The role of green chemistry activities in fostering secondary school students' understanding of acid–base concepts and argumentation skills
Mageswary
Karpudewan
*a,
Wolff
Michael Roth
b and
Devananthini
Sinniah
c
aUniversiti Sains Malaysia – School of Educational Studies USM Penang, Penang, Penang 11800, Malaysia. E-mail: kmageswary@usm.my; mageswary_karpudewan@yahoo.com
bUniversity of Victoria Victoria, British Columbia, Canada
cUniversiti Sains Malaysia Minden, Pulau Pinang, Malaysia
First published on 1st June 2016
Abstract
In a world where environmental degradation is taking on alarming levels, understanding, and acting to minimize, the individual environmental impact is an important goal for many science educators. In this study, a green chemistry curriculum—combining chemistry experiments with everyday, environmentally friendly substances with a student-centered approach that includes student–student discussion—was tested for its potential to increase the understanding of acid–base concepts and argumentative skills. A quasi-experimental design was chosen intended to take into account teacher/school nested effects. The study involved three classes of 150 16 year old Form Four students (1 experimental, N = 50; 2 control, N = 100) from two Schools A and B serving students from the same sociocultural and economic backgrounds taught by two teachers (Teacher A in School A taught 1 experimental and 1 control; Teacher B in School B taught 1 control). An ANCOVA with a pre-test as a covariate showed a statistically significant treatment effect as measured by an acid–base concept understanding test. Additionally, qualitative analysis of an Argumentation Skill Test (AST) shows that the experimental students used higher levels of argumentation skills following treatment than their peers in the two control classes. Implications are discussed for integrating green chemistry into the secondary school chemistry curriculum to teach the content on acid–base and green chemistry as a tool to assist the construction of arguments.
There are few individuals who would doubt that humanity is facing an increasing environmental crisis (Perlingieri, 2009). Pollution is not only an important outcome of many industrial processes but also—as shown in the widespread appeals of many municipalities in industrial nations to individual recycling and composting—the outcome of practices at the personal level. Not surprisingly, there have been calls for engaging students in environmentally friendly practices and even activism (e.g., Roth and Barton, 2004). Taking action, increasing the individual's power to act in personally relevant settings and allowing individual students to become interested in making a difference in their local environment appear to foster environmental argumentation (Oliveira et al., 2012). When students have opportunities to engage in argumentation, their competencies in context-related discourses increase due to the requirement to evaluate, support, question, and critique ideas in the process of reaching consensus about the issues at hand (Weinberger and Fischer, 2006; Kim and Roth, 2014). Therefore, the importance of including argumentative discourses in the school science has been stressed in many studies (Erduran et al., 2004; Simon et al., 2006; Kuhn, 2010). Unsurprisingly, there are calls for more research to be conducted on the tools and pedagogical strategies that can help teachers and students in the construction and evaluation of arguments (Berland and Reiser, 2010; Eskin and Ogan-Bekiroglu, 2013).
This study was designed to investigate whether a green chemistry curriculum—which combines the use of environmentally friendly, everyday substances to conduct chemistry experiments with a student-centered context that fosters debate and making arguments—leads to a better understanding of chemical concepts—here related to acid–base chemistry—and increased argumentation skills than a regular chemistry course taught in a teacher-centered approach together with the traditional laboratory tasks and chemical substances.
Study background
Green chemistry
Green chemistry is also known as sustainable chemistry, a form of chemistry designed to prevent pollution. It emphasizes the use of materials, processes, or practices that reduce or eliminate the creation of pollutants and waste materials. It includes practices that reduce the use of hazardous and non-hazardous materials, energy, water, or other resources and protect natural resources through efficient use (Anastas and Warner, 1998). Green chemistry is a platform to discuss sustainable development in the classroom (Wardencki et al., 2005). This is so because green chemistry provides a connection between the subject matter of chemistry and students' everyday lives (Braun et al., 2006) and creates spaces for students to address in a fair manner the environmental issues faced by the local and global community (Haack et al., 2005). In fact green chemistry has been suggested to be an integral part of students' training from the very early stage because it is a tool to promote their competencies to participate in societal debates about the application of chemistry and technology (Eilks and Rauch, 2012).One possible way to bring green chemistry into education is through adapting green chemistry principles to science education laboratory work (Burmeister et al., 2012). Despite various documented challenges—including paucity of literature on organic perspectives and green chemistry as optional material rather than as main topic—multiple effective teaching methods have been suggested (Andraos and Dicks, 2012). These include presenting green chemistry through real-world case studies in stand-alone courses or as cross-curricular interventions (with laboratory resources). Additionally, green chemistry has been presented using problem-based case study approaches, whereby the students are exposed to the challenges to developing alternatives (e.g. to chlorofluorocarbon) (Heaton et al., 2006). On the other hand, product life cycle analysis constitutes an approach that unites green chemistry principles, sustainable chemistry, and engineering. Such an approach was employed to improve the argumentation skills of 15-year-old Finnish students (Juntunen and Aksela, 2014). In another case study, chemistry and biology undergraduate majors and minors became more competent in using green chemistry metrics, assessing the traditional and greener products and have better grasp on the role of toxicology as a result of learning a non-laboratory green chemistry course (Marteel-Parrish, 2014).
Previous research shows that chemical concepts taught in the context of green chemistry allow students to make connections with their local economic, environmental, and societal context; these connections are thought to underlie the advanced conceptual understanding that students develop compared to their peers who are instructed in the same concepts but in a traditional teacher-centered approach (Karpudewan et al., 2012a, 2012b, 2012c; Karpudewan et al., 2015). In these studies, the green chemistry curriculum resulted not only in conceptual gains but also gains in other areas. For example, green chemistry curricula led to improving pre-service teachers' self-determined environmental motivation (Karpudewan et al., 2012a), pro-environmental attitudes (Karpudewan et al., 2012b), environmental values (Karpudewan et al., 2012d), and understanding of traditional environmental concepts and sustainable development concepts (Karpudewan et al., 2009). In the present study, the acid–base part of the traditional chemistry curriculum established by the Malaysian Ministry of Education was redesigned in the same manner that other aspects had been redesigned and tested in previous research.
The aforementioned green chemistry activities have been developed based on a socio-constructivist approach. The curricula as a whole and the student-centered activities and experiments specifically require collaboration and joint decision-making while solving real-world (authentic) problems. For instance in the thermochemistry lessons, students worked in small groups to prepare biodiesel using cooking oil obtained from various sources. Upon producing the biodiesel they need to compare the life cycle of biodiesel and petroleum diesel. This is followed by calculating the efficiency of the fuels in terms of consumption to travel for 100 km and the cost of doing so. Students worked in smaller groups in solving problems such as “Is it worthwhile to opt for biodiesel?” or “Is opting for biodiesel really green?” Students tended to debate their answers within their groups and between groups in whole class sessions. In the process, students arrived at explanations through connecting the claims and evidence and being socially involved in generating, evaluating, critiquing, and refining the claims and evidence in the attempt to explain the situation. When students engage in learning in this way, their science learning is deliberately enhanced (Grooms et al., 2014). For instance, using a case study approach, lessons on biodiesel introduced to grade 10–12 students encouraged discussion, sharing and improved students' ability to reflect on societal and ecological consequences of biodiesel production and its usage compared to the crude oil (Eilks, 2002). This led us to hypothesize that students may also develop greater argumentation skills compared to their peers in teacher-centered courses even without special instruction, a hypothesis that is further grounded in existing research that demonstrated how discussions of familiar situation enhances argumentation skills (Kim and Roth, 2014).
Argumentation and acid–base concepts
Argumentation not only is a key aspect characterizing science (Osborne et al., 2004) but also appears to enhance and deepen thought processes (Zohar and Nemet, 2002). An increasing number of studies points to the positive effects that arise for science learning in classrooms that foster argumentation skills (Berland and Reiser, 2009; Berland and McNeill, 2010; Garcia-Mila et al., 2013; Grooms et al., 2014). Such studies provide evidence that a focus on argumentation improves students' conceptual understanding (Venville and Dawson, 2010; Aydeniz et al., 2012; Walker and Sampson, 2013; Cetin, 2014) and quality of argumentation (Erduran et al., 2004; Garcia-Mila et al., 2013). In all of these studies, instructional strategies have been used to teach argumentation skills explicitly (Simon et al., 2006). However, it is unknown whether students develop argumentation skills in a context that fosters debate without explicitly teaching the skills. The above-mentioned study of biodiesel lessons revealed that students' communicative skills did indeed improve without explicit instruction (Eilks, 2002).Acids, bases, and their interactions are key topics in chemistry. Research shows that students tend to have a variety of misunderstandings concerning acids and bases (Demircioglu et al., 2005) often despite and perhaps because of teaching (Chiu, 2007; Artdej et al., 2010). Studies based on the argumentation approach show that students developed an enhanced understanding of chemistry generally and acid–base concepts specifically when compared to students in regular, teacher-centered courses (Sampson and Walker, 2012; Kaya, 2013). A study investigating a socio-constructivist approach (e.g. green chemistry experiments and activities) that fosters debate over controversial issues also revealed an enhanced student understanding of chemistry concepts (Karpudewan et al., 2015). However, research on the socio-constructivist approach (particularly on green chemistry) and understanding about acid–base concepts is limited.
Research methods
Design
In this study, a quasi-experimental design—which randomly assigns whole classes to treatment and control (Campbell and Stanley, 1963)—was used to test the effectiveness of a student-centered green chemistry curriculum against the regular teacher-centered chemistry curriculum. To address the possibility that the students in different treatments start off differently and to ascertain that there are no treatment-by-group interactions, methodologists recommend the use of pre-tests employed as covariates (Shadish et al., 2002), resulting in the basic designwhere O1 and O2 refer to pretest and post-test and T1 and T2 to experimental and control treatment (curriculum). When the same teacher teaches both experimental and traditional curriculum, an alternative hypothesis to effects arising from the curriculum is the possibility that vested interests lead to bias, favoring/disadvantaging the two treatment groups. On the other hand, if one teacher in one school teaches the experimental curriculum and another teacher in a second school teaches the control curriculum, then the alternative hypothesis arising from the nesting of treatment in teacher and school cannot be eliminated. To address both issues, the following design was used
where T1 refers to Teacher A in School A teaching the green chemistry curriculum in a student-centered context, T2 refers to Teacher A in School A using a traditional teacher-centered approach with text-book laboratories, and T3 refers to Teacher B in School B using the same teacher-centered approach. School B and Teacher B were chosen to match School A and Teacher A on many attributes (see below) for the purpose of improving the validity of the inferences. An analysis of covariance (ANCOVA) approach was used to detect treatment effects. Planned contrasts—between (a) treatment and control groups and (b) the two control groups—were employed using a Bayesian approach to assess the relative probability of null and alternative effects.
Pretests (O1) were administered during the first week of the study immediately prior to the acid–base unit of the chemistry course. The experimental and control curricula (T1, T2, T3) were taught over the course of Weeks 2 to 6. The post-tests (O2) were administered during Week 7. During the final week, interviews were conducted with randomly selected students designed to collect information concerning their understanding of acid–base concepts.
Although this design excludes most hypotheses other than curriculum as the difference-producing factor, it cannot eliminate the possibility of interactions that might occur if the experimental treatment also were to occur in another context such as School B.
Participants
Ethics
Firstly, approval to carry out the study in the secondary schools has been obtained from the Evaluation Planning Research Department of the Ministry of Education. As the students were below 18 years old at the time of the study, an approval form was distributed to the parents of the students participating in this study. Additionally, to ensure that the control group students were not deprived from the benefits of the treatment employed to teach the experimental group the control group students were provided an opportunity to experience green chemistry activities after the data collection session (Taber, 2014).Interventions: experimental and control curricula
This study was conducted in the context of the students' 10 hour (5-week) acid–base unit covering (a) characteristics of acids and bases, (b) strength of acids and bases, (c) concentration of acids and bases, (d) neutralization processes, and (e) acids and bases in daily life. Control and experimental curricula were taught according to the standard, textbook-oriented approach or according to the student-centered approach developed for the implementation of green chemistry in previous studies (e.g., Karpudewan et al., 2015).Similar to previous studies (e.g., Karpudewan et al., 2012a, 2012c), the experimental green chemistry curriculum supported greater student engagement. Students engaged in comparing answers/results and questioned when there were differences. When differences could not be resolved, students tended to pose questions to the teacher. In the green chemistry curriculum, the students responded to questions concerning familiar, real-life substances and issues, which generally encouraged them to develop arguments about the topic. Since they were familiar with the relevant issues, they tended to relate the data collected in the experiments to what they experienced at home. In the case of discrepant data or experiences, the student-centered approach encouraged debating differences and providing arguments and counter-arguments.
Data sources
Standard statistical approaches in experimental psychology have been subject to considerable critique accompanied by recommendations to use Bayesian forms of hypothesis testing. In contrast to standard statistical approaches where the null hypothesis is or is not rejected, the Bayesian approach establishes the probabilities of null and alternative hypotheses and, thereby, the possibility to test for invariances across groups, a standard test in the natural sciences (Rouder et al., 2009). Therefore, in this study we performed the usual frequentist approach and further established the probability of the occurrences of null hypothesis against alternative hypothesis. We conducted the Bayesian analysis because it tells us more about the amount of the evidence for H0 and H1. For this purpose we conducted two planned comparisons: (a) between experimental and control groups for establishing the probability of the hypothesis that the treatment causes any effect; and (b) between the two control groups to test the alternative hypotheses of differences arising because of teacher and school effects. Web-based software (http://pcl.missouri.edu/bf-two-sample) was used to calculate the Bayes factor.
In the present study, students responded in writing to two questions:
Question 1: Which food contains high level of acidity: tea, egg, fish or carbonated drinks? What do these substances have in common? Can you think of other types of foods that have acidic characteristics?
Question 2: A mixture of acid and base will result in neutralization. If this is the case, why is milk of magnesia commonly available in pharmacies frequently subscribed by the doctors as treatment for gastritis?
Students need to understand the issues raised and provide arguments supporting their answers. The written responses to these questions were classified as belonging to one of five levels (Table 1). Responses that contain only a claim were classified as Level 1 of argumentation. Statements were categorized as Level 2 of argumentation if they contained claims supported by data, warrant, or backing but do not include a rebuttal. Level 3 statements included claims supported by data, warrant, or backing with occasional weak rebuttal. Statements in the written responses categorized in Level 4 included clear rebuttals. Finally, Level 5 statements contain extended rebuttals. Three chemistry teachers and two of the authors independently analyzed students' responses. After completing individual analysis the teachers and researchers met to resolve any differences in their categorization until consensus was reached. Based on the responses provided students were categorized into five argument levels. Transition matrices were developed for each group to illustrate the changes in the number of students moved from one level to another level between the pre- and post-test. Additionally, similar to other studies (Osborne et al., 2004; Simon et al., 2006) the number of arguments for each level has been counted by considering the responses provided for both the questions. Later the frequency has been converted into percentages.
| Level | Descriptions | Sample answers |
|---|---|---|
| 1 | Simple claim. |
• Acidic, carbonated drinks
• Not clear how to differentiate between acid and base. • To neutralize the acid in the stomach. |
| 2 | Claim with data but no warrants or backing and rebuttals. |
• Yes acidic because these substances can change the color of litmus from blue to red. Another example is tamarind.
• The milk of magnesia has alkaline properties because of this the alkaline can neutralize the acid. |
| 3 | Series of claims with warrants, or backing. |
• All these substances are similar because these are acidic (claim). Based on my observation during the activity just now (the student is referring to GCAs) these substances can change the color of litmus from blue to red (data). This is because these substances produce H+ions which actually exhibit acidic properties (warrant). Other examples are vinegar, orange and pineapple juices.
• Milk of magnesia possesses alkaline properties (claim). I learnt from the activity on neutralization that acid will neutralize the base (data). The reaction of milk magnesia and acid in the stomach neutralized with each other and reduced the level of acid in the stomach hence reducing the pain (backing). |
| 4 | A claim with warrants, or backing, rebuttals and qualifiers. |
• They are acidic (claim). The similarity is that all these substances can change the color of litmus from blue to red (data). However, the change in color occurs only when the litmus is wet (rebuttal). These occur because all acidic substances produce H+ions (backing). If the substances were alkaline it produces OH−ions and changes the color of litmus from red to blue (qualifier). Other examples are vinegar and tamarind.
• Milk of magnesia is alkaline (claim). Based on my observation during the activity acid and base neutralizes each other (data). Stomach excretes hydrochloric acid and the milk of magnesia is alkaline. The mixture of acid and base causes the H+ions and OH−ions to react and produces water molecules (warrant/backing). The pain will stop when the hydrochloric acid (H+) excreted in the stomach is neutralized using alkali from the milk (OH−) (qualifier). |
| 5 | An extended argument with one or more rebuttal. |
• The similarities between these substances are they are acidic (claim). Their acidic properties are due to the presence of H+ions (warrant). These substances can convert the color of litmus from blue to red in the presence of water (data). However, for the color change to occur the litmus paper must be wet (rebuttal). The acidic properties are only shown in water because the H+ions exist as free radicals in water.
• The mixture of acid and base neutralizes between each other (data). Hydrochloric acid is produced in the stomach and milk of magnesia is alkaline (claim). Aqueous hydrochloric acid produces free radical H+ions and milk of magnesia produces OH−ions. Both these ions combine and produce water molecules whereby they neutralize between each other (warrant and backings). The pain in the stomach would not stop if the amount of OH−ion is not enough to react with H+ions (rebuttal). H+ions still remain despite consuming milk of magnesia. |
Results
This study was designed to test two hypotheses: (a) green chemistry activities requiring argumentation will lead to better acid–base concept understanding as measured by the ABCT than the regular chemistry curriculum; and (b) green chemistry activities requiring argumentation will lead towards developing better arguments (as measured by the AST) than the regular chemistry curriculum. In the following two subsections, we provide the results of our study in terms of acid–base understanding and changes in the level of arguments.Acid–base understanding
The analysis of covariance (ANCOVA) requires the homogeneity of regression slopes. The test shows that this requirement was satisfied. The ANCOVA results (F(2,146) = 846.67, p < 0.00001) reveal a statistically reliable effect for treatments (Table 2). The experimental group (MEXP-TA = 16.42, SDEXP-TA = 0.97) taught by Teacher A outperformed both control groups, that taught by Teacher A (MCON-TA = 9.32, SDCON-TA = 1.57) as well as that taught by Teacher B in the second school (MCON-TB = 8.94, SDCON-TB = 1.22) (Fig. 1). A Bayesian test was used for two planned comparisons that evaluate the evidence for null and alternative hypotheses. First, comparing the treatment to the two control groups, there is decisive evidence for the alternative hypothesis of treatment differences (JZS = 7020.11), that is, the alternative hypothesis is over 7020 times as likely as the null hypothesis (i.e., p(H1|data)/p(H0|data) = JZS = 7020.11). A comparison of the two control groups shows that the null hypothesis is nearly three times as likely as the alternative hypothesis (p(H0|data)/p(H1|data) = 1/JZS = 1/0.361 = 2.77). This JZS value indicates that there is marginal evidence for H0, i.e., that there is marginal evidence for an invariant property across the two groups. These results therefore suggest that the innovative green chemistry curriculum implemented in a social context fostering argumentation leads to higher concept knowledge outcomes when compared to the regular, teacher-centered way of teaching the same acid–base concepts.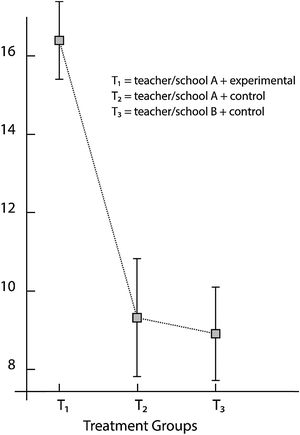 | ||
| Fig. 1 Posttest mean scores of groups taught by Teacher 1 (experimental group), Teacher 2 and Teacher 3 (control groups) for the acid–base understanding test. | ||
Changes in the levels of arguments
To investigate whether the experimental curriculum led to higher levels of argumentation, responses to the argumentation skill test were classified and are presented in Table 1. The effect of a curriculum on students' quality of discourse can be assessed using transition matrices (Roth and Lucas, 1997). To establish the effects of the control group's teacher dominated curriculum and the experimental curriculum on students' argumentation skills, transition matrices were constructed (Table 3). The transition matrices show that in the control group from school A, 35 students moved from Level 1 to Level 2 and 5 students moved from Level 1 to Level 3. Six students moved from Level 2 to Level 3 and 4 students remained at Level 3. This amounts to a mean per student level change of ΔLA = 1.02. Transition matrices of the control group from school B shows that 42 students moved from Level 1 to Level 2, and two students from Level 1 to Level 3. Six students made Level 2 statements prior to the study and moved to making argumentations at Level 3 following the curriculum. This amounts to a mean per student level change of ΔLB = 1.04. The transition matrix of the experimental group is distinctly different from that of both control groups. Thus, among the 45 students constructing statements at Level 1 prior to the experimental curriculum, 32 responded to the AST with Level 3 statements and 13 students made statements at Level 4 of argumentation. The five students making Level 2 statements prior to the study, two and three were writing statements at Level 4 and Level 5, respectively. This amounts to a mean per student level change of ΔLE = 2.32. That is, although the students in control and experimental groups were nearly equal in using statements at Level 1 and Level 2, the post-test results were very different.| Pretest | Post-test | ∑ | ||||
|---|---|---|---|---|---|---|
| L1 | L2 | L3 | L4 | L5 | ||
| a | ||||||
| L1 | 35 | 5 | 40 | |||
| L2 | 6 | 6 | ||||
| L3 | 4 | 4 | ||||
| ∑ | 35 | 15 | 50 | |||
| b | ||||||
| L1 | 42 | 2 | 44 | |||
| L2 | 6 | 6 | ||||
| ∑ | 42 | 8 | 50 | |||
| c | ||||||
| L1 | 32 | 13 | 45 | |||
| L2 | 2 | 3 | 5 | |||
| ∑ | 32 | 15 | 3 | 50 | ||
These results show that prior to the interventions, control and experimental group students are nearly equally distributed between Level 1 (control 84% vs. experimental 90%) and Level 2 (control 12%, experimental 10%), with some additional control group students being in Level 3 (4%). After the treatments, however, 36% of the experimental group students but none of the control group students argue at Levels 4 and 5. Whereas 77% of the control group students argue at Level 2 following treatment, none of the experimental students argue below Level 3. The treatment likely brought about dramatic differences in terms of the levels of argumentation that the students in the different groups produce, allowing the experimental students to advance, on average, about 2.3 levels whereas the control students advanced by about 1 level.
Discussion and conclusion
It is evident from our quantitative analysis of ABCT that students exhibited improved understanding about acid and base concepts when instructed by means of a green chemistry curriculum, which provides for (a) making connections with everyday understanding and experience and (b) debating the impact of green and traditional chemistry on the students' daily environment. In addition, as shown by the analysis of the written argumentation test, the green chemistry learning environment also improved students' argumentation skills over those of students taught using a more conventional method. Although shown to have general benefits, our research does not allow discerning the independent effects of connections with the everyday life or increased argumentation. However, the outcomes of this research parallel those of previously reported studies, which suggested that argumentation-driven pedagogy improves students' understanding of scientific concepts and argumentation skills (Cross et al., 2008; Walker and Sampson, 2013; Cetin, 2014). Argumentation-based interventions significantly strengthened the acquisition of scientific reaction rate-related concepts and positively impacted the structure and complexity of pre-service teachers' argumentation (Cetin, 2014). In the context of this study, green chemistry was introduced as chemistry laboratory work (Burmeister et al., 2012) and the principles were integrated into practice during the laboratory work on acid–base permitting the presentation of acid–base concepts as real world issues (Andraos and Dicks, 2012). This encouraged students to actively participate, critically think, discuss, and debate issues to arrive at conclusions and solutions (Eilks, 2002; Marteel-Parrish, 2007, 2014). In the green chemistry curriculum argumentation was not explicitly taught. But, as in other studies (Braun et al., 2006; Cross et al., 2008), the curriculum content itself created a platform that encouraged students to take and defend personal positions over the societal relevant issues. These circumstances considerably led students to acquire better understanding about the content (acid–base concepts).The green chemistry curriculum used in this study emphasizes student-centered inquiry involving chemical substances as well as their use in students' everyday out-of-school environments. Students were provided with real-world problems relevant to their own lives, such as identifying the pH of standard household items. Connections to the everyday environment provide a sense of authenticity to green chemistry (Cacciatore and Sevian, 2006). Previous investigations suggest that students who engage in inquiry-oriented experiments—as opposed to following the “cookbook” approach of standard textbook series—are more enabled to engage in argumentation than their counterparts in traditional curricula (e.g., Hofstein et al., 2004). The benefits accruing for our green chemistry students may therefore derive in part from the inquiry-oriented aspect of the curriculum.
Connections to the everyday world may also provide students with a sense of security for making claims, provide appropriate evidence, and argue for the reasoning that justify the evidence. According to Driver et al. (2000), a major barrier in developing young people's argumentation skills in science is the lack of opportunity to engage in debates in areas that students are familiar with. Green chemistry supports learning science concepts in relation to student's everyday life. Green chemistry activities provide space for the students to solve the problem and construct their own ideas while debating differences in experimental results or different responses to environmental problems arising from the everyday use of chemical substances.
Contemporary science education calls for active participation of the students during science lessons. Argumentation shifts the emphasis of science teaching from imparting content towards educating the students on the importance applying the content in and to real life contexts (Osborne et al., 2004). Most studies, however, appear to teach argumentation skills independently of the particular scientific concepts and students' familiarity with everyday contexts where the taught concepts are relevant. The present study shows that green chemistry activities not only enhance student understanding, consistent with previous research, but also that there are gains over regular instruction with respect to the levels of argumentation skills that students develop. This is possible because the nature of the course on green chemistry itself permits students to communicate their ideas while making decision about the green processes in parallel to the increasing depth of knowledge and awareness of sustainable initiatives in other countries (Marteel-Parrish, 2014). This nature of green chemistry is in line with the Argument-Driven Instructional (ADI) pedagogy employed in other studies (Sampson and Walker, 2012; Grooms et al., 2014). As in the ADI instructional strategy, while engaged in green chemistry lessons students collaboratively involved in generating questions, students in the present curriculum developed arguments based on the data obtained and in turn used evidence to support their claims. They constantly engaged in collaboratively reflecting the findings of the lab work with their real life scenario. Regarding this, the present study revealed outcomes similar to another one focusing on global climate change where evidence and claims dominated student discourse and where students frequently refuted or supported others' views (McNeill and Pimentel, 2010). Experiencing a similar situation, the present students probably were able to develop better arguments. As such, the introduction of green chemistry in a socio-constructivist approach addresses the call for helping teachers and students to teach and learn science in more meaningful ways (Eskin and Ogan-Bekiroglu, 2013).
The results of this study are particularly salient in the country where this study has been conducted because the general politics are oriented towards improving its positioning in the upcoming TIMSS and PISA assessments. The present study shows that green chemistry activities are a viable means of enhancing student understandings over those exhibited by their peers instructed in the traditional manner.
In this study, the green chemistry curriculum is the likely causal factor that enhanced both concept understanding and argumentation skills. A quasi-experimental design with two control groups was used to exclude the possibility that the teacher instructing under the experimental conditions might be biased against the group under the control conditions. Two control groups were used to eliminate the possible nested school context and teacher effects under the control conditions. However, this leaves open the possibility that there is school or teacher by treatment interactions. Such effects can be excluded in matched-pair tandem designs where both teachers teach experimental and control groups (Randler and Bogner, 2008). To ascertain that differences are due to the treatment alone, future research should be designed so as to eliminate effects arising from the nested aspects. In addition, “green chemistry” is a label for a complex intervention. Future research might be designed, where possible, to investigate the independent effect of the holistic teaching–learning environment on student's understanding, argumentation skills, and the changes in other student characteristics reported in previous research.
References
- Anastas P. T. and Warner J. C., (1998), Green chemistry: theory and practise, Oxford, UK; Oxford University Press.
- Andraos J. and Dicks A. P., (2012), Green chemistry teaching in higher education: a review of effective practices, Chem. Educ. Res. Pract., 13(2), 69–79.
- Artdej R., Ratanaroutai T., Coll R. K. and Thongpanchang T., (2010), Thai grade 11 students' alternative conceptions for acid-base chemistry, Research in Science & Technological Education, 28(2), 167–183.
- Aydeniz M., Pabuccu A., Cetin P. S. and Kaya E., (2012), Argumentation and students' conceptual understanding of properties and behaviours of gases, International Journal of Science and Mathematics Education, 10(6), 1303–1324.
- Berland L. K. and McNeill K. L., (2010), A learning progression for scientific argumentation: understanding student work and designing supportive instructional contexts, Sci. Educ., 94(5), 765–793.
- Berland L. K. and Reiser B. J., (2009), Making sense of argumentation and explanation, Sci. Educ., 93(1), 26–55.
- Berland L. K. and Reiser B. J., (2010), Classroom communities' adaptations of the practice of science argumentation, Sci. Educ., 95(2), 191–216.
- Braun B., Charney R., Clarens A., Farrugia J., Kitchens C., Lisowski C., Naistat D. and O'Neil A., (2006), Completing our education, J. Chem. Educ., 83(8), 1126–1129.
- Burmeister M., Rauch F. and Eilks I., (2012), Education for sustainable development (ESD) and chemistry education, Chem. Educ. Res. Pract., 13(2), 59–68.
- Cacciatore K. L. and Sevian H., (2006), Teaching lab report writing through inquiry: a green chemistry stoichiometry experiment for general chemistry, J. Chem. Educ., 83(7), 1039–1041.
- Campbell D. T. and Stanley J. C., (1963), Experimental and quasi-experiment designs for research, Boston: Houghton Mifflin Company.
- Cetin P. S., (2014), Explicit argumentation instruction to facilitate conceptual understanding and argumentation skills, Research in Science & Technological Education, 32(1), 1–20.
- Chiu M. H., (2007), A national survey of students' conceptions of chemistry in Taiwan, Int. J. Sci. Educ., 29(4), 421–452.
- Cross D., Taasoobshirazi G., Hendricks S. and Hickey D. T., (2008), Argumentation: a strategy for improving achievement and revealing scientific identities, Int. J. Sci. Educ., 30(6), 837–861.
- Demircioglu G., Ayas A. and Demircioglu H., (2005), Conceptual change achieved through a new teaching programs on acids and bases, Chem. Educ. Res. Pract., 6(1), 36–51.
- Driver R., Newton P. and Osborne J., (2000), Establishing the norms of scientific argumentation in classrooms, Sci. Educ., 84(3), 287–312.
- Eilks I., (2002), Teaching biodiesel: a sociocritical and problem oriented approach to chemisty teaching and students first views on it. Chem. Educ. Res. Pract., 3, 77–85.
- Eilks I. and Rauch F., (2012), Sustainable development and green chemistry in chemistry education, Chem. Educ. Res. Pract., 13(2), 57–58.
- Erduran S., Simon S. and Osborne J., (2004), TAPping into argumentation: developments in the application of Toulmin's argument pattern for studying science discourse, Sci. Educ., 88(6), 915–933.
- Eskin H. and Ogan-Bekiroglu F., (2013), Argumentation as a strategy for conceptual learning of dynamics, Res. Sci. Educ., 43, 1939–1956.
- Garcia-Mila M., Gilabert S., Erduran S. and Felton M., (2013), The effect of argumentative task goal on the quality of argumentative discourse, Sci. Educ., 97(4), 497–523.
- Grooms J., Sampson V. and Golden B., (2014), Comparing the effectiveness of verification and inquiry laboratories in supporting undergraduate science students in constructing arguments around socioscientific issues, Int. J. Sci. Educ., 36(9), 1412–1433.
- Haack J. A., Hutchison J. E., Kirchhoff M. M. and Levy I. J., (2005), Going green: lecture assignments and lab experiences for the college curriculum, J. Chem. Educ., 82(7), 974.
- Heaton A., Hodgson S., Overton T. and Powell R., (2006), The challenge to develop CFC (chlorofluorocarbon) replacements: a problem based learning case study, Chem. Educ. Res. Pract., 7, 280–287.
- Hofstein A., Shore R. and Kipnis M., (2004), Providing high school chemistry students with opportunities to develop learning skills in an inquiry laboratory: a case study, Int. J. Sci. Educ., 26(1), 47–62.
- Juntunen M., K. and Aksela M., K., (2014), Improving students' argumentation skills through a product life-cycle analysis project in chemistry education, Chem. Educ. Res. Pract., 15, 639–649.
- Karpudewan M., Ismail Z. H. and Mohamed N., (2009), The integration of green chemistry experiments with sustainable development concepts in pre-service teachers' curriculum: Experiences from Malaysia, International Journal of Sustainability in Higher Education, 10(2), 118–135.
- Karpudewan M., Ismail Z. and Roth W.-M., (2012a), Fostering pre-service teachers' self-determined environmental motivation through green chemistry experiments, J. Sci. Teacher Educ., 23(6), 673–696.
- Karpudewan M., Ismail Z. and Roth W.-M., (2012b), Promoting pro-environmental attitudes and reported behaviors of Malaysian pre-service teachers using green chemistry experiments, Environ. Educ. Res., 18(3), 375–389.
- Karpudewan M., Ismail Z. and Roth W.-M., (2012c), Ensuring sustainability of tomorrow through green chemistry integrated with sustainable development concepts (SDCs), Chem. Educ. Res. Pract., 13, 120–127.
- Karpudewan M., Ismail Z. and Roth W.-M., (2012d), The efficacy of a green chemistry laboratory-bases pedagogy: Changes in environmental values of Malaysia pre-service teachers, International Journal of Science and Mathematics Education, 10(3), 497–529.
- Karpudewan M., Roth W. M. and Ismail Z., (2015), The effects of “Green Chemistry” on secondary school students' understanding and motivation, The Asia-Pacific Education Researcher, 25, 35–43.
- Kaya E., (2013), Argumentation practices in classroom: Pre-service teachers' conceptual understanding of chemical equilibrium, Int. J. Sci. Educ., 35(7), 1139–1158.
- Kim M. and Roth W.-M., (2014), Argumentation as/in/for dialogical relation: a case study from elementary school science, Pedagogies, 9(4), 300–321.
- Kuhn D., (2010), Teaching and learning science as arguments, Sci. Educ., 94(5), 810–824.
- Marteel-Parrish A. E., (2007), Toward the greening of our minds: a new special topics course, J. Chem. Educ., 84(2), 245.
- Marteel-Parrish A. E., (2014), Teaching green and sustainable chemistry: a revised one-semester course based on inspirations and challenges, J. Chem. Educ., 91(7), 1084–1086.
- McNeill K. L. and Pimentel D. S., (2010), Scientific discourse in three urban classrooms: the role of the teacher in engaging high school students in argumentation, Sci. Educ., 94(2), 203–229.
- Oliveira A. W., Akerson V. L. and Oldfield M., (2012), Environmental argumentation as sociocultural activity, J. Res. Sci. Teach., 49(7), 869–897.
- Osborne J., Erduran S. and Simon S., (2004), Enhancing the quality of argumentation in school science, J. Res. Sci. Teach., 41(10), 994–1020.
- Perlingieri S., (2009), The worldwide environmental crisis gone missing: the precautionary principle. Accessed December 15, 2014 at http://www.globalresearch.ca/the-worldwide-environmental-crisis/12268.
- Randler C. and Bogner F. X., (2008), Planning experiments in science education research: comparison of a quasi-experimental approach with a matched pair tandem design, International Journal of Environmental and Science Education, 3(3), 95–103.
- Roth W.-M. and Barton A. C., (2004), Rethinking scientific literacy, New York, NY: Routledge.
- Roth W.-M. and Lucas K. B., (1997), From “truth” to “invented reality”: a discourse analysis of high school physics students' talk about scientific knowledge, J. Res. Sci. Teach., 34(2), 145–179.
- Rouder J. N., Speckman P. L., Sun D., Morrey R. D. and Iverson G., (2009), Bayesian t tests for accepting and rejecting the null hypothesis, Psychonomic Bulletin & Review, 16, 225–237.
- Sampson V. and Walker J. P., (2012), Argument-driven inquiry as a way to help undergraduate students write to learn by learning to write in Chemistry, Int. J. Sci. Educ., 34(10), 1443–1485.
- Simon S., Erduran S. and Osborne J., (2006), Learning to teach argumentation: Research and development in the science classroom, Int. J. Sci. Educ., 28(2–3), 235–260.
- Shadish W. R., Cook T. D. and Campbell D. T, (2002), Experimental and quasi-experimental designs for generalized causal inference, Boston, New York: Houghton Mifflin Company.
- Taber S. K., (2014), Ethical considerations of chemistry education research involving human subjects, Chem. Educ. Res. Pract., 15, 109–113.
- Venville G. J. and Dawson V. M., (2010), The impact of a classroom intervention on grade 10 students' argumentation skills, informal reasoning, and conceptual understanding of science, J. Res. Sci. Teach., 47(8), 952–977.
- Walker J. P. and Sampson V., (2013), Learning to argue and arguing to learn: argument-Driven inquiry as a way to help undergraduate chemistry students learn how to construct arguments and engage in argumentation during a laboratory course, J. Res. Sci. Teach., 50(5), 561–596.
- Wardencki W., Curylo J. and Namiesnik J., (2005), Green chemistry-current and future issues, Pol. J. Environ. Stud., 14(4), 389–395.
- Weinberger A. and Fischer F., (2006), A framework to analyze argumentative knowledge construction in computer-supported collaborative learning, Comput. Educ., 46(1), 71–95.
- Zohar A. and Nemet F., (2002), Fostering students' knowledge and argumentation skills through dilemmas in human genetics, J. Res. Sci. Teach., 39(1), 35–62.
| This journal is © The Royal Society of Chemistry 2016 |


