Using a word association test for the assessment of high school students' cognitive structures on dissolution
Ayşegül
Derman
*a and
Ingo
Eilks
b
aGaziantep University, Faculty of Education, Elementary Education Department, Gaziantep, Turkey. E-mail: aderman1977@gmail.com
bUniversity of Bremen, Department of Biology and Chemistry, Institute for Science Education, Bremen, Germany. E-mail: ingo.eilks@uni-bremen.de
First published on 16th June 2016
Abstract
Understanding students' cognitive structures in a specific knowledge domain helps to determine the “what, how and why” features of such knowledge, so that we can take these structures into consideration in teaching. The purpose of the present study was to identify students' cognitive structures about solution and dissolution concepts. The study utilized mixed methods within a non-experimental descriptive design. The data source for this study consisted of 157 11th grade students' written accounts of solution chemistry via a Word Association Test (WAT). A response frequency mapping method was used to identify the students' cognitive structures. To determine the nature of students' cognitive structures, sentences were analyzed in terms of misconceptions, lacking understanding, and vague understanding. Overall the study shows a very diverse picture in cognitive structures existing among the students, including many poorly-developed concepts. Implications for teaching are given.
Introduction
One of the main goals of chemistry education is to support students in developing an understanding of concepts related to the nature of matter. This includes properly applying these concepts in new and relevant situations. According to Uzuntiryaki and Geban (2005), one major obstacle to solving chemistry problems is often a lack in understanding of basic chemistry concepts. It has been long known that students frequently have difficulties solving chemistry problems caused by insufficient knowledge, e.g. in terms of lack of knowledge or misconceptions (e.g.Nakhleh, 1992; Taber, 2002). But they also lack in understanding of the basic nature of chemical knowledge, encompassing the phenomenological, sub-microscopic and symbolic representational levels (Johnstone, 1991, 2000).There are several requirements for effective chemistry learning, including cognition and perspicacity. Students need logical and abstract thinking skills to achieve comprehension in chemistry (Blake and Norland, 1978), since the content is full of abstract and theoretical concepts such as the particulate nature of matter (Liu and Lesniak, 2005; Adadan et al., 2010; Eilks, 2013) and chemical bonding theory (Othman et al., 2008; Levy Nahum et al., 2010). Dissolution and the nature of solutions is also a domain which has been repeatedly described as being difficult (Stavridou and Solomonidou, 1989; Abraham et al., 1994; Çalık et al., 2005; Çalık et al., 2007; Adadan and Savasci, 2012; Adadan 2014). In any case, comprehension of basic chemical processes like dissolution is crucial to continuous learning in high school and university chemistry classes (Ebenezer, 2001). Since a great number of students do not understand dissolution processes, they may also fail to understand more advanced topics later on, including acid–base chemistry, electrochemistry, and chemical equilibrium (Çalık et al., 2005; Uzuntiryaki and Geban, 2005; Adadan and Savasci, 2012). According to Hewson and Hewson (1983), the knowledge students possess before explicit instruction plays an important role as a source of potential learning difficulties. Language use (Prieto et al., 1989; Ebenezer and Erickson 1996; Markic et al., 2013), logical thinking skills, and foreknowledge (Haidar and Abraham 1991; Abraham et al., 1994; Uzuntiryaki and Geban, 2005) all influence the development of students' conceptual learning related to solution chemistry, a topic which is a fundamental part of almost every junior and senior high school science curriculum. There is already some research about student understanding and misconceptions in learning about solutions/dissolution (e.g.Haidar and Abraham, 1991; Adadan and Savasci, 2012). However research about how concepts, terms and processes are related to each other and form students' cognitive structures is still limited in general and for the case of Turkey in particular. This study aims to contribute to the existing literature in this field. It attempts to develop a better understanding of students' cognitive structures related to solution chemistry by using a Word Associations Test. The study was guided by the following research questions:
(1) Which concepts related to dissolution and the nature of solutions do 11th grade students possess in their cognitive structures?
(2) Do any misconceptions (MC), lacking understanding (LU) or vague understanding (VU) related to dissolution and the nature of solution exist in students' cognitive structures? If so, what are they?
Theoretical framework
Student learning is influenced by the learner's original and individual knowledge, epistemology, and ability levels (Tyson et al., 1997). Constructivistic learning theory describes the importance of the interaction between the learner and the environment, as well as the construction of concepts and schemata through life experience based on a priori knowledge (Ausubel, 1968 cited in Wilson, 1990). The networks formed by these interactions and connections are the cognitive structures belonging to the individual. According to Tsai (2001) student knowledge gained from science courses is stored in the long term memory hierarchically and represented as a cognitive structure. Though there is no single accepted definition of the term ‘cognitive structure’ and there is also very limited amount of information available on how these structures are formed (Taber, 2008) the term is, however, still used in many studies (e.g.White, 1985; West and Pines, 1985; West et al., 1985).There are different interpretations like structural knowledge (Jonassen et al., 1993 cited in Liu and Ebenezer, 2002) or knowledge structure (Nakiboglu, 2008) which are used to describe cognitive structures. Knowledge about how concepts within a domain are interrelated forms structural knowledge. It is also defined as the content-specific cognitive structure dealing with the essential element of students' conceptual understanding and differs from declarative knowledge. Studies on structural knowledge focus on how conceptual understanding is structured in terms of interrelationships between and among concepts, instead of describing students' concepts based on categories (Liu and Ebenezer, 2002).
In the last decade different techniques such as word association tests, concept mapping, interviewing, etc. have been used as research techniques to examine the nature of cognitive structures in learners' knowledge (Lee, 1986, 1988). White (1985) explored some of these issues, for example the interactive nature of the purpose, the assumed model and the extent of cognitive structures of concern, and the methodologies employed. Additionally, it is possible that cognitive structures are modified while they are being investigated. Introspection is likely to cause some change in a respondent's cognitive structure due to methods based on interviews or the detailing of thought processes.
Cognitive structures, like the mental connections between terms, concepts and process, as forming the focus of this study, are not easy to identify. Çalık et al. (2005) mention several difficulties in finding effective research methods for examining students' cognitive structures in the area of solution chemistry and related topics. Correspondingly, a wide variety of methods is applied. Fensham and Fensham (1987) conducted research by using clinical interviews on students' concepts of the nature of solutions based on three phenomena: solids dissolving in water, reactions between chemicals in solution, and the influence of various factors on the rate of reactions (cited in Çalık et al., 2005). Ebenezer and Erickson (1996) investigated 11th grade students' conceptions of solubility and first year chemical engineering students' conceptions of energy in solution process, respectively, by using a “phenomenographic” tradition of individual interviews. Pinarbaşi et al. (2006) used a multiple choice concept test (“Solution Concepts Test”) to investigate the effect of conceptual change due to text-oriented instruction as compared to traditional instruction on students' understanding of solution processes (e.g., dissolving, solubility, factors affecting solubility, concentrations of solutions, types of solutions, physical properties of solutions). In another study, Adadan (2014) examined the progression of particular solution chemistry concepts (e.g., saturated and supersaturated solutions) with the aid of interviews. As a final example, Liu and Ebenezer (2002) descriptively and structurally explored 7th and 12th grade students' concepts of solutions by using a free writing technique. The variety of methods applied reveals the complexity of the effort to delve into students' cognitive structures in chemistry.
According to Nakhleh (1992), lacking or incorrect understanding of phenomena will hinder later learning when the learner attempts to connect new information into a cognitive structure already containing scientifically unreliable structures. Misinterpretation of and misconceptions about the concept may arise. Because of the resilience of alternative conceptions (or misconceptions) that are embedded in larger conceptual frameworks, teaching targeting the development of a scientifically reliable conceptual understanding is very difficult to achieve in many domains (Treagust and Duit, 2008). However, the variety of studies on students' alternative understanding provides us with a good starting point to search for more highly networked understanding of students' cognitive structures, e.g., in the case of dissolution and related chemistry concepts.
Abraham et al. (1994) identified a concept of dissolution containing five original concepts. The study employed a sample of 9th grade physical science students, 11th and 12th grade high school chemistry students, and college students who had enrolled in their first semester of general chemistry courses at a university. The researchers observed that only a few students at the college level had a valid comprehension of chemical change, periodicity, and phase changes, whereas the use of particulate terms (atoms, molecules and ions) increased across the grade levels. More specifically, they identified the fact that many learners possess alternate conceptions of both the solution process and the diverse aspects of solution chemistry (Stavridou and Solomonidou, 1989; Haidar and Abraham 1991; Çalık, 2005; Çalık et al., 2005; Uzuntiryaki and Geban, 2005; Çalık et al., 2007; Adadan and Savasci, 2012). In New Zealand, Cosgrove and Osborne (1981) interviewed secondary school students to determine their ideas of the dissolution process. When the students were shown a tea spoon of sugar dissolving in water, they were asked “What happens to the sugar?” This study revealed that younger students generally interpreted dissolving as melting while older learners connected the process with the suitable technical terms, but had no sound scientific concepts supporting the terms. In a similar study, Longdon et al. (1991) compared students between the ages of eleven and fourteen on the topic of dissolution. They found that understanding of the particle interpretation of matter grew with advancing age. Mulford and Robinson (2002) accentuated the fact that learners held to the misconception that total mass of the solution could be lighter than sum of the masses of solute and solvent.
Another focus was given by Prieto et al. (1989) who also investigated 11- to 14-year-old students' understanding of the nature of solutions. They reported that the students used the concepts of density and absorption to explain the solution process. Other studies have also suggested that pupils stated a mutual effect between the solute and the solvent in the dissolution process. They considered this process to be a chemical change (Prieto et al., 1989; Stavridou and Solomonidou 1989; Haidar and Abraham 1991; Abraham et al., 1994). In another study based on 12- to 18-year-old learners, Blanco and Prieto (1997) described the effects of stirring and temperature changes on the dissolution of a solid in a liquid. They found poor progress in learners' development of chemical understanding in terms of the effects studied. Furthermore, they concluded that students by direct observation of mechanical events started believing that dissolution does not occur without mechanical events. The resulting development of chemical concepts was rather poor. Pupils in this and other studies began to explain dissolution as a process of “melting” or a result of “density” (Haidar and Abraham 1991; Abraham et al., 1992; Lee et al., 1993; Ebenezer and Erickson 1996; Ebenezer, 2001). Learning can also be hindered by the terms that are used. Many chemistry words have both scientific and colloquial meanings (dual meaning vocabulary) (Markic et al., 2013). This also is the case for terms like ‘solving’ or ‘solution’.
Overall, understanding the nature of solutions seems to be a very basic but nevertheless difficult topic for many students. Many details are known about misinterpretations and mistakes when asking students to solve tasks centering around solution chemistry. However, much less is known about the cognitive structures and their degree of complexity.
Method and sample
Word association test (WAT)
Word Association Tests (WAT) were originally suggested by Johnson in the 1960s (Johnson, 1967, 1969: as discussed in Gunstone, 1980). In recent years, they became common tools in science education research to help determine and map concepts in student understanding, including the relationships between knowledge and cognitive structures (Shavelson, 1972, 1974; Bahar et al., 1999; Bahar and Hansell, 2000; Nakiboğlu, 2008; Schizas et al., 2013). The WAT represents a tool for exploring aspects of the content and structure of an individual's knowledge in a specific content area. Word associations also allow insights in the structure and work of the human memory (Thomson and Tulving, 1970; Petrey, 1977).Johnson (1967, 1969) as discussed in Gunstone (1980) carried out a series of studies with high school students taking physics courses. He found a positive correlation between learners' problem solving performance and the number of associations related to the problems. Many related studies have also shown that using WATs is a robust method, since it reveals the types and numbers of concepts in the learners' cognitive structures. It also reveals the relationships between them, allows for cultural differentiation (Isa and Maskill, 1982), and identifies the development of cognitive structures in certain domains. Examples in chemistry education can be found, e.g., on atomic structure (Nakiboğlu, 2008) or decomposition (Schizas et al., 2013).
Research design and instrument
The current study used a mixed-method approach within a non-experimental descriptive design. It was based on qualitative data collection, combined with quantitative and qualitative data analysis procedures (Johnson and Onwuegbuzie, 2004). The central tool of the study is a Word Association Test (WAT). Eight keywords were provided to participants as stimulus words. Each student received a booklet with eight pages, each providing one of the stimulus words in the following order: solvent, solute, solution, concentration, dissolution, solubility, temperature and pressure. To assure the content validity of the WAT, the first author examined the high school chemistry curriculum that covered all these terms. Furthermore, three experienced chemistry teachers and a general chemistry instructor were asked to express their opinions about the stimulus words used in the study and they confirmed that they were suitable for the study.The WAT booklets were given to the participants, who were then asked to write as many terms associated with the stimulus words as they could. To prevent a chain-reaction effect, which may be caused if someone is distracted by superfluous information, all the stimulus words were written on a single page with enough blank space around them to write down any thoughts. The participants were also asked to write a sentence including each one of the stimulus words and their response words. According to the literature (Shavelson, 1972; Gunstone, 1980; Nakiboğlu 2008; Ayas, 2011) the students were given about 70 seconds per item. Most students completed the WAT booklet in approximately 10 minutes.
Data analysis
The responses for each stimulus word were counted to summarize the data gathered by the WAT (Shavelson, 1974; Bahar et al., 1999). In order to provide inter-judgmental reliability, the data from the first 50 participants were also analyzed independently by a second expert in the fields of physical chemistry and chemistry education. The criterion of counting the total number of different response words was employed to compare the analyses. The inter-judgmental reliability of the consensus and consistency estimates of raters was reflected to the agreement rage as suggested by Miles and Huberman (1994). The agreement on the total number of different response words was found to be higher than 90% throughout (solvent 94%, solute 95%, solution 95%, concentration 93%, dissolution 94%, solubility 95%, temperature 92% and pressure 93%). Based on this the whole data set was evaluated by Excel.The response frequencies' map method proposed by Nakiboğlu (2008) was used for the data analysis of the WAT. This is an integrated method based on Gussarsky and Gorodetsky's (1988) relatedness coefficient method and Bahar et al. (1999) response frequencies' method. In this case, a frequency table including stimulus and response words was formed and a cognitive map obtained according to the frequency values. Frequency tables determine the directions of the arrows and strength of the associations shown on the map. According to Nakiboğlu (2008) this method has more explanatory power and is better-suited to present both the directions and strength of associations found in students' knowledge structures. In the current study, a content analysis was conducted before starting to form the map. Students' response words which were meaningful and valid in the context of dissolution were transferred to an Excel sheet. After screening the data, a frequency table was constructed by counting the response words for each stimulus word. Because this study has a large number of response words, the full table is not provided in this paper. A short version of the frequency table is given as a sample below (see Table 2) in order to help understanding how the frequencies' map was built. In this sample, the stimulus words are given in the first row. Response words gathered from WAT are given in the first column.
A frequencies' map was then constructed to represent the data. The frequency ranges and vertical directions for weak or strong correlations in the map were determined according to the association frequency scores between stimulus and response words. If a stimulus word was obtained from students as a response word, it was added in a frame. If a response word was obtained as a new word different from the stimulus words, it was not added in a frame in the map. The frequency score of an association between a stimulus word and its response word regulates the width of the frames and the arrows. The width represents the strength of the associations. The widest arrows show the highest frequency scores and they are put in the first cell. The arrows come from the first row to the responses in the first column (see Table 2). The direction of the arrows is in parallel with the direction of the relationships. Diverging from Nakiboğlu's (2008) original mapping scheme, reverse relations between the concepts are also shown by double-sided arrows in the frequency range in which they occurred. Concepts interpreted as misconceptions were shown with square dotted arrows in the frequency range in which they occurred. According to Nakiboğlu (2008), this type of maps helps researcher to show both the strength and direction of the associations and thereby infer the relations between concepts and students' cognitive structures.
Qualitative content analysis was applied for the analysis of the sentences written by the students in the second part of the WAT. Qualitative content analysis was applied by the first author of this study for the analysis of the sentences written by the students in the second part of the WAT based on findings from related studies. The sentences were examined individually to identify whether they represent misconceptions (MC) (Cosgrove and Osborne, 1981; Ebenezer and Erickson, 1996; Çalık et al., 2005; Pinarbaşi et al., 2006), lacking understanding (LU) or vague understanding (VU) (Pinarbaşi et al., 2006; Adadan and Savasci, 2012; Adadan, 2014). Samples were selected as excerpts to illustrate the findings and discussion.
Educational background and sample
In Turkey, science in grades 4 to 8 is taught as an integrated subject encompassing chemistry, biology and physics. Students first encounter dissolution and the concepts related to solutions (e.g. solute, solvent, solution, factors effecting solution rate such as temperature, heterogeneous mixtures, contact surface, etc.) in grade 7. Chemistry as a stand-alone subject starts in grade 9 and continues for another four years (age range 15–18). Solution chemistry is part of the curriculum, it includes teaching units on “mixtures” in grade 10, “liquid solutions” and “reaction speed and stoichiometry” in grade 11, and “chemistry and electricity” in grade 12. Data was collected from 157 students in grade 11. All students were taught the units “liquid solutions” and “reaction speed and stoichiometry” before data was collected. The students came from four different state high schools located in the southeastern region of Turkey. Data was collected during spring of the academic year 2013/14. Each student completed WAT booklet approximately within 10 minutes. Basic demographic information about the participants is presented in Table 1. A total of 45.2% of the participants were female, 54.8% were male, the mean age was 16.9 (range 16 to 18 years). All participants were informed about the nature and methods of the study. They all agreed to participate in the study on a voluntary basis.| Demographic feature | f | % |
|---|---|---|
| Gender | ||
| Female | 71 | 45.2 |
| Male | 86 | 54.8 |
| Total | 157 | 100 |
| Age | ||
| 16 | 24 | 15.3 |
| 17 | 125 | 79.6 |
| 18 | 8 | 5.1 |
| Total | 157 | 100 |
| Mean | 16.9 | |
| Std. Dev. | 0.441 | |
Findings
Table 2 gives the frequency of response words associated with the stimulus words.| Response words | Stimulus words | |||||||
|---|---|---|---|---|---|---|---|---|
| Solvent | Solute | Solution | Concentration | Dissolution | Solubility | Temperature | Pressure | |
| Solvent | — | 16 | 23 | 7 | 21 | 13 | 6 | 5 |
| Solute | 18 | — | 29 | 8 | 17 | 17 | 10 | 6 |
| Solution | 21 | 13 | — | 13 | 20 | 8 | 7 | 14 |
| Concentration | 8 | 9 | 13 | — | 7 | 20 | 10 | 6 |
| Dissolution | 6 | 3 | 6 | 3 | — | 20 | 20 | 11 |
| Solubility | 6 | 3 | 2 | 6 | 11 | — | 26 | 17 |
| Temperature | 12 | 7 | 7 | 8 | 13 | 48 | — | 18 |
| Pressure | 2 | 1 | 3 | 5 | 6 | 32 | 17 | — |
| Alcohol | 65 | 42 | 6 | 1 | 1 | 6 | — | — |
| Alcohol–water | — | — | 15 | — | 6 | 1 | — | — |
| Non-polar | 7 | 4 | 1 | 33 | 2 | — | — | |
| Acid | 26 | 3 | 7 | 10 | 5 | 11 | — | — |
| Supersaturated | — | — | 4 | 24 | 1 | — | — | — |
| Melting | 5 | 4 | — | 2 | 25 | 2 | 1 | — |
| Gasoline | 16 | 2 | — | — | — | — | — | — |
| Precipitation | 5 | 3 | 7 | 3 | 22 | 4 | 5 | 4 |
| Water | 137 | 22 | 8 | 13 | 17 | 21 | — | — |
| Salt water | 10 | — | 96 | 12 | 26 | 2 | — | — |
| Salt | — | 125 | — | — | 12 | 13 | — | — |
| Sugar | — | 119 | — | — | — | 8 | — | — |
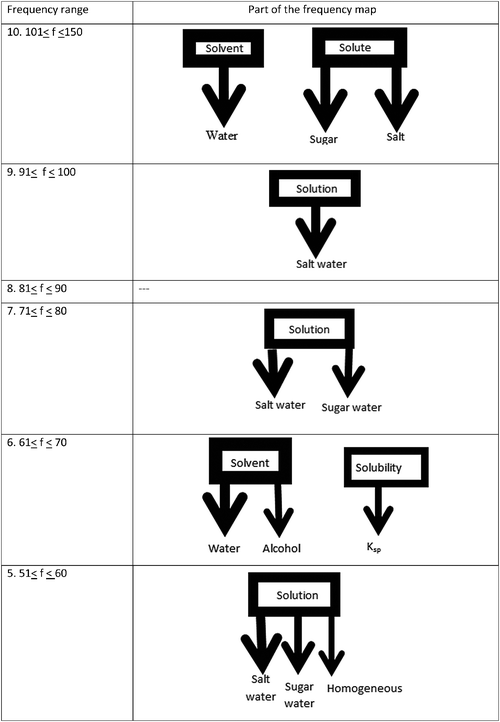
A frequency map was formed to shows the relations of the stimulus and associated words. The map represents ten different frequency range categories (see Fig. 1–3). The thickest arrows are placed in the tenth category, which showed the highest frequency range (see Fig. 1–3). Since the “water” response word was associated with the “solvent” stimulus word 137 times (see Table 2), it was placed in tenth (the strongest) frequency range. Since there were no frequency scores between 81 ≤ f ≤ 90 in Table 2, no associations were made in this frequency range. The directions of the arrows were taken from stimulus words in the first row and correspond to the response words placed in the first column in Table 2. In the frequency map the directions of the arrows represent the direction of the relationships. Double-sided arrows show reverse relations among the stimulus words. Moreover, the square-dotted arrow indicates misconceptions related to dissolution embedded in the cognitive structures of students (Table 3).
Fig. 1 shows that two different set of cells for the stimulus words “solvent” and “solute” appeared at the strongest association level of students' cognitive structures between the 101 ≤ f ≤ 150 frequency range. Solvent was associated with only one response word. Solute was associated with two response words. At the 9th level (91 ≤ f ≤ 100) we can observe that there is only one cell for the stimulus word “solution” and one response word for it. No concepts emerged at the 8th level. At the 7th level the stimulus word “solution” appeared again and two words were observed as response words. “Solvent” and “solubility” appeared as two separate set of cells at the 6th level. The association of the stimulus word “solvent” increased to two; “solubility” appeared with only one word. At the 5th level, the stimulus word “solution” came onto the scene again as a set cell due to increasing association.
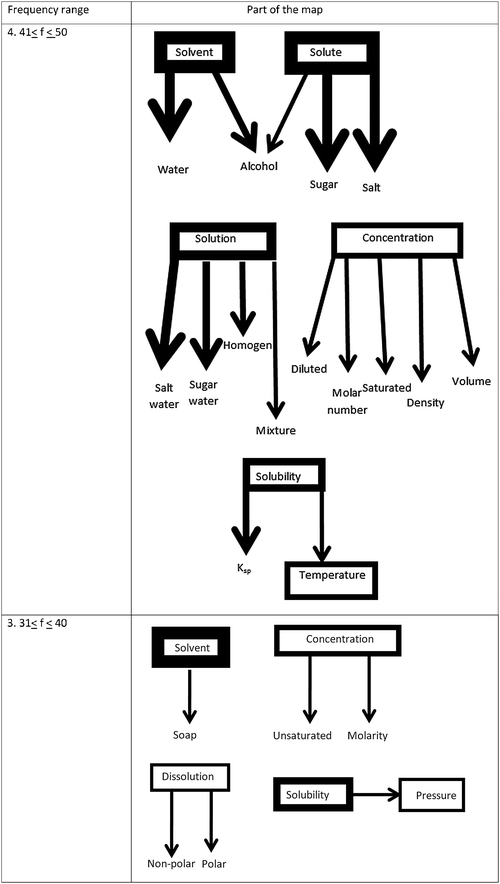
The stimulus words “solvent, solute, solution, concentration, solubility and temperature” appeared as separate cells which included one-way associations at the 4th level. The stimulus words “solvent, concentration, solubility, dissolution, pressure” appeared as separate cells having one-way associations at the 3rd level.
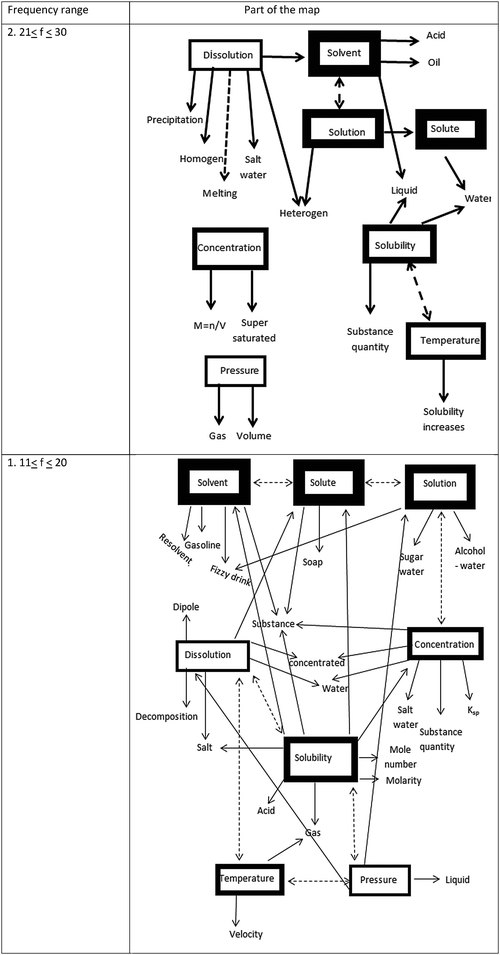
When it comes to the 1st and 2nd levels, all the stimulus words appeared. However, the associations found at the second level were mostly one-way associations. It was observed that reverse associations among stimulus words increased. The same response words were associated with different stimulus words, thus showing more complex and dynamic structure at the first level.
In addition to the graphic representations shown in Fig. 1–3, some qualitative findings were also obtained. The first findings are based on the assumption that learners construct meaning through their already held concepts while acquiring new knowledge. In the WAT it is suggested that each response word related to any stimulus word represents such a concept. Thus, the number of different response words for each stimulus word was determined (Table 4). The number of the various response words varied between 215 and 716. According to Schaefer (as cited in Bahar et al., 1999), a word without any associations in a cognitive structure does not have a meaning and the meaning of that word is extended when more associations are formed. The more association a stimulus has the richer meaning it has. Table 4 gives insight in the frequency of response words to the different stimulus words. We can see that solvent, solution, concentration, solubility, solute, dissolution were better connected to other concepts than temperature and pressure.
| Stimulus word | Total number of different response words |
|---|---|
| Solvent | 716 |
| Solute | 618 |
| Solution | 677 |
| Concentration | 640 |
| Dissolution | 616 |
| Solubility | 638 |
| Temperature | 268 |
| Pressure | 215 |
The students' response sentences which are provided by the students in the second part of the WAT are generally more complex in nature when compared to one word responses (Gunstone, 1980). The findings obtained from the WAT sentence analysis revealed that students possessed some MC, LU and VU related to dissolution (see below).
Discussion
The aim of the current study was to determine students' cognitive structures in the field of dissolution chemistry. Two research questions were formed; the first one was “Which concepts related to dissolution have 11th grade students acquired and incorporated into their cognitive structures?”. Only the concepts of solvent and solute emerge in the 101 ≤ f ≤ 150 frequency range, which is the strongest level in terms of the appearance (Fig. 1) in students' cognitive structures. Additionally, it was observed that one or two stimulus words emerged in all ranges from the 91 ≤ f ≤ 100 to the 41 ≤ f ≤ 50 the frequency range. The number of the words associated with these stimulus words is, however, limited to only one or two. A static organization exists in which response words are associated with only one stimulus word and no interactive relation among stimulus words appears. Within 41 ≤ f ≤ 50 and 31 ≤ f ≤ 40 frequency ranges, respectively, six stimulus words (solvent, solute, solution, concentration, solubility and temperature) and five stimulus words (solvent, concentration, dissolution, solubility and pressure) emerged. Nonetheless, non-interactive associations were observed at this level.Within the cognitive structures almost all of the connections (e.g. mixture, homogeneity, saturated, dilute, etc.) were seen as already experienced or learned concepts (i.e. salt water and sugar water are known as homogenous mixtures…) of dissolution. Their knowledge about “polar, nonpolar and dipole” concepts was at a declarative level (Liu and Ebenezer, 2002) as it emerged in “polar dissolves better in polar, nonpolar dissolves better in nonpolar…”. In parallel with Mayer (1975), this situation can be interpreted as students having limitations in networking their knowledge in solution chemistry concepts. However, Uzuntiryaki and Geban (2005) accentuated that students' foreknowledge and their reasoning abilities helped them to understand solution concepts.
Students' cognitive structures for the topic of solution are static, non-interactive and limited in terms of external connectedness (Gunstone, 1980). It seems that the students did not meaningfully integrate any potential foreknowledge (i.e. of the particulate nature of matter) with topics from solution chemistry. This situation impedes students' meaningful learning and their ability to form scientifically reliable cognitive structures related to the domain of dissolution.
All the eight stimulus words emerged at the first (11 < f < 20) and second (21 < f < 30) level of cognitive structure. We observed that one-way (dissolution–solvent, solution–solute) and two-way (solvent–solution, solubility–temperature) interactions were barely present. At the first level, which is the strongest level in terms of cognitive structure, we determined that both one-way (dissolution–solute, pressure–solution, solubility–concentration etc.) and two-way (solution–concentration, dissolution–solubility, dissolution–temperature, solubility–pressure, temperature–pressure etc.) interactions increased. The same response words were associated with different stimulus words (e.g. substance response word associated with solute, solvent, concentration, and solubility stimulus words). Unfortunately, these levels are the weakest in terms of frequency range in the cognitive map. However, they are the richest and most highly-developed in terms of cognitive organization when compared to all the other levels.
As seen in Fig. 2 and 3, students associated the stimulus word “temperature” with dissolution and solubility and stated that temperature increased the solubility. From the sentence part of the WAT we see that they were not able to develop a comprehensive and solid understanding of this connection (Vogue Understanding 1a, b- see Table 5) and did not meaningfully connect it to the relationship between “solubility, dissolution rate and enthalpy change” (Pinarbaşi et al., 2006).
| Codes | Excerpt | Frequency |
|---|---|---|
| MC1a | Sugar that I put into water melted… | 9 |
| MC1b | Sugar and salt melted in water… | 16 |
| LU1 | Mixtures are one form of solution… | 7 |
| LU2 | Solutions provide electrical conductivity… | 5 |
| MC2a | Dissolution is examined under a chemical event… | 6 |
| MC2b | Dissolution is a chemical event… | 7 |
| LU3 | Dissolution is the ionization of a solute in a solvent… | 7 |
| MC3 | The solute is the substance which breaks down, shrinks and disappears… | 10 |
| During dissolution evaporation, burning and disappearing can occur… | ||
| MC4 | A precipitate occurs only if the solution is supersaturated… | 5 |
| MC5 | The amount of a substance affects the solubility… | 10 |
| LU4 | In chemistry, the best thing that dissolves substances is water… | 8 |
| VU1a | While temperature increases dissolution rate in an endothermic dissolution process, it decreases dissolution rate in an exothermic dissolution process… | 38 |
| VU1b | A rise in temperature increases or decreases the solubility depending on whether solution enthalpy is endothermic or exothermic. | 24 |
The least number of response words was produced “pressure.” The two-way interaction for both this term and for “solubility” occurred at the first frequency range. This is the weakest level in the cognitive structure map. This was also obvious in the following student excerpts: “Pressure is a factor affecting dissolution…”, “Pressure affects solubility…“and “Solubility of gases is directly proportionate to pressure.” The nature of these associations is almost completely in the form of declarative knowledge as building blocks.
In light of these findings, we can state that most of students' knowledge tends to remain at the declarative knowledge level. In addition, the concepts of volume and temperature were both tied to the stimulus word “pressure” and show characteristics of external connectedness. The nature of external connectedness is expressed by “when the temperature rises, pressure rises, too… pressure is inversely correlated to volume…”. This shows that students could not meaningfully associate temperature and pressure concepts with the other concepts in solution chemistry.
The second research question of the present study was “Are there any misconceptions (MCs), lacking (LUs) and vogue understanding (VUs) related to solution topic in their cognitive structures? If so, what are they?” One misconception occurred in the association of dissolution with the process of melting. This was found at the second level of student cognitive structure (21 ≤ f ≤ 30). This misconception occurred in sentences such as “sugar that I put into water melted…”, “Sugar and salt melted in water…” (MC1a, MC1b). The synonymous use of the terms “dissolution” and “melting” by the participants is one of the most common misconceptions found in almost all solution chemistry studies (e.g.Cosgrove and Osborne, 1981; Çalık et al., 2005). A second misconception found in this study, namely that “dissolution is a chemical event…” (MC2a,b), also belongs to the list of most common misconceptions found worldwide (Ebenezer and Erickson, 1996; Pinarbaşi et al., 2006). Findings in the present study (LU1, LU2, LU3, MC3, VU1a, b, MC4, MC5, LU2, LU4) correspond with the findings of similar studies (Pinarbaşi et al., 2006; Adadan and Savasci, 2012; Adadan, 2014).
Conclusions
Solution chemistry is a basic chemistry concept which has repercussions influencing a wide variety of other topics in the high school chemistry curriculum. Topics related to the solution process are taught in advanced contexts according to grade level, for example units on ‘mixtures’ in grade 10 and ‘liquid solutions’ in grade 11 in Turkey. Despite this fact, students' cognitive structure networks often remain weak and non-interactive. This finding reveals that students have difficulties in comprehending solution chemistry concepts and incorporating them into their cognitive structures. This finding has also been suggested by other research studies examining solution chemistry (e.g.Çalık, 2005; Çalık et al., 2005; Uzuntiryaki and Geban, 2005; Çalık et al., 2007; Adadan and Savasci, 2012).We can say that students' cognitive structures related to solution chemistry in the case of this sample of students from Turkey are rather limited. The current findings overlap with other studies on solution chemistry in Turkey and other countries (Ebenezer and Erickson, 1996; Ebenezer, 2001; Liu and Ebenezer, 2002; Çalık, 2005; Çalık et al., 2005; Uzuntiryaki and Geban, 2005; Çalık et al., 2007; Adadan and Savasci, 2012; Adadan, 2014) but this study provides a further, deeper view into students' understanding of solution chemistry by providing a qualitative mapping of students' cognitive structures elaborated by the WAT. In the related literature, these unfavorable conditions is generally associated with certain instructional strategies and with gaps in the teaching and learning environment (e.g.Eilks et al., 2012). Ebenezer (2001) and Johnstone (2000) emphasize that students have difficulties in associating submicroscopic explanations of chemical systems with their personal macroscopic experiences in the everyday world. Therefore, in order to help learners build deeper and more structured understanding of concepts related to chemistry, applicable teaching strategies should be developed and evaluated in chemistry classroom contexts, e.g. concept mapping or conceptual change strategies. As has been previously mentioned in various studies (Ebenezer, 2001; Taber, 2008; Talanquer, 2011; Adadan, 2014), once the learners are given opportunities to link their personal experiences (their macroscopic images) to their visualizations (by using both dynamic and static visual particulate representations) in an atmosphere that provides opportunities for them to explain and discuss, they can improve their scientific comprehensions for particular scientific concepts. However, this needs to be performed with sufficient care, so that students are not confused or misled by ill-defined explanations or faulty visualizations (Eilks et al. 2009, 2012).
When students develop incorrect concepts and connect them with their cognitive structures, the resulting misconceptions are extremely persistent and will hinder the further development of scientifically reliable knowledge (Nakhleh, 1992). Thus, the undesired domino effect obstructs the construction of meaningful cognitive structures and effectively hinders meaningful learning. Appropriate teaching techniques need to be applied in the context of the chemistry classroom in order to help students to prevent or minimize misconceptions and to encourage meaningful learning and the construction of well-structured cognitive networks (Anderson and Botticelli 1990; Ebenezer, 2001; Eilks, 2013; Schizas et al., 2013).
Based on the findings discussed above, we can conclude that chemistry curriculum developers, textbook writers and teachers need to continue implementing carefully designed teaching materials and activities to promote the formation of highly associated and reliable cognitive structures. This includes experiments, visualizations, or the use of a chemistry knowledge scale (macroscopic, multi-particle, molecular, atomic, etc…). These designs should take careful account of the different dimensions (composition/structure, energy, and time) and approaches (mathematical, conceptual, contextual, and historical) to teaching solution chemistry (Talanquer, 2011). They also need to carefully select and deal with appropriate models, explanations and illustrations, while simultaneously considering any potential risks and misleading factors as they were found in textbooks and Internet resources, e.g., by Eilks et al. (2009, 2012). Teaching should focus on developing a coherent curricular structure, especially when it comes to particle explanations (Eilks, 2013). This might help students to achieve well-developed cognitive structure for solution chemistry.
We also conclude that teachers should develop intimate knowledge of their pupils' cognitive structures as part of their pedagogical content knowledge before starting a course. This will allow teachers to tailor the teaching process to build scientifically reliable student knowledge. Identifying students' prior knowledge can also aid teachers to help learners to undergo conceptual change and promote knowledge growth (Nakiboğlu, 2008). Chemistry teachers can apply the WAT in order to determine their students' existing knowledge, misconceptions and cognitive structures and to design their instruction accordingly. They can also use the WAT to self-reflect on their own teaching both before and after instruction occurs (Bahar et al., 1999; Nakiboğlu, 2008).
Limitations
The present study investigated students' cognitive structures in groups instead of observing individual cognitive structures (Gilbert and Watts 1983; Taber, 2008). This was done by establishing a model of students' collective cognitive structures. Therefore, the current study cannot find all forms existing in individual cognitive structures. The nature of WAT tests leads studies to a type of limited modeling of students' individual cognitive structures and to the common designs which point to a beneficial model of these commonalities (Nakiboğlu, 2008). The method used in this study is effective in terms of determining the overall direction and strengths of the associations and presenting them in a map. However, some limitations in the nature of the associations presented in the map in Fig. 1–3 also exist. These limitations were eliminated to some extent by the findings obtained from the sentence part of the WAT However, corresponding studies need to be supported or combined with other techniques such as concept mapping or free writing in order to fully eliminate this limitation.Acknowledgements
The authors would like to acknowledge the technical support (in drawing the cognitive structure map in computer) provided by lecturer Rıdvan Şirin.References
- Abraham M. R., Gryzybowski E. B., Renner J. W. and Marek A. E., (1992), Understanding and misunderstanding of eighth graders of five chemistry concepts found in textbooks, J. Res. Sci. Teach., 29, 105–120.
- Abraham M. R., Williamson V. M. and Westbrook S. L., (1994), A cross-age study of the understanding five concepts, J. Res. Sci. Teach., 31, 147–165.
- Adadan E., (2014), Investigating the influence of preservice chemistry teachers' understanding of the particle nature of matter on their conceptual understandings of solution chemistry, Chem. Educ. Res. Pract., 15, 219–238.
- Adadan E. and Savasci F., (2012), An analysis of 16–17-year-old students' understanding of solution chemistry concepts using a two-tier diagnostic instrument, Int. J. Sci. Educ., 34, 513–544.
- Adadan E., Trundle K. C. and Irving K. E., (2010), Exploring grade 11 students' conceptual pathways of the particulate nature of matter in the context of multirepresentational instruction, J. Res. Sci. Teach., 47, 1004–1035.
- Anderson O. R. and Botticelli S., (1990), Quantitative analysis content organization in some biology texts varying in textual composition, Sci. Educ., 74, 167–182.
- Ayas A., (2011) and Kavram Öğrenimi, in Çepni S. (ed.) Kuramdan Uygulamaya Fen ve Teknoloji Öğretimi, Ankara: Pegem Akademi, 126–151.
- Bahar M. and Hansell M. H., (2000), The relationship between some psychological factors and their effect on the performance of grid questions and word association tests, Educ. Psychol., 20, 349–364.
- Bahar M., Johnstone A. H. and Sutcliffe R. G., (1999), Investigation of students' cognitive structure in elementary genetics through word association tests, J. Biol. Educ., 33, 134–141.
- Blake A. J. and Norland F. H., (1978), Science instruction and cognitive growth in college students, J. Res. Sci. Teach., 15, 413–419.
- Blanco A. and Prieto T., (1997), Pupils' views on how stirring and temperature affect the dissolution of a solid in a liquid: a cross-age study (12 to 18), Int. J. Sci. Educ., 19, 303–315.
- Çalık M., (2005), A cross-age study of different perspectives in solution chemistry from junior to senior high school, Int. J. Sci. Math. Educ., 3, 671–696.
- Çalık M., Ayas A. and Ebenezer J. V., (2005), A review of solution chemistry studies: insights into students' conceptions, J. Sci. Educ. Technol., 14, 29–50.
- Çalık M., Ayas A. and Coll R. K., (2007), Enhancing pre-service primary teachers' conceptual understanding of solution chemistry with conceptual change text, Int. J. Sci. Math. Educ., 5, 1–28.
- Cosgrove M. and Osborne R., (1981), Physical Change (Working Paper No. 26), Learning in Science Project, University of Waikato, Hamilton, New Zealand.
- Ebenezer J. V., (2001), A hypermedia environment to explore and negotiate students' conceptions: animation of the solution process of table salt, J. Sci. Educ. Technol., 10, 73–91.
- Ebenezer J. V. and Erickson L. G., (1996), Chemistry students' conception of solubility: a phenomenograpy, Sci. Educ., 80, 181–201.
- Eilks I., (2013), Teachers' ways through the particulate nature of matter in lower secondary chemistry teaching: a continued change of different models vs. a coherent conceptual structure?, in Tsaparlis G. and Sevian H. (ed.), Concepts of matter in science education, Dordrecht: Springer, pp. 213–230.
- Eilks I., Witteck T. and Pietzner V., (2009), A critical discussion of the efficacy of using visual learning aids from the internet to promote understanding, illustrated with examples explaining the Daniell voltaic cell, EURASIA J. Math. Sci. Technol. Educ., 5, 145–152.
- Eilks I., Witteck T. and Pietzner V., (2012), The role and potential dangers of visualisation when learning about sub-microscopic explanations in chemistry education, Centre Educ. Policy Stud. J., 2, 125–145.
- Fensham P. and Fensham N., (1987), Description and frameworks of solutions and reactions in solutions, Res. Sci. Educ., 17, 139–148.
- Gilbert J. K. and Watts D. M., (1983), Concepts, misconceptions and alternative conceptions: changing perspectives in science education, Stud. Sci. Educ., 10, 61–98.
- Gunstone F. R., (1980), Word association and the description of cognitive structure, Res. Sci. Educ., 10, 45–53.
- Gussarsky E. and Gorodetsky M., (1988), On the chemical equilibrium concept: constrained word associations and conception, J. Res. Sci. Teach., 25, 319–333.
- Haidar H. A. and Abraham R. M., (1991), A comparison of applied and theoretical knowledge of concept based on the particulate nature of matter, J. Res. Sci. Teach., 28, 919–938.
- Hewson M. G. and Hewson P. W., (1983), Effect of instruction using students' prior knowledge and conceptual change strategies on science learning, J. Res. Sci. Teach., 20, 731–743.
- Isa A. M. and Maskill R., (1982), A comparison of science word meaning in the classrooms of two different countries: scottish integrated science in Scotland and in Malaysia, Br. J. Educ. Psychol., 52, 188–198.
- Johnson P. E., (1967), Some psychological aspects of subject matter structure, J. Educ. Psychol., 58, 75–83.
- Johnson P. E., (1969), On the communication of concepts in science, J. Educ. Psychol., 60, 32–40.
- Johnson R. B. and Onwuegbuzie A. J., (2004), Mixed methods research: a research paradigm whose time has come, Educ. Res., 33, 14–26.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assist. Lear., 7, 75–83.
- Johnstone A. H., (2000), Teaching of chemistry – logical or psychological? Chem. Educ. Res. Pract., 1, 9–15.
- Lee K. W., (1986), Problem solving in electrochemistry: variables, strategies and teaching and learning, Unpublished PhD thesis, Monash University, Australia.
- Lee K. W., (1988), Two non- traditional measures of chemistry learning: word association and idea association, Res. Sci. Educ., 18, 169–176.
- Lee O., Eichinger C. D., Anderson W. C., Berkhemier D. G. and Blakeslee T. D., (1993), Changing middle school students' conceptions of matter and molecules, J. Res. Sci. Teach., 30, 249–270.
- Levy Nahum T., Mamlok-Naaman R., Hofstein A. and Taber K. S. (2010). Teaching and learning the concept of chemical bonding, Stud. Sci. Educ., 46, 179–207.
- Liu X. and Ebenezer J., (2002), Descriptive categories and structural characteristics of students' conceptions: an exploration of the relationship, Res. Sci. Techn. Educ., 20, 111–132.
- Liu X. and Lesniak K., (2005), Students' progression of understanding the matter concept from elementary to high school, Sci. Educ., 89, 433–450.
- Longdon K., Black P. and Solomon J., (1991), Children's interpretation of dissolving, Int. J. Sci. Educ., 13, 59–68.
- Markic S., Broggy J. and Childs P., (2013), How to deal with linguistic issues in the chemistry classroom, in Eilks I. and Hofstein A. (ed.) Teaching chemistry - a studybook, Rotterdam: Sense, pp. 127–152.
- Mayer R. E., (1975), Different problem-solving competencies established in learning computer programming with and without meaningful models, J. Educ. Psychol., 67, 725–734.
- Miles M. B. and Huberman A. M., (1994), Qualitative data analysis: an expanded sourcebook, Thousend Oaks: Sage.
- Mulford D. R. and Robinson W. R., (2002), An inventory for alternate conceptions among first-semester general chemistry students, J. Chem. Educ., 79, 739–744.
- Nakhleh M. B., (1992), Why some students don't learn chemistry, J. Chem. Educ., 69, 191–196.
- Nakiboğlu C., (2008), Using word associations for assessing non major science students' knowledge structure before and after general chemistry instruction: the case of atomic structure, Chem. Educ. Res. Pract., 9, 309–322.
- Othman J., Treagust D. F. and Chandrasegaran A. L., (2008), An investigation into the relationship between students' conceptions of the particulate nature of matter and their understanding of chemical bonding, Int. J. Sci. Educ., 30, 1531–1550.
- Petrey S., (1977), Word associations and the development of lexical memory, Cognition, 5, 57–71.
- Pinarbaşi T., Canpolat N., Bayrakceken S. and Geban O., (2006), An investigation of effectiveness of conceptual change text-oriented instruction on students' understanding of solution concepts, Res. Sci. Educ., 36, 313–335.
- Prieto T., Blanco A. and Rodriguez A., (1989), The ideas of 11 to 14-year-old students about the nature of solutions, Int. J. Sci. Educ., 11, 451–463.
- Schizas D., Katrana E., Stamou G., (2013), Introducing network analysis into science education: Methodological research examining secondary school students' understanding of ‘decomposition’, Int. J. Environ. Sci. Educ., 8, 175–198.
- Shavelson R. J., (1972), Some aspects of the correspondence between content structure and cognitive structure in physics instruction, J. Educ. Psychol., 63, 225–234.
- Shavelson R. J., (1974), Methods for examining representations of a subject matter structure in a student's memory, J. Res. Sci. Teach., 11, 231–249.
- Stavridou H. and Solomonidou C., (1989), Physical phenomena-chemical phenomena: do pupils make distinction? Int. J. Sci. Educ., 11, 83–92.
- Taber K. S., (2002), Chemical misconceptions – prevention, diagnosis and cure: theoretical background, vol. 1, London: RSC.
- Taber K. S., (2008), Conceptual Resources for Learning Science: Issues of transience and grain-size in cognition and cognitive structure, Int. J. Sci. Educ., 30, 1027–1053.
- Talanquer V., (2011), Macro, Submicro, and Symbolic: the many faces of the chemistry “triplet”, Int. J. Sci. Educ., 33, 179–195.
- Thomson D. M. and Tulving E., (1970), Associative encoding and retrieval: weak and strong cues, J. Exp. Psychol., 86, 255–262.
- Treagust D. F. and Duit R., (2008), Conceptual change: a discussion of theoretical, methodological and practical challenges for science education, Cult. Stud. Sci. Educ., 3, 297–328.
- Tsai C. C., (2001), Proping students' cognitive structures in science: the use of a flow map method coupled with a meta listening technique, Stud. Educ. Eval., 27, 257–268.
- Tyson L. M., Venville G. J., Harrison A. G. and Treagust D. F., (1997), A multidimensional framework for interpreting conceptual change events in the classroom, Sci. Educ., 81, 387–404.
- Uzuntiryaki E. and Geban Ö., (2005), Effect of conceptual change approach accompanied with concept mapping on understanding of solution concepts, Instr. Sci., 33, 311–339.
- West L. and Pınes L., (1985), Cognitive structure and conceptual change, Orlando, FL: Academic Press.
- West L., Fensham P. and Garrand J., (1985), Describing the cognitive structures of learners following instruction in chemistry, in West L. and Pınes L. (ed.) Cognitive structure and conceptual change, Orlando, FL: Academic Press, pp. 29–49.
- White R. T., (1985), Interview protocols and dimensions of cognitive structure, in West L. and Pınes L. (ed.) Cognitive structure and conceptual change, Orlando, FL: Academic Press, pp. 51–59.
- Wilson J. M., (1990), Chemistry concepts and group cognitive structure: a study of undergraduate nursing students, Res. Sci. Educ., 20, 292–299.
| This journal is © The Royal Society of Chemistry 2016 |