constructive comments on an earlier version of this manuscript. We also thank the Office of Education Research (National Institute of Education, Nanyang Technological University) for the award of a research grant (OER 40/08RS) to the authors.References
- Adams W. K. and Wieman C. E., (2011), Development and validation of instruments to measure learning of expert-like thinking, Int. J. Sci. Educ., 33(9), 1289–1312.
- Akkus H., Kadayifçi H., Atasoy B. and Geban Ö., (2003), Effectiveness of instruction based on the constructivist approach on understanding chemical equilibrium concepts, Res. Sci. Technol. Educ., 21, 210–227.
- Arslanab H. A., Cigdemogluc C. and Moseleyd C., (2012), A three-tier diagnostic test to assess pre-service teachers' misconceptions about global warming, greenhouse effect, ozone layer depletion, and acid rain, Int. J. Sci. Educ., 34(11), 1667–1686.
- Aydin S., Aydemir N., Boz Y., Cetin-Dindar A. and Bektas O., (2009), The contribution of constructivist instruction accompanied by concept mapping in enhancing pre-service chemistry teachers' conceptual understanding of chemistry in the laboratory course, J. Sc. Educ. Tech., 18(6), 518–534.
- Bain K. and Towns M. H., (2016), A review of research on the teaching and learning of chemical kinetics, Chem. Educ. Res. Pract., 17(2), 246–262.
- Bektaşli B. and Çakmakci G., (2011), Consistency of Students' Ideas about the Concept of Rate across Different Contexts, Educ. Sci., 36(162), 273–287.
- Bilgin İ. and Geban Ö., (2006), The effect of cooperative learning approach based on conceptual change condition on students' understanding of chemical equilibrium concepts, J. Sci. Educ. Technol., 15(1), 31–46.
- Cakmakci G., (2010), Identifying alternative conceptions of chemical kinetics among secondary school and undergraduate students, Turk. J. Chem. Educ., 87(4), 449–455.
- Çakmakci G. and Aydogdu C., (2011), Designing and evaluating an evidence-informed instruction in chemical kinetics, Chem. Educ. Res. Pract., 12, 15–28.
- Çakmakci G., Leach J. and Donnelly J., (2006), Students' ideas about reaction rate and its relationship with concentration or pressure, Int. J. Sci. Educ., 28(15), 1795–1815.
- Caleon I. and Subramaniam R., (2010a), Do students know what they know and what they do not know? Using a four-tier diagnostic test to assess the nature of students' alternative conceptions, Res. Sci. Educ., 40, 313–337.
- Caleon I. and Subramaniam R., (2010b), Development and application of a three-tier diagnostic test to assess secondary students' understanding of waves, Int. J. Sci. Educ., 32, 939–961.
- Çalik M., Kolomuç A. and Karagölge Z., (2010), The effect of conceptual change pedagogy on students' conceptions of rate of reaction, J. Sci. Educ. Technol., 19, 422–433.
- Cetin-Dindar A. and Geban O., (2011), Development of a three-tier test to assess high school students' understanding of acids and bases, Procedia – Soc. Behav. Sci., 15, 600–604.
- Hackling M. W. and Garnett P. J., (1985), Misconceptions of chemical equilibrium, Int. J. Sci. Educ., 7, 205–214.
- Justi R., (2003), Teaching and learning chemical kinetics, in Gilbert J. K., De Jong O., Justi R. Treagust D. and Van Driel J. H. (ed.) Chemical Education: Towards Research-based Practice, Dordrecht: Kluwer, pp. 293–315.
- Kaya E. and Geban Ö., (2012), Facilitating conceptual change in rate of reaction concepts using conceptual change oriented instruction, Educ. Sci., 37, 216–225.
- Kıngır S. and Geban Ö., (2012), The effect of conceptual change approach on students' understanding of reaction rate concepts, H. Ü. J. Educ., 43, 306–317.
- Kolomuç A. and Tekin S., (2011), Chemistry teachers' misconceptions concerning concept of chemical reaction rate, Eur. J. Phys. Chem. Educ., 3(2), 84–101.
- Kurt S. and Ayas A., (2012), Improving students' understanding and explaining real life problems on concepts of reaction rate by using a four step constructivist approach, Energy Educ. Sci. Technol. Part B: Soc. Educ. Stud., 4(2), 979–992.
- Laidler K. J., (1988), Rate controlling step: a necessary or useful concept? J. Chem. Educ., 65(3), 250–254.
- Lundeberg M. A., Fox, P. W., Brown, A. C. and Elbedour, S., (2000), Cultural influences on confidence: country and gender, J. Educ. Psychol., 92(1), 152–159.
- McClary L. M. and Bretz S. L., (2012), Development and assessment of a diagnostic tool to identify organic chemistry students' alternative conceptions related to acid strength, Int. J. Sci. Educ., 34(15), 2317–2341.
- Morrison J. A. and Lederman N. G., (2000), Science teachers' diagnosis of students' perceptions. Presented at the annual meeting of the National Association for Research in Science Teaching, New Orleans, LA.
- Popham W. J. and Husek T. R., (1969), Implications of criterion-referenced measurement, J. Educ. Meas., 6(1), 1–9.
- Seçken N. and Seyhan H. G., (2015), An analysis of high school students' academic achievement and anxiety over graphical chemistry problems about the rate of reaction: the case of Sivas province, Procedia, Soc. Behav. Sci., 174, 347–354.
- Sözbilir M., Pınarbaşı T. and Canpolat N., (2010), Prospective chemistry teachers' conceptions of chemical thermodynamics and kinetics, Eur. J. Math., Sci. Technol. Educ., 6(2), 111–120.
- Sreenivasulu B. and Subramaniam R., (2013), University students' understanding of chemical thermodynamics, Int. J. Sci. Educ., 35(4), 601–635.
- Sreenivasulu B. and Subramaniam R., (2014), Exploring undergraduates' understanding of transition metals chemistry with the use of cognitive and confidence measures, Res. Sci. Educ., 44(6), 801–828.
- Stankov L. and Crawford J. D., (1997), Self-confidence and performance on test of cognitive abilities, Intelligence, 25(2), 93–109.
- Supasorn S. and Promarak V., (2015), Implementation of 5E inquiry incorporated with analogy learning approach to enhance conceptual understanding of chemical reaction rate for grade 11 students, Chem. Educ. Res. Pract., 16(1), 121–132.
- Tan K. C. D., Goh N. K., Chia L. S. and Treagust D., (2002), Development and application of a two-tier multiple choice diagnostic instrument to assess high school students' understanding of inorganic chemistry qualitative analysis, J. Res. Sci. Teach., 39(4), 283–301.
- Tastan-Kırık Ö. T. and Boz Y., (2012), Cooperative learning instruction for conceptual change in the concepts of chemical kinetics, Chem. Educ. Res. Pract., 13, 221–236.
- Treagust D. F., (1988), Development and use of diagnostic tests to explore students' misconceptions in science, Int. J. Sci. Educ., 10, 159–170.
- Turányi T. and Tóth Z., (2013), Hungarian university students' misunderstandings in thermodynamics and chemical kinetics, Chem. Educ. Res. Pract., 14, 105–116.
- Van Driel J. H., (2002), Students' corpuscular conceptions in the context of chemical equilibrium and chemical kinetics, Chem. Educ. Res. Pract., 3(2), 201–213.
- Voska K. W. and Heikkinen H. W., (2000), Identification and analysis of student conceptions used to solve chemical equilibrium problems, J. Res. Sci. Teach., 37(2), 160–176.
- Yalçınkaya E., Taştan-Kırık Ö., Boz Y. and Yıldıran D., (2012), Is case-based learning an effective teaching strategy to challenge students' alternative conceptions regarding chemical kinetics? Res. Sci. Technol. Educ., 30(2), 151–172.
| This journal is © The Royal Society of Chemistry 2016 |