An ontological and epistemological analysis of the presentation of the first law of thermodynamics in school and university textbooks
Joaquín Castillo
Poblete
,
Rocío Ogaz
Rojas
,
Cristian
Merino
and
Waldo
Quiroz
*
Pontificia Universidad Catolica de Valparaiso, Instituto de Quimica, Campus Curauma. Avenida Universidad 330, Valparaiso, Chile. E-mail: waldo.quiroz@pucv.cl
First published on 30th July 2016
Abstract
Considering the relevance of thermodynamics to the scientific discipline of chemistry and the curriculum of the Western school system, the philosophical system of Mario Bunge, particularly his ontology and epistemology, is used herein to analyze the presentation of the first law of thermodynamics in 15 school and university textbooks. The ontological analysis shows that the concepts heat “q” and work “w” are categorized as processes, while the concepts of internal energy “U”, pressure “P”, volume “V” and temperature “T” are categorized as properties. The results reveal that 8 of the 15 textbooks incorrectly present work “w” as a property, while 7 textbooks incorrectly present heat “q” as a property. Furthermore, 3 textbooks present the concept of energy as a property and assign it a merely operational definition as the capacity to do work. The analysis also examined patterns of causality and the mechanism used to explain the connection between the variables of cause and effect in three thermodynamic systems. The results indicated that only 2 textbooks contain such a mechanism.
Introduction
In the natural sciences, the concepts of theory, hypothesis and law are generally confused by both professional scientists and science teachers (Lederman et al., 2002). One of the most influential philosophies for defining these concepts within the natural sciences is scientific realism, whose principal exponent, according to the journal Science, is Mario Bunge (Michel et al., 2011). One of the most notable features of this philosophy is the fact that it is not simply epistemological but also logical, ontological, axiological and metaphysical (Bunge, 1974a, 1974b; Bunge, 1977, 1979, 1983a, 1983b).The proposal defines the concepts of theory, law and hypothesis as follows. A scientific hypothesis is an idea, i.e., a statement, regarding a given material reality, which should have a basis and be empirically verifiable. Supporting evidence will then validate the idea as a scientifically legitimate hypothesis. A law is a subcategory of a hypothesis and identifies a pattern of causality and a proven mechanism that connects the variables of cause and effect. Finally, a scientific theory is a system comprising hypotheses logically connected by a relationship of deductibility, wherein the most general hypotheses are known as posits or axioms (Bunge, 2007).
Therefore, in this philosophy, the concepts of hypothesis, law and theory are closely linked, not only in how they are formed (epistemology) but also in their natural or material reference points (ontology and metaphysics). The ontology and the materialist metaphysics of this system offer important semantic tools for clarifying scientific concepts for teaching purposes. For example, in 2000, Mario Bunge clarified the concept of energy from an epistemological, an ontological and a metaphysical perspective. He categorized energy as the only property applicable to all material objects, thereby implying the mutability of reality (Bunge, 2000).
Using ontology and realist epistemology to analyze scientific concepts, in a previous study, our research group analyzed Boyle's Law from an ontological perspective, identifying the variables of pressure and volume as properties of a gaseous system, where the volume is the cause and the pressure of the gas is the effect. This perspective is consistent with the proven mechanism of molecular collisions. Analyzing 14 university-level general chemistry books from this perspective showed that 13 of the books contained serious ontological errors, such as omitting the pattern of causality or presenting it erroneously, namely by identifying pressure as the cause and volume as the effect (Quiroz and Rubilar, 2015). In a subsequent study, our group analyzed the concept of osmosis and its presentation in university-level biology and chemistry textbooks. The results showed that more than 50% of the books identifies osmosis as a process associated only with the property of concentration, thus reducing it to a mere diffusional process, without mentioning the state of equilibrium and the property of osmotic pressure (Spinelli Barria et al., 2016).
Many complex concepts can benefit from Bunge's ontology and metaphysics to ensure their correct presentation. It is clear that the evaluation of Mario Bunge's systematic philosophy provides an opportunity for science education (Matthews, 2012). However despite its great influence on the philosophy of science, the application of this philosophical system for the analysis of scientific concepts in science education studies has been comparatively minor and limited to a few works of Deleporte about the concept of “specie” in biology (Deleporte, 2012), a Bunge work on the concept of energy (Bunge, 2000) and our previous works on Boyle′s law (Quiroz and Rubilar, 2015) and the concept of “Osmosis” (Spinelli Barria et al., 2016). In more general terms, beyond the philosophical system of Mario Bunge, the use of ontology as a tool for the analysis of scientific concepts in science education studies is mostly limited to the work of Chi and colleagues on conceptual change and its relationship with ontology (Chi, 2008; Chi et al. 2012). The same problem arises in the case of the use of epistemology for the analysis of scientific laws where the only work we have found in the literature is from Kipnis related to Ohm's law (Kipnis, 2009). Considering the core relevance of classical thermodynamics to chemistry as a scientific discipline and the importance of chemistry knowledge in the Western school system, we deemed it relevant to apply this tool for analyzing the presentation of these concepts in science textbooks. Accordingly, this study aims to ontologically and epistemologically analyze the presentation of the first law of thermodynamics in chemistry textbooks used in universities and schools.
Classical thermodynamics
Logically and epistemologically, classical thermodynamics is a theory comprising four general scientific hypotheses, or axioms, all of which are laws. The first two laws are known as the zeroth and first laws and are mentioned in most general chemistry textbooks and the Chilean secondary education system.To analyze the presentation of these laws in scientific and school textbooks, we must first establish their ontology, i.e., the material references of their concepts, and their patterns of causality.
The zeroth law of thermodynamics states that a thermodynamic property called temperature exists. This law defines the state of thermal equilibrium as the point at which the temperatures of the two systems in contact are identical (Engel et al., 2007). The first law defines a second property of a system, namely the internal energy. Depending on the conditions, changes in internal energy (ΔU) can occur from or to the environment through the processes of heat and/or work. Mathematically, these changes are presented in the following way in most textbooks, as shown in eqn (1).
| ΔU = q + w | (1) |
The present study aims to visualize how the first law of thermodynamics is presented in university and school textbooks, while, if possible, discerning whether the law is presented with the correct pattern of causality and the mechanism responsible for generating a particular effect. It is also important in this study to identify the ontological definition attributed to this law and how concepts associated with it are used, such as internal energy, heat, work, volume, pressure and temperature. Considering the universal application of this law, our analysis focuses on perfect gases and reversible processes, which are the most common systems used to develop and explain this law in chemistry textbooks.
Ontological classification of the concepts of internal energy, temperature, heat and work
Mario Bunge's philosophy defines the following five ontological categories: “things or material objects”, “properties”, “processes”, “events” and “states” (Bunge, 1977). As explained below, the two categories relevant to the laws of thermodynamics are properties and processes.A property refers to a characteristic or an attribute of an object or a thing. Therefore, properties do not exist of their own accord. In the zeroth law, the concepts of temperature and internal energy “U” are properties because, as Mario Bunge explains, energy does not exist of its own accord; rather, an object with energy exists. This explanation is consistent with the evolution of caloric theory, which regards energy as an object, whereas contemporary thermodynamics regards it as a property (Levine, 2014). The same rationale holds for the concept of temperature “T”. That is, temperature does not exist; instead, objects with temperature exist, as specified by this law.
The most complex concepts to categorize ontologically are heat and work.
The concept of heat “q” can be explained as follows:
[⋯] the term “heat” is that it's like “hotness.” “Hotness,” as we illustrated above, refers to molecular motion, and motion is a Process. But the technical term heat, although a noun, actually refers not just to the motion of the molecules, but to the transfer of “hotness.” That is, heat is defined as “the transfer of energy” or energy in transit from one object or substance to another, and is therefore a Process. (Chi, 2008).
Given our perspective, we classified both work and heat as processes. For Mario Bunge, a process is a successive and sequential change in the state of an object, whereas a state is a set of properties that define an object at a given moment.
The concept of heat “q” therefore refers to a process of energy transfer that occurs when a system moves from an initial state of thermal disequilibrium to a final state of thermal equilibrium. Clearly, this concept is associated with states, the properties of energy and temperature and changes over time. Our classification is aligned with this reasoning. The concept of heat is not classified ontologically as a “form of energy”, as this classification would imply that heat is a property, i.e., an attribute of a material object, similarly to pressure or volume. Heat characterizes the change from a state of thermal disequilibrium to a state of thermal equilibrium. Thus, it is not an attribute of an object, but a manifestation of a change in internal energy based on differences in temperature. Heat refers to the process, whereas temperature and internal energy are properties of the different states, and the material object is the physiochemical system in which the process occurs.
The concept of work is also related to changes in states within a thermodynamic system. These states can be clearly identified in certain cases, for example, in the case represented below by the graph of volume “V” vs. pressure “P”, both of which variables, together with temperature T, describe the current state of the system. These variables change over time due to energy exchanges. As with the concept of heat, the concept of work is intrinsically connected to states, properties and changes. Accordingly, our classification of work as a process stems from this understanding of connectedness.
Therefore, proposing that a body “possesses” heat is as incorrect as stating that a body possesses work. Properties, not processes, are possessed, and they are possessed by material objects. Our ontological classification is summarized in Table 1.
| Variable | Ontological classification |
|---|---|
| U | Property |
| q | Process |
| w | Process |
| T | Property |
| P | Property |
| V | Property |
Pattern of causality of the zeroth law
The zeroth and first laws of thermodynamics hypothesize a pattern of causality. A semantic analysis of these laws within the framework of the materialist ontology of Mario Bunge's philosophy establishes the variables of cause and effect and the mechanisms responsible for forming the patterns of causality.For the zeroth law, the analysis is simple in that the law states that given two systems in contact and of identical temperature, thermal equilibrium is established, thereby implying that given two bodies with the same temperature (cause), the effect is that the difference in internal energy is zero (ΔE = 0), assuming that no other energy transfer occurs.
Similarly, given two bodies with different values of mass, m1 and m2, and different temperatures, T1 and T2, such that T1 > T2, an energy transfer will occur from the body with higher temperature to the body with lower temperature until thermal equilibrium is achieved at a final temperature Tf, as expressed by eqn (2):
| m2c2(T2 − Tf) = m1c1(Tf − T1) ≡ q | (2) |
Pattern of causality of the first law of thermodynamics
Because the first law explicitly involves three variables, ΔU, q and w, and implicitly involves T, P and V, analyzing its pattern of causality is complex and cannot be performed without considering the specific material system. The following section analyzes the patterns of causality for different specific systems.System with a mobile adiabatic wall
Consider a system consisting of an occluded gas in a chamber with a mobile but thermally isolated wall. Here, energy exchange does not occur via heat. Therefore, q = 0, so the first law is reducible to eqn (3):| ΔU = w | (3) |
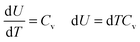 | (4) |
| w = −PdV | (5) |
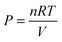 , yields
, yields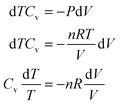 | (6) |
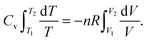 | (7) |
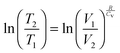 | (8) |
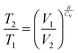 | (9) |
| ΔU(effect) ← ΔV(cause) |
A system with rigid diathermal walls
In the case of an occluded gas within rigid but thermally conductive walls, w = 0 because the volume does not vary. If this system is placed in contact with a system of a different temperature, then an energy transfer will occur via the heat process, represented by eqn (10):| ΔU = q | (10) |
| dqv = mcvdT | (11) |
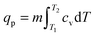 | (12) |
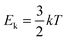 | (13) |
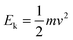 | (14) |
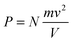 | (15) |
Hence, it is evident that if the system is isolated and has rigid walls, then the variation in internal energy is zero because the internal energy of the system remains constant, as specified by eqn (16).
| ΔU = 0 | (16) |
A closed system with mobile and thermally conductive walls
In a closed system with mobile and thermally conductive walls in which heat and work can be present in different proportions, the first law in eqn (1) is a valid mathematical expression in that the two processes, heat and work, are generated based on the initial and final conditions of the states involved.The cause variable for this type of system is established, which in certain cases, may be V, where a variation of this property generates an energy transfer via w, consequently varying the internal energy U. Thus, if the initial and final temperatures of the system are not identical, then the heat process is initiated.
The latter occurs because the original cause variable can be temperature or volume, depending on the conditions of the given material system. That is, a difference in temperature will spur an energy transfer, culminating spontaneously upon attaining thermal equilibrium. Depending on the final gas volume, which will depend on the external pressure, work “w” will be generated, and its magnitude will in turn depend on the distance the wall moves. The remaining energy will increase or decrease q and internal energy U. Table 2 shows the patterns of causality and the mechanisms on which they are based within the context of this proposal.
Teaching thermodynamics in the school system
In teaching chemistry, how a scientific idea is communicated to students is crucial, particularly when the goal is the scientific literacy of the public, in other words:A process of “focused investigation” that ignores conceptual reductionism to allow students to participate in the scientific adventure of facing relevant problems and (re)building scientific knowledge that teaching normally presents already built, thus favoring more efficient and more significant learning (Sabariego del Castillo, 2006).
The current science teaching focuses on transmitting established scientific knowledge. The challenge today, however, is ensuring this transmission includes processes of reflection and constant questioning whereby students can internalize the acquired knowledge, while rebuilding and using their initial conceptions as starting points to gain a fuller understanding of a particular topic. “The presence of these ideas in students is thus very relevant in the process of building knowledge, given that students learn based on what they already know” (Mahmud and Gutiérrez, 2010).
Students formulate different conceptions of a subject, based on their interaction with reality through their senses, the means by which common knowledge increasingly acquires its basis and structure and which will eventually be expressed through everyday language and thus communicated within society. Can we use everyday language to refer to scientific concepts, facts or phenomena? We know the answer, and we know that the use of everyday language, on many occasions, is incompatible with the concepts representing scientific knowledge. Thus, the language used to express a scientific idea must be articulated to communicate the underlying concept correctly so that the concept can be clearly and precisely understood, as described by Sanmartí et al. (1999): “A characteristic of this type of language is the specific vocabulary it possesses”. Terms are often used whose everyday connotation refers to the precise object. Moreover, in specific cases, such as heat and temperature, terms are used in the same contexts, but no differentiation is made in everyday language. This lack of differentiation can influence the ideas students form about the concepts.
About the concepts underlying the first law of thermodynamics it is important to mention that the ontological relationships between energy, work and heat is complicated and have evolved from the work of joule in the 19 century (Rosenberg, 2010). Therefore is not surprising that both teachers and students generate alternative ideas about the concepts of energy, work and heat.
For example, Kean et al. demonstrate that a significant number of seniors in chemical and mechanical engineering do not understand how temperature and energy are related (Kean et al., 2008). On the other hand Niaz evaluated the ability of science major freshman students to differentiate between heat energy and temperature demonstrating that even after having studied thermochemistry, students still have considerable difficulty in differentiating those thermodynamics concepts (Niaz, 2006). In 2013 Wattanakasiwich et al. developed a conceptual survey for assessing the understanding of more than 2000 student from Australia and Thailand of fundamental principles in thermodynamics. The results demonstrate that students have more difficulties in the integration of concepts related to the first law of thermodynamics and processes related (Wattanakasiwich et al., 2013). In another study of Laburu and Niaz they proposed a Lakatosian philosophical approach for the differentiation between heat energy in 32 ninth-grade students in a public school in Londrina, Brazil. The results demonstrate that some students were able to question the “hard-core” of their beliefs about heat, energy and temperature to construct a transitory model that increases progressively in their heuristic/explanatory model further towards the scientific model (Laburú and Niaz, 2002).
A study among students aged 12 to 23 years on alternative ideas related to the concepts of heat and temperature was performed and it was learned that a large proportion of students had incorrect prior conceptions, such as “the body has heat”, and “temperature is heat”. The study concluded that the influence of everyday language persists in the use and verbalization of many ideas associated with heat and temperature (Mahmud and Gutiérrez, 2010). This study is noteworthy because the first quote highlights the ontological confusion between a process (heat) and a property that is emphasized by the phrase “has heat”. The second quote equates temperature and heat, thus revealing an ontological confusion between property (temperature) and the process that triggers it (heat).
A study conducted at Ahi Evran University found that “of a sample of 60 second grade students at the department of sciences of the Faculty of Education, 38% do not comprehend the difference between the concepts of heat and temperature, using the different contexts indistinctly, which is in agreement with several studies in this area” (Kartal et al., 2011). Finally in an excellent review of Wong, Chu and Yap on alternative conceptions of the concept of heat they find that it can be classified into five categories: “residing in object,” “ontological”, “movement,” “cause and effect,” and “condition” which may be traceable to the linguistic usage or definitions in textbooks. In this work they demonstrate that even among scientists and science educators there are still some disagreement on the definition of heat considering it as an adjective, a noun, a verb, a process, a “form of energy”, thermal energy and molecular kinetic energy (Wong et al., 2016).
According to Carrascosa (2005), the origins of the alternative conceptions vary, but they can nevertheless be classified as follows:
The influence of everyday experiences in which the reiterative, sensorial and direct nature of these experiences and, fundamentally, the habitual way they are interpreted though the use of ordinary thought, leads to the internalization of certain explanations as unquestionable evidence.
As mentioned, the influence of verbal, visual and written communication is based on the premise that common language comprises words whose meaning is the fruit of everyday experiences transmitted by previous generations. Therefore, occasionally, this process of transmission can engender or perpetuate alternative ideas.
Thus, these conceptions are articulated coherently through our mental structure. They remain persistent in a field and resist conceptual change, thus prompting the following question: how can we refocus these conceptions and build a coherent scientific notion based on science? Although answering this question requires analysis from several different viewpoints, in the current context, one viewpoint is particularly relevant. This viewpoint encourages correctly using scientific language in science classes. It simultaneously recommends providing adequate tools and strategies to contend more critically and more reflexively with the reality presented in the literature or in scientific textbooks, based on the idea that all knowledge can be questioned and that questioning information obtained from different sources is the process that has generated scientific knowledge throughout history (Greenbowe and Meltzer, 2003).
We have discussed in general how articulating scientific language acquires importance in science teaching and how alternative conceptions can arise through personal experience. It is important to mention a third origin of these conceptions, as it is the focus of the present study, namely the conceptual errors or omissions in textbooks in the body of the text, images, or the captions accompanying the images. Conceptions can arise in secondary education and persist throughout higher education. They can also be reinforced by specific university textbooks such that the concepts formed prior to university learning are not refocused into accepted scientific knowledge, but remain generating alterative conceptions. Therefore, it is important to analyze a scientific text from a more critical and reflective viewpoint, which teachers should likewise encourage by providing students with adequate conceptual tools.
Because this study focuses, moreover, on the ontological classification of concepts, it is important to analyze the relevance of ontology and student misconceptions based on the existing literature (Chi et al., 2012) propose:
[⋯] that misconceptions are largely flawed inter-level causal explanations of the patterns of processes, and they are flawed in “structure” (and perhaps also incorrect in other ways, such as the technical details). By flawed in “structure”, we mean that the “type” of explanations might manifest an inter-level attribute that is ontologically inappropriate [⋯]
Building on this argument, Slotta, Chi and Joram propose that:
“students may classify science concepts according to these ontological categories (Material substances and processes) and then rely on these classifications in subsequent learning as a source of inference or sense making. To the extent that students are mistaken in their ontological categorization of a particular concept, we propose that they exhibit characteristic misconceptions” (Slotta et al., 1995).
It is clear that thermodynamics is a central issue in education in physics, chemistry, and biology. About that Dreyfus et al. state that “coordination between and among disciplines would be fruitful” (Dreyfus et al., 2015). We believe that the common discourse among these three scientific disciplines can be achieved through a materialistic ontology and also this ontology allows us to understand how introductory-level undergraduate students understand the central concepts to thermodynamics.
We believe that the ontological categories implicitly assigned to scientific concepts may generate alternative conceptions. For instance, it can be difficult to correctly learn that the concept of heat refers to a process of energy transfer, and not to a material object or a property.
The current science curriculum provides a large amount of information, prompting the following questions: Is it necessary to provide so much information? Are we encouraging student learning? Are we prioritizing significant and/or sustainable learning or mere memorization? We could continue asking similar questions that should be asked by anyone in the teaching profession.
In the context of thermodynamics and considering how its curriculum is defined, we identify several subject areas and concepts that are presented to students in the penultimate year of secondary education. These concepts include energy, heat, work, and temperature. They are defined in the area of physical chemistry and constitute the basis for comprehending several macroscopic phenomena and a wide range of physical and chemical processes.
Therefore, they must be taught correctly so that other chemistry concepts can be understood with a solid, correct and coherently structured conceptual basis, thereby allowing the student to more effectively comprehend other science subjects in the curriculum and thus build a body of knowledge that is adapted to the current demands of science and society. All of the named teaching considerations constitute a challenge for anyone involved in delivering science teaching and must be addressed to achieve significant advancements in the scientific literacy of the general public.
Text analysis
Textbook selection criteria
The criteria used to select textbooks were based on the work of Binn and Bell (Binns and Bell, 2015), Vesterinen et al. (Vesterinen et al., 2013) and Niaz and Fernandez (2008). The criteria are as follows:(a) The availability of the textbooks in universities, nearby libraries and high schools
(b) Textbooks that were used in previous years
(c) Textbooks that have been published in several editions and have been accepted by the science education community
(d) Consultations with colleagues teaching in different parts of the world revealed that various textbooks selected for this study are used as translations
(e) Various studies published in science education journals have used these textbooks
(f) Textbooks are regularly used by science teachers in the Chilean school system
The present study aims to evaluate the ontological classification and patterns of causality of the first law of thermodynamics by analyzing the presentation of the concepts of energy, internal energy, heat and work in the textbooks. From a pedagogical standpoint, it is also necessary to reflect on how this subject matter in specific physical chemistry textbooks may promote inferior levels of education because of possible errors contained in the textbooks and by identifying the major differences between the textbooks. Although the content of the university and school textbooks cannot be evaluated using identical criteria, due to the expected use of the books, we accept the idea that in science, concessions (e.g., simplifications) are permitted so that a scientific idea can be understood on different educational levels. However, this allowance is acceptable only when the underlying concept remains unaltered in an explanation and its meaning and interpretation do not generate errors and eventual alternative conceptions.
To achieve this aim, 15 textbooks from different educational levels were selected. As Table 3 shows, four textbooks are used in physical chemistry, eight textbooks are used in general chemistry, and three textbooks are used in secondary school education.
| Text | Publisher | Author | Year | Pages | ID |
|---|---|---|---|---|---|
| a These textbooks are school-level chemistry books. | |||||
| Química física | Pearson Educación | Engel T., Reid P. and Hehre W. J. | 2006 | 13–18, 22–23 | L1 |
| Química física | Médica Panamericana | De Paula A. and De Paula J. | 2007 | 28–40 | L2 |
| Fisicoquímica | McGraw-Hill Interamericana de España S.L. | Chang R. | 2008 | 76–84 | L3 |
| Fisicoquímica | Compañía Editorial Continental | Laidler K. J. and Meiser J. H. | 1997 | 45–54 | L4 |
| Principios de química: los caminos del descubrimiento | Médica Panamericana | Atkins P. W. and Jones L. | 2006 | 198–207 | L5 |
| Principios y reacciones | Thomson Paraninfo | W. L. Masterton and C. N. Hurley | 2004 | 212, 229–231 | L6 |
| Química la Ciencia Central | Pearson educación | H. E. L. Theodore L. Brown, Jr., Bruce E. Bursten and Julia R. Burdge | 2004 | 152–162 | L7 |
| Química y reactividad química | Thomson Paraninfo | J. C. Kotz, P. M. Treichel and G. C. Weaver | 2005 | 203–220 | L8 |
| Química | McGraw-Hill | K. W. Whitten, K. D. Gailey and R. E. Davis | 2008 | 547–551, 566–569 | L9 |
| Química | McGraw-Hill | R. Chang | 2007 | 224–234 | L10 |
| Principios de química | Panamericana | P. Atkins and L. Jones | 2012 | 235–249 | L11 |
| Química | McGraw-Hill | R. Chang and K. Goldsby | 2014 | 231–241 | L12 |
| Texto del estudiante química 3°–4° medio | Ediciones Calycanto | Cabello M. | 2015 | 21–22, 26, 30–36 | L13a |
| Química III medio | Empresa Editora Zig-Zag, S.A. | M. Contreras, G. Cordano, M. Rojas and J. Valenzuela | 2010 | 16–25 | L14a |
| Química III medio | Santillana Bicentenario | N. Arancibia, O. Ortega, W. Figueroa, S. Torres and R. Pérez | 2012 | 12–25 | L15a |
Results and discussion
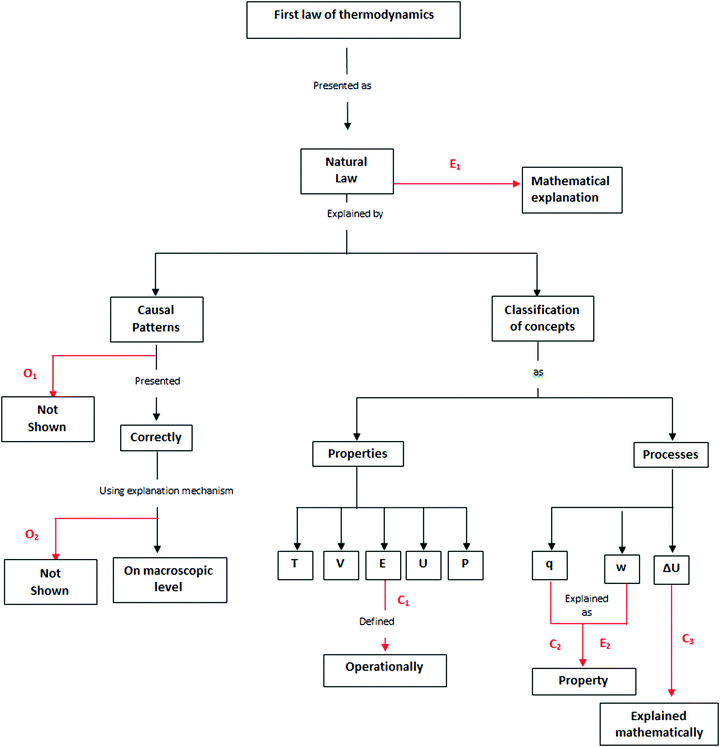
To systematically analyze the presentation of the first law of thermodynamics, a tool was created to aggregate the relevant information and classify it according to the ontologically and epistemologically defined categories presented at the beginning of the article. This aggregation was performed by identifying the relevant concepts and assigning a category based on the results. The results of the categories are shown in Table 4, and the tool is shown in Fig. 1.| Classification | |
|---|---|
| Errors | |
| E1 | This error is epistemological. In the mathematical presentation of the law in association with different material systems, the variables of cause and effect are confused. |
| E2 | This error is ontological and arises from identifying concepts that refer to processes (i.e., q or w) as being properties (i.e., T, U). |
| Contradictions | |
| C1 | This contradiction refers to defining internal energy as a system property, whereas the concept of energy is operationally “the capacity to do work”. |
| C2 | This contradiction refers to defining the concepts of heat and work as processes, though when used within the text, they are explicitly identified as forms of energy. |
| C3 | This contradiction occurs when the state of a system is claimed as depending only on the thermodynamic properties it possesses, whereas when the process of the variation of internal energy is presented, the processes of heat and work are mentioned, thus neglecting to discuss the properties that define these states, namely P, V or T. |
| Omissions | |
| O1 | This omission occurs when the law and the underlying processes are disconnected from causal patterns. |
| O2 | This omission occurs when the law is presented, but not in association with an explanation mechanism that links the cause and the effect variables. |
Tool validation
A main obstacle to detecting ontological or epistemological errors, omissions or contradictions in textbooks is guaranteeing the objectivity and reproducibility of the classification. To verify both types of typological testing, a double codification of the same textbook was performed by two independent observers. A correlation between both classifications was calculated, following Eltinge and Roberts (Eltinge and Roberts, 1993) and based on the statistical tool developed by Kappa de Fleiss, Cohen, and Everitt (Fleiss et al., 1969) which has been widely used in several text analyses (Lemoni, Lefkaditou, Stamou, Schizas and Stamou, 2013; Lemoni, Stamou and Stamou, 2011).The kappa coefficient is a measure of agreement between two observers. It was calculated within a range of 0 to 1, where 0 indicates no agreement, and 1 indicates full agreement.
The kappa statistic is calculated by constructing a contingency table based on the classifications of each observer. Eqn (17) specifies how to calculate the Kappa statistic determination.
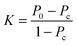 | (17) |
The Kappa coefficient obtained by two independent observers was 0.89, which is close to 1. Thus, we have a high degree of confidence in our results.
Textbook analysis of the presentation of the first law of thermodynamics in perfect gas systems
The possibilities of conducting an ontological and epistemological analysis of scientific concepts are numerous. Both authors and the aforementioned investigations demonstrate some of the possibilities, for example, analyzing the evolution of the textbooks of different editions (Quiroz and Rubilar, 2015) and studying the presentation of the same concept in different scientific disciplines (Spinelli Barria et al., 2016).The analysis of the information in the selected textbooks is summarized in two tables, with different categories, thus exemplifying the first approach to the proposed study. Table 5 contains two categories of analysis (CAs). The first category indicates whether the text correctly defines the concepts of energy (E), internal energy (U), heat (q) and work (w) in accordance with the ontological categories previously defined (A). The second category identifies the contradictions in the textbooks (B) regarding the processes of variation in internal energy (ΔU), heat and work. Analogously, Table 6 shows the analysis of the first law of thermodynamics in the following three systems: adiabatic with mobile walls, diathermal with rigid walls, and closed with mobile and thermally conductive walls. The analysis examines how the variation in internal energy is presented and whether an explanation mechanism (M) connecting the cause (Ca) and effect (Ef) is presented for the variation.
| CA | L1 | L2 | L3 | L4 | L5 | L6 | L7 | L8 | L9 | L10 | L11 | L12 | L13 | L14 | L15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O1: no causal pattern is provided; P: presented in the correct form; C1: concept of energy is understood operationally; E2: error in ontological classification. C2: contradiction of processes as forms of energy; C3: mathematical explanation without reference to causal patterns. | ||||||||||||||||
| A | E | C1 | C1 | P | P | C1 | C1 | C1 | C1 | C1 | C1 | C1 | C1 | P | C1 | C1 |
| U | P | P | P | P | P | P | P | P | P | P | P | P | P | P | P | |
| q | E2 | P | P | E2 | P | E2 | E2 | E2 | P | P | E2 | P | E2 | P | P | |
| w | E2 | E2 | P | E2 | E2 | E2 | E2 | E2 | P | P | P | P | E2 | P | P | |
| B | ΔU | P | P | C3 | C3 | P | C3 | C3 | C3 | C3 | C3 | P | C3 | C3 | C3 | P |
| q | P | C2 | C2 | P | C2 | P | P | C2 | C2 | C2 | C2 | C2 | P | C2 | C2 | |
| w | P | P | P | P | P | P | P | C2 | C2 | P | C2 | P | P | C2 | C2 | |
| Adiabatic system | Diathermal system | Closed system | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ca | M | Ef | Ca | M | Ef | Ca | M | Ef | |
| E1: error in epistemological classification; P: presented correctly; O1: no causal patterns provided; O2: no explanation mechanism provided. | |||||||||
| L1 | P | P | P | P | P | P | P | P | P |
| L2 | P | P | P | P | O2 | P | E1 | P | P |
| L3 | P | O2 | P | E1 | O2 | P | P | P | P |
| L4 | P | P | P | E1 | O2 | P | E1 | P | P |
| L5 | P | P | P | P | P | P | E1 | O2 | P |
| L6 | O1 | O2 | O1 | E1 | O2 | P | E1 | O2 | P |
| L7 | O1 | O2 | O1 | O1 | O2 | O1 | E1 | O2 | P |
| L8 | O1 | O2 | O1 | E1 | O2 | P | E1 | O2 | P |
| L9 | O1 | O2 | O1 | E1 | O2 | P | E1 | O2 | P |
| L10 | O1 | O2 | O1 | E1 | O2 | P | E1 | O2 | P |
| L11 | P | P | P | P | P | P | P | P | P |
| L12 | O1 | O2 | O1 | E1 | O2 | P | E1 | O2 | P |
| L13 | O1 | O2 | O1 | O1 | O2 | O1 | E1 | O1 | P |
| L14 | O1 | O2 | O1 | O1 | O2 | O1 | E1 | O1 | P |
| L15 | O1 | O2 | O1 | O1 | O2 | O1 | P | P | P |
Table 5 shows that the most frequent results are “C1” and “E2”, pertaining to the ontological classification of the concepts E, U, q and w (A). For example, 11 of the 15 university textbooks define the concept of energy merely operationally, without linking it ontologically to a property. This presentation contrasts with that of the concept of internal energy, whose ontological connection to a property is present in all the textbooks. Regarding the concepts q and w, w is erroneously defined as a property in 8 of the 15 textbooks, and the same error (E2) occurs with q in 7 of the 15 textbooks.
As described above, energy is a property possessed by all things, i.e., a universal property that also allows the mutability of all things, i.e., change. Because material properties do not exist in isolation from things or material objects, reference will always be made to the energy possessed by a certain thing. In thermodynamics, this energy is internal energy (U) (the energy of a thermodynamic system). Therefore, defining energy as the “capacity to do work” limits comprehension of the concept as merely the consequence of a body possessing energy, thus severing the concept from the definition and ontological classification that years of research have established.
Some textbooks classify the concepts of heat and work as “forms of energy” (E2), evidenced by sentences such as “… The energy is transferred in the form of heat or work …” (Atkins and Jones, 2006).
This classification construes these concepts as properties. This construal is not arbitrary because if we understand energy as a property, then its manifestations acquire an identical ontological classification. However, our classification indicates that “q” and “w” are processes of energy transfer.
If we use the definition of heat and work as forms of energy and therefore, properties, we can state that a body “possesses heat or work” or that it is able to transfer heat and/or work. However, this statement generates a misunderstanding of the nature of these concepts due to an incorrect ontological classification and the incorrect use of the concept.
Similarly, regarding heat, phrases such as the following classify heat ontologically as a “thing” in claiming that it can flow or pass between systems or between the system and its surroundings: “[⋯] During the reaction, heat flows out of the system (q < 0), and internal bath temperature increases to Tf [⋯] (Engel et al., 2006); and [⋯] When two systems are at different temperatures, heat can pass one to another directly [⋯]” (Laidler and Meiser, 1997).
Assigning the characteristic of flowability to heat echoes the assertion of caloric theory, which regards heat as a material substance and claims that its transfer causes temperature variations in the system. This theorization is also consistent with the following statement:
Early chemists described heat according to the caloric theory, in which an object's temperature was proportional to the quantity of caloric contained in the object. This early view, which attributed to the heat a substance ontology, was seen as a process of molecular excitation (Slotta et al., 1995).
With regard to the presence of a pattern of causality for the processes ΔU, q and w, it can be observed that these processes are mostly presented correctly in the textbooks. That is, the textbooks explicitly state that U will vary depending on the variation of properties that define the state of the system (T, P, V). They also specify that q will accompany a state of thermal disequilibrium and that “w” will arise only when the pressures between the system and the environment differ. However, analyzing the results presented in Table 5 (B), it can be stated that many of the textbooks contain contradictions, mainly regarding what the processes “ΔU”, “q” and “w” refer to.
With regard to the first of these three processes, the most frequent contradictions relate to internal energy. Ten textbooks present a type-3 contradiction (C3) in which the internal energy of a system is correctly defined as a property that depends on the current system state, which is determined by the properties T, P V and n. Subsequently, however, the textbooks explain that the variation in the state results from heat being absorbed or released by the system or the work performed by or upon the system, disconnected from the properties that cause the variation itself. For example, consider the following statements: “The first law can be expressed by saying that the change in the internal energy of a system, ΔU, is the sum of the heat q that enters the system and the work w that is done upon it: ΔU = q + w” (Laidler and Meiser, 1997); and “if work is done on a system or if heat is given to it, its energy will increase” (Masterton et al., 2004). In these statements, the first law is connected only with the processes causing variation in internal energy, while the remaining disconnected from the causes of the variation (q and w) that generate E1. The concept of heat is also classified as other than a process in claiming “heat that is given” and alluding to heat as transferrable between systems or between the system and the environment. Thus, heat is classified as a property.
We can therefore ask the following question: how are the variables (T, P, V and n) connected to the variation in internal energy in the presentation of the first law? Does the variation in the internal energy depend directly on q and w, or do these processes constitute part of the mechanism that generated this variation in the system? These questions are being answered throughout this article; however, it is important to reflect on them and ensure that this reflection allows us to identify the optimal presentation of the first law of thermodynamics, including the causal patterns that cause the change in the state of a particular system.
Regarding “q” and “w”, the contradictions found in the analysis are mostly of type C2, as the concepts are described as “forms of energy”. Why are these descriptions contradictory? They are consistent with the correct ontological classification, but when a concept is used in an explanation, either in an image or within the text itself, it refers to an incorrect ontological classification. For example, the most common case is when heat and work are presented as processes of energy transfer and then used in statements such as:
– “The work transferred to or from the system” (Kotz et al., 2005)
– “The other component of the internal energy is the Heat q” (Chang and Herranz, 2007)
– “Therefore, heat and work with properties of direction or path and not states” (Laidler and Meiser, 1997)
– “If the gas absorbs heat from the environment” (Chang, 2008)
All of these examples insinuate that “q” and “w” are properties.
In 10 of the 15 textbooks, this contradiction occurs for the concept of q and in 5 textbooks for the concept of w. As previously noted, in science, and in this case, chemistry, concessions are made. That is, certain statements help simplify explanations and allow people who do not possess in-depth knowledge of a subject area to nonetheless understand it. Notwithstanding the usefulness of concessions for learning, we believe that it is important to obtain a consensus regarding a concept and its explanation. With this consensus, we can avoid altering the correct definition and/or the ontological classification, thus ensuring that the meaning of the concept remains unchanged. Otherwise, conceptual errors appear because of didactic concessions. For example, several textbooks state that q is a process, but use the concept to discuss the flow of heat, thereby leaving the concept amenable to interpretation and distortion, as heat can thus be understood as a “thing” transferrable from one system to another system or a property of thermodynamic systems that describes their state.
Table 6 indicates that the presentation of the first law of thermodynamics as applied to the three types of systems exhibits a common pattern across the textbooks. Namely, no significant differences were observed in how the information is presented. Although the degree of complexity varies depending on the educational level of the students and their knowledge of the area, the errors, omissions and contradictions discovered in the analysis are similar across textbooks used in university-level physical chemistry and general chemistry courses and even in school-level textbooks.
The results show a high frequency of the presentation of the law in these three systems without an explanation mechanism connecting the variables of cause and effect (O2). That is, the effect (ΔU) is associated with a cause, but this effect does not correspond to the properties determining the state of the system (T, V, P). Moreover, the cause described in the text is the sum of heat and work, so the first law is presented in a merely mathematical way (E1). Hence, it is impossible to clearly visualize how the internal energy of a system changes and identify which properties cause the change. Therefore, the pattern of causality is unfounded, and it is unclear which variable is the cause and which variable is the effect.
Additionally, several textbooks do not refer to the application of the first law for systems with mobile adiabatic walls or for systems with rigid diathermal walls (designated O1 because the causal patterns of these processes are not given). This lack of reference prompts the following question: is it important to explain this law in relation to other types of systems? We would answer that it is necessary to extend the presentation and explanation of the first law to other systems because although the law is the same, its explanation mechanism differs depending on the characteristics of a particular system (see Table 2). Therefore, if the law and thus the mechanism linking the cause and effect variables of the system are not noted for different types of systems, it is impossible to understand the nature of the change in internal energy, i.e., to understand the actual cause of the change and the process generating the effect. Although internal energy is a function of the state and depends on not the path pursued, but the initial and final states of the system, when presenting the law and discerning its epistemological referent, the presentation of the law (a scientific hypothesis that defines a pattern of causality and links the latter to a mechanism of explanation) must clearly explain how the law manifests in material reality by specifying the cause and the associated mechanism and effect. Without these specifications, the presentation of the law remains merely a scientific hypothesis.
Presentation of the first Law of thermodynamics' proposal
Now we will make a proposal for the presentation of the first law using the appropriate ontological concepts based on our philosophical framework.The internal energy is that state of a thermodynamic system that represents other forms of energy (kinetic and potential energy) and that merely depends on the variables/properties that define the state of the system (P, V and T). Thermodynamics is in charge of studying the changes in internal energy that a system undergoes, directly related to noticeable macroscopic evidence related to the properties mentioned above.
The first law of thermodynamics states that the internal energy of a system can vary through the implementation of two processes, work and heat, as they are expressed in the following equation:
| ΔU = q + w |
(Considering a system with diathermic mobile walls).
Both processes are triggered by the variation of some of the properties that define the state of the system (T, P and V). In relation to the heat process, it is correct to claim that it will take place, if and only if, an initial state of thermal imbalance exists between two systems, or between the system and its surrounding. In the case of the external temperature being higher than the one of the system (T surrounding > T system) the energy transference will occur from the zone of higher temperature to the one with a lower temperature, so the system's temperature will increase with the purpose to reach a state of thermal balance. When this happens, the internal energy of the system will increase. In relation to the working process, it is correct to affirm that it will take place, if and only if, there is a difference in pressure between the system and its surrounding. In the case in which the external pressure is higher in comparison to that of the system (P surrounding > P system) an energy transference will occur in the system with the purpose of reaching a balance between both pressures, so the decrease in the volume of the system will cause an increase in the pressure and gas temperature. When this happens, the energy in the system will increase.
Considering this, it is possible to establish an expression that represents a sign that acquires the energy transferred through those processes and the relation that they have with the variation of the internal energy of a thermodynamic system.
The internal energy of a system will increase when:
– The system increases its temperature by the transference of energy through the heat process. The energy transferred acquires a positive value and can be represented as: q > 0
– The system increases its temperature as a product of the energy transference through the work process, producing a decrease in the volume and pressure rise of the gas. The energy transferred acquires a positive value and can be represented as: w > 0
On the other hand, the internal energy of a system will decrease, when:
– The system will decrease its temperature as a product of the transference of energy to its surrounding through the heat process. The energy transferred acquires a negative value and can be represented as: q < 0
– The system will decrease its temperature as a product of the transference of energy to its surrounding through the work process, producing an increase in the volume and therefore a decrease in pressure of the system. The transferred energy acquires a negative value and can be represented as: w < 0
By making reference to a closed system with diathermic and mobile walls, both processes of transference of energy can take place, so the variation of the internal energy of the system will depend on the sign that acquires the transferred energy according to what has been discussed beforehand.
Conclusion
The study of science education across all scientific disciplines can develop tools based on ontology and epistemology to analyze the presentation of scientific concepts in textbooks. The epistemology and ontology of Mario Bunge's philosophical system are sufficiently general and orienting for this purpose.Our results indicate that the thermodynamic concepts heat “q” and work “w” must be presented as processes, while considering their underlying dynamic nature. The concepts of energy, internal energy “U”, volume “V”, pressure “P” and temperature “T” must be presented explicitly as properties.
To accurately present the first law of thermodynamics, we propose specifying in the text that patterns of causality and the mechanisms of this law depend considerably on the systems to which this law applies. The first law must be presented with the mechanisms and patterns of causality in the context of at least one particular system.
We believe that the ontological categorization of concepts provides one way to make learning concepts more significant because it helps establish a direct relationship between the concept and its material basis.
Ontological analysis aims to examine concepts and propositions and is a process of combining ideas within certain propositions and using ontological categories (thing, state, event, property and process) to imbue the provided information with meaning. Our ontological analysis of the concepts of heat and work indicates that these concepts are widely classified as properties either in their definition or in their application in explaining a thermodynamic unit. The concepts are explained as forms of energy and thus are classified as properties rather than processes. Consequently, the nature of the concept is incorrectly presented, as its ontological classification is erroneous for one of two reasons. First, the concept may be defined correctly and assigned the correct ontological classification. Subsequently, however, its application construes its nature as belonging to a different ontological category. Second, it is incorrectly defined, and this incorrect classification is then used to explain a particular system.
Natural laws corresponding to an epistemological category indicate patterns of causality linked through a mechanism that generates a determined effect. However, the textbooks analyzed did not completely provide this indication as follows: the first law of thermodynamics was assigned attributes that are merely mathematical or operational, thus hindering reflection on the subject matter by providing only a formula in which to substitute values and calculate the outcome of variables presented without their ontological references, i.e., without the properties and processes that arise from them. These textbooks present the first law of thermodynamics in terms of the processes that cause variations in the internal energy of the system. However, no direct link is established between the properties that describe the system state and that of the original variables, which precede changes in the state and therefore, variations in internal energy.
It was also common for the textbooks to define energy from an operational perspective, thus failing to clearly represent its ontological classification. Although energy is connected with material objects as described above, the concept of energy is associated with not a universal property, but the mutability that it supplies material systems, i.e., the changes observed in these systems following the variation of one or more properties of that system. This mutability is indicated in the definition of energy as the capacity to do work.
Due to these difficulties that have been previously recognized, there is necessity to modify the way in which the first law of thermodynamics is presented appears, whether in scientific texts or at the moment to teach it to high-school or higher education students. To do so, we consider relevant at the moment to present the first law of thermodynamics, the following aspects be explicitly mentioned:
To establish a relation between the type of system that experiences a change, the mechanism associated with the process that is taking place, and the causative patterns implied that give origin to it.
– To connect the process of energy transference to the properties of the system (P, V and T)
– To present heat and work as transference of energy processes, where causative patterns are the properties of the system.
We believe that it is also important to be able to identify the relationship between the three levels of representation of a subject. Only by doing so is it possible to generate integrated knowledge, namely by establishing connections between perceivable facts (phenomena) and non-perceivable facts, which comprise most facts relating to reality. Although such facts are not perceived, they can be understood by encouraging actions such as conjecture, model creation, and the construction of connections between symbols and between micro- and macroscopic levels.
Finally, if we aim to make our students learn the sciences, then we must help them acquire more adequate, clearer and more precise language to ensure they are able to communicate ideas coherently and articulately. They also require the correct tools to comprehend reality from a critical and analytical viewpoint, such that questioning plays a fundamental role in their lives, consistent with the principle that all knowledge can be questioned and perfected. From our perspective, this pedagogical path is optimal.
Acknowledgements
This scientific product is derived from FONDECYT research 1150659. The authors gratefully acknowledge sponsors from CONICYT – CHILE and project DREAMS 0.37.307/2015.References
- Atkins P. W. and Jones L., (2006), Principios de química: los caminos del descubrimiento, Médica Panamericana.
- Binns I. C. and Bell R. L., (2015), Representation of Scientific Methodology in Secondary Science Textbooks, Sci. Educ., 24(7–8), 913–936, DOI: http://10.1007/s11191-015-9765-7.
- Bunge M., (1974a), Semantics II: Interpretation and Truth, Springer.
- Bunge M., (1974b), Treatise on Basic Philosophy: Semantics I: Sense and Reference, Netherlands: Springer.
- Bunge M., (1977), Treatise on Basic Philosophy: Volume 3: Ontology I: The Furniture of the World, D. Reidel.
- Bunge M., (1979), Treatise on Basic Philosophy: Ontology II, Netherlands: Springer.
- Bunge M., (1983a), Epistemology & Methodology I: Exploring the World, Netherlands: Springer.
- Bunge M., (1983b), Treatise on Basic Philosophy: Volume 6: Epistemology & Methodology II: Understanding the World, Netherlands: Springer.
- Bunge M., (2000), Energy: between physics and metaphysics, Sci. Educ., 9(5), 457–461.
- Bunge M., (2007), La investigación científica.
- Carrascosa J., (2005), El problema de las concepciones alternativas en la actualidad (parte I). Análisis sobre las causas que la originan y/o mantienen, Revista Eureka sobre enseñanza y divulgación de las ciencias, 2, 183–208.
- Chang R., (2008), Fisicoquímica, McGraw-Hill Interamericana de España S.L.
- Chang R. and Herranz R. Z., (2007), Química, McGraw-Hill.
- Chi M. T. H., (2008), Three Types of Conceptual Change: Belief Revision, Mental Model Transformation, and Categorical Shift In, in Vosniadou S. (ed.) International Handbook of research on conceptual change, Hillsdale, NJ: Erlbaum, pp. 61–82.
- Chi M. T. H., Roscoe R. D., Slotta J. D., Roy M. and Chase C. C., (2012), Misconceived causal explanations for emergent processes, Cognit. Sci., 36(1), 1–61, DOI: http://10.1111/j.1551-6709.2011.01207.x.
- Deleporte P., (2012), The Systemist Emergentist View of Mahner and Bunge on ‘Species as Individuals’: What Use for Sci. Educ.? Sci. Educ., 21(10), 1535–1544, DOI: http://10.1007/s11191-012-9438-8.
- Dreyfus B. W., Geller B. D., Meltzer D. E. and Sawtelle V., (2015), Resource Letter TTSM-1: Teaching Thermodynamics and Statistical Mechanics in Introductory Physics, Chemistry, and Biology, Am. J. Phys., 83(1), 5–21, DOI: http://10.1119/1.4891673.
- Eltinge E. M. and Roberts C. W., (1993), Linguistic content analysis: a method to measure science as inquiry in textbooks, J. Res. Sci. Teach., 30(1), 65–83, DOI: http://10.1002/tea.3660300106.
- Engel T., Reid P. and Hehre W. J., (2006), Química física, Pearson Educación.
- Engel T., Reid P., Hehre W., Rodríguez A. R., Román J. Z. and Pascual A. B., (2007), Introducción a la fisicoquímica: termodinámica, Pearson Educación.
- Fleiss J. L., Cohen J. and Everitt B. S., (1969), Large sample standard errors of kappa and weighted kappa, Psychol. Bull., 72(5), 323–327, DOI: http://10.1037/h0028106.
- Greenbowe T. J. and Meltzer D. E., (2003), Student learning of thermochemical concepts in the context of solution calorimetry, Int. J. Sci. Educ., 25(7), 779–800.
- Kartal T., Öztürk N. and Yalvaç H. G., (2011), Misconceptions of science teacher candidates about heat and temperature, Science Direct, 15, 2758–2763.
- Kean A., Miller R., Self B., Moore T., Olds B. and Hamilton E., (2008), Identifying robust student misconceptions in thermal science using model-eliciting activities. Paper presented at the 2008 ASEE Annual Conference and Exposition, Pittsburg, PA.
- Kipnis N., (2009), A Law of Physics in the Classroom: The Case of Ohm's Law, Sci. Educ., 18(3), 349–382, DOI: http://10.1007/s11191-008-9142-x.
- Kotz J. C., Treichel P. and Weaver G. C., (2005), Química y reactividad química, America: Cengage Learning Latin.
- Laburú C. E. and Niaz M., (2002), A Lakatosian framework to analyze situations of cognitive conflict and controversy in students' understanding of heat energy and temperature, J. Sci. Educ. Technol., 11(3), 211–219.
- Laidler K. J. and Meiser J. H., (1997), Fisicoquímica, Compañía Editorial Continental.
- Lederman N. G., Abd-El-Khalick F., Bell R. L. and Schwartz R. S., (2002), Views of Nature of Science Questionnaire: Toward Valid and Meaningful Assessment of Learners' Conceptions of Nature of Science, J. Res. Sci. Teach., 39(6), 497–521, DOI: http://10.1002/tea.10034.
- Lemoni R., Stamou A. G. and Stamou G. P. (2011), “Romantic”, “Classic” and “Baroque” Views of Nature: An Analysis of Pictures About the Environment in Greek Primary School Textbooks-Diachronic Considerations, Research in Science Education, 41(5), 811–832, DOI: http://10.1007/s11165-010-9191-4.
- Lemoni R., Lefkaditou A., Stamou A. G., Schizas D. and Stamou G. P., (2013), Views of Nature and the Human-Nature Relations: An Analysis of the Visual Syntax of Pictures about the Environment in Greek Primary School Textbooks-Diachronic Considerations, Research in Science Education, 43(1), 117–140, DOI: http://10.1007/s11165-011-9250-5.
- Levine I. N., (2014), Principios de fisicoquímica, McGraw Hill.
- Mahmud M. and Gutiérrez O., (2010), Estrategia de Enseñanza Basada en el Cambio Conceptual para la Transformación de Ideas Previas en el Aprendizaje de las Ciencias, Formación Universitaria, vol. 3, No. 1, DOI: http://10.4067/S0718-50062010000100003.
- Masterton W. L., Hurley C. N., Llopis M. V. and Cuenya P. R., (2004), Química: principios y reacciones, Paraninfo, Editorial S. A.
- Matthews M. R., (2012), Mario Bunge, Systematic Philosophy and Science Education: An Introduction, Sci. Educ., 21(10), 1393–1403, DOI: http://10.1007/s11191-012-9530-0.
- Michel J. B., Kui Shen Y., Presser Aiden A., Veres A., Gray M. K., Pickett J. P., Aiden E. L., et al. (2011), Quantitative analysis of culture using millions of digitized books, Science, 331(6014), 176–182, DOI: http://10.1126/science.1199644.
- Niaz M., (2006), Can the study of thermochemistry facilitate students' differentiation between heat energy and temperature? J. Sci. Educ. Technol., 15(3–4), 269–276, DOI: http://10.1007/s10956-006-9013-7.
- Niaz M. and Fernández R., (2008), Understanding quantum numbers in general chemistry textbooks, Int. J. Sci. Educ., 30(7), 869–901, DOI: http://10.1080/09500690701217337.
- Quiroz W. and Rubilar C. M., (2015), Natural laws and ontological reflections: the textual and didactic implications of the presentation of Boyle's law in general chemistry, Chem. Educ. Res. Pract., 16(3), 447–459, DOI: 10.1039/c4rp00251b.
- Rosenberg R. M., (2010), From joule to caratheodory and born: A conceptual evolution of the first law of thermodynamics, J. Chem. Educ., 87(7), 691–693, DOI: http://10.1021/ed1001976.
- Sabariego del Castillo J., (2006), Alfabetización Científica. Congreso Iberoamericano de Ciencia, Tecnología, Sociedad e Innovación CTS+I.
- Sanmartí N., Izquierdo M. and García P., (1999), Hablar y escribir. Una condición necesaria para aprender ciencias, Cuadernos de pedagogía, 281, 54–58.
- Slotta J. D., Chi M. T. H. and Joram E., (1995), Assessing Students' Misclassifications of Physics Concepts: An Ontological Basis for Conceptual Change, Cogn. Instr., 13(3), 373–400, DOI: http://10.1207/s1532690xci1303_2.
- Spinelli Barria M., Morales C., Merino C. and Quiroz W., (2016), Realist ontology and natural processes: a semantic tool to analyze the presentation of the osmosis concept in science texts, Chem. Educ. Res. Pract., DOI: 10.1039/c5rp00219b.
- Vesterinen V. M., Aksela M. and Lavonen J., (2013), Quantitative Analysis of Representations of Nature of Science in Nordic Upper Secondary School Textbooks Using Framework of Analysis Based on Philosophy of Chemistry, Sci. Educ., 22(7), 1839–1855, DOI: http://10.1007/s11191-011-9400-1.
- Wattanakasiwich P., Taleab P., Sharma M. D. and Johnston I. D., (2013), Development and implementation of a conceptual survey in thermodynamics, International Journal of Innovation in Science and Mathematics Education, 21(1), 29–53.
- Wong C. L., Chu H. E. and Yap K. C., (2016), Are alternative conceptions dependent on researchers' methodology and definition?: a review of empirical studies related to concepts of heat, Int. J. Sci. Math. Educ., 14(3), 499–526, DOI: http://10.1007/s10763-014-9577-2.
| This journal is © The Royal Society of Chemistry 2016 |