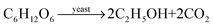Development of analytical thinking ability and attitudes towards science learning of grade-11 students through science technology engineering and mathematics (STEM education) in the study of stoichiometry
Patcharee
Chonkaew
a,
Boonnak
Sukhummek
*a and
Chatree
Faikhamta
b
aKing Mongkut's University of Technology Thonburi, Thailand. E-mail: b.sukhummek@gmail.com
bKasetsart University, Thailand
First published on 19th May 2016
Abstract
The purpose of this study was to investigate the analytical thinking abilities and attitudes towards science learning of grade-11 students through science, technology, engineering, and mathematics (STEM) education integrated with a problem-based learning in the study of stoichiometry. The research tools consisted of a pre- and post-analytical thinking ability test, a science learning attitude test, classroom observations, student reflective journals, and semi-structured interviews. The findings indicated that STEM learning activities based on problem-based learning successfully developed analytical thinking abilities and attitudes towards science learning. Consequently, the students realized how important theories are, and were able to integrate their knowledge from various fields to solve problems and to create new innovations. About 80% of the students showed higher analytical thinking ability scores above the prescribed criterion of 70% of the full score. After learning, the scores of the students were higher than those before learning at a confidence level of 0.01. The attitudes towards science learning were higher than those before learning at a confidence level of 0.01. The successful activities of STEM started with offering knowledge to students through an inquiry-based process until they could construct the knowledge on their own. After that, the teacher initiated a problem situation and allowed each group of students to create a useful product adopted from the experimental results via integrating STEM knowledge to modify their creative works.
Introduction
It is commonly known that the achievements of industrialized nations and many developing countries rely mainly on the effective use of science and technology (UNESCO, 2010). These countries have an increasing demand for qualified researchers and technicians who are able to identify, adapt, and effectively use knowledge in science and technology to develop their own unique technologies (Kennedy and Odell, 2014; Meng et al., 2014). But nowadays many countries around the world are experiencing decreasing numbers of students choosing to study science, technology, engineering, and mathematics (STEM) even in the United States and European countries (Osborne and Dillon, 2008; National Science Board [NSB], 2012). Many findings reveal that interest, motivation, attitudes, beliefs, self-confidence, and self-efficacy of students towards science and technology decline with passing school years, especially from elementary to middle school (Crawley and Black, 1992; Potvin and Hasni, 2014). These affective components play an important role in students' decisions to engage in STEM. However, the literature reports that students' attitudes towards science learning still remains positive in secondary school (DeWitt et al., 2014). The question “How can science and technology be taught in school to change students' attitudes towards and aspirations in science and technology?” has become a crucial issue (Kahveci, 2015). The objectives of science education are concerned not only with student cognition but also with student attitudes (Reiss, 2005).Regarding progress in science and technology, human brain research (Caine and Caine, 1990), and necessary skills for people in the 21st century include global awareness, creativity and innovation, critical thinking and problem solving, communication and collaboration, information literacy (The Partnership for 21st Century Skills, 2009), media literacy, and technology literacy. These have caused many changes in education strategies for all grade levels (Kennedy and Odell, 2014). Educators say that education in the 21st century should teach learners to master higher-order thinking skills. Moreover, learners should be able to use technology as an instrument to gain knowledge and apply it in their daily lives (Sanders, 2009). However, the findings of science educators and scholastic documents show that there have been numerous problems that impede the success of higher-order thinking skill development for Thai students (Wiratchai et al., 2004; Ministry of Education, 2006). The recent evidence that reflected the recession of the Thai education system was the result of the Programme for International Student Assessment (PISA) conducted among the member countries of the Organisation for Economic Co-operation and Development (OECD), which aimed to study the education systems of each member country to investigate whether they have provided their youth with enough skills to face their futures. The assessment focused on how learners applied their knowledge to solve problems in their real lives rather than in their school curriculums. The results of the latest year (2012) revealed that Thai students scored higher than they had previously in every subject, but they were still lower than the mean scores of the most participating countries—Thailand was ranked 50th among the 65 participating countries (OECD, 2012). The main cause of low scores was that Thai students were weak in analytical thinking skills. Perhaps one method of developing the higher-order thinking skills is through improving student’s understanding of science, technology, engineering, and mathematics (Fan and Yu, 2015). Moreover, teachers should be provided with appropriate professional development opportunities that will enable them to lead their students towards acquiring STEM literacy and continuously develop their teaching strategies (Crow et al., 2013). Integrative approaches among science, technology, engineering, and mathematics, normally performed on problem-solving activities in assignments, tests, and exams, have a positive effect on student learning especially by increasing and improving student interest and learning in STEM (Becker and Park, 2011).
STEM education has become an international topic over the past decade. It was first introduced in the United States when the American government found that the country's capacity for competition had gradually fallen in various areas, and they brought STEM policy into practice. STEM education is a cross-discipline teaching method, integrating four subjects together: science, technology, engineering, and mathematics. Over time, they found that STEM education could improve the American students' perceptions and increase enrolments in STEM subjects of the students (Gonzalez and Kuenzi, 2012). In general, students have to apply the nature and good qualifications of each subject to solve the problems they face, and develop new innovations to suit the present world (Breiner et al., 2012; Defarnette, 2012; Wayne, 2012). Integrated STEM education can make learning more relevant and meaningful for students. It contributes to deepening the students' understanding of content via teaching in a rich applied environment such as engineering and/or technology (Dugger, 1993; Kuenzi, 2008). Consequently, it can improve students' attitudes toward STEM subjects, enhance their level of thinking skills, and increase achievement (Stohlmann et al., 2013). In addition, STEM learning experiences can prepare students for the global economy of the 21st century (Cullum et al., 2007; Hynes and Santos, 2007). It also provides opportunities to turn abstract science and mathematics concepts into concrete real-life applications. STEM education mostly combines problem solving, analytical, critical, creative thinking, teamwork, and communication skills as a pedagogical strategy (Shahali et al., 2015). It can also improve student learning concerning the application of scientific and mathematics knowledge (Cantrell et al., 2006; Schnittka and Bell, 2011; Wendell and Rogers, 2013). However, teachers' attitudes and beliefs also play an important role in the success of STEM education (Ernest, 1989; Handal and Herrington, 2003; Wilkins and Ma, 2003). Teachers who learn more about science and mathematics concepts feel more comfortable in teaching STEM (Nadelson et al., 2012). Teaching STEM will be effective if teachers or facilitators have a deep understanding of their subject matter and can explain concepts and procedures from multiple perspectives (Ejiwale 2012; Halim et al., 2014). Their level of subject knowledge and understanding is directly linked to their ability to effectively teach STEM integration (Nadelson et al., 2012; Stohlmann et al., 2013). However, content knowledge alone is not sufficient be effective in STEM teaching. Teachers need to know about pedagogical strategies that support students in active participation in classroom activities: guiding students in scientific inquiry, designing experiments, and illuminating STEM topics (Ejiwale, 2012). An effective approach for helping students, especially those who lack prior knowledge, to achieve success in increasing their STEM knowledge and understanding, problem prediction, and analysis capabilities, is the most positive effect of integrative STEM education (Fan and Yu, 2015).
For science students, chemistry is a compulsory component of their curriculum. Chemical understanding mainly relies on making sense of invisible and untouchable things (Barak and Hussein-Farraj, 2013). There are four levels of understanding: macroscopic, microscopic, symbolic, and process. A good understanding in chemistry requires the ability to link among these four levels and explain changes at the macroscopic level in terms of interactions between individual atoms and molecules (Dori and Hameiri, 2003; Barak and Dori, 2005). Researchers have shown that many students struggle to properly link the different levels of understanding (Dori and Barak, 2001; Chandrasegaran et al., 2008). For example, they had insufficient understanding of the macroscopic and microscopic representations of molecules, the meaning of the symbols, and the formulas in chemical equations (Kaberman and Dori, 2009). Other researchers explain that conceptual understanding is the students' ability to implement a new concept in an unfamiliar situation, to link the new concept to concepts already known, and to explain and draw conclusions using a new concept (She, 2004; Barak et al., 2007). Conceptual understanding is a crucial result of the educational process and knowledge construction (She, 2004). Creating an appropriate learning environment in order to develop conceptual understanding is one of the most important goals in teaching (Smith et al., 1993). To develop a correct conceptual understanding of science issues, students should be able to execute the processes of reflection and discuss their understandings of scientific concepts as they develop them (Coll et al., 2005). Concerning the subject of chemistry, one group of researchers introduced a strong integrated STEM regarding the concept of biodiesel fuel production, having two core concepts of biology (biochemistry of algae) and chemistry (transesterification). With a real-world lab application, the students could visualize how all STEM fields were interrelated and how they supplemented each other. This approach could encourage and engage students in creating and investigating their own experiments. It also affected high school students' skills and attitudes in relation to STEM subjects (Burrows et al., 2014). For meaningful learning of a topic, the teacher has to organize all related information in an appropriate sequence with effective instructional strategies (Novak, 2003).
Research goal and questions
The present study intended to develop students' analytical thinking ability and positive attitudes towards science learning through integrating STEM with problem-based learning (PBL) activities in stoichiometry. This aim raised the following two questions:1. Can and to what extent can integrating STEM with problem-based learning activities in stoichiometry improve students' analytical thinking ability and positive attitudes towards science learning?
2. What are the characteristics of integrating STEM with problem-based learning activities for teaching stoichiometry?
Methodology
This action research based on Kemmis and Mc Taggarts' theoretical framework through the PAOR (Plan–Act–Observe–Reflect) circle (Kemmis and Mc Taggart, 1988). A new learning unit was developed, introducing STEM integrated with PBL and connecting learning to students' daily lives via stoichiometry, which is an extremely important concept for introductory chemistry courses. The learning unit was developed in order to deepen students' conceptual understanding about the relationship between substances in a chemical equation. It was composed of six problem-based lessons, taking approximately 21 periods, 50 minutes per period, in eight weeks. They were: (a) Chemical Equation, (b) Masses of Substances in a Chemical Reaction, (c) Volume of Gases in a Chemical Reaction, (d) Relationship between Quantities of Substances in a Chemical Equation, (e) Limiting Reagent and Calculation from Multi-Chemical Equations, and (f) Percent Yield. Each lesson was introduced using the PAOR circle that was designed to encourage students to participate in learning, hands-on exploration and experimental activities, and develop their conceptual understanding and analytical thinking abilities. The lesson plans were validated by a group of 5 experts in chemistry and chemistry education. To assess student ability in analytical thinking, at the final stage of each lesson, students had to exhibit their analytical thinking by explaining or showing their ideas or items to express their conceptual understanding and ability to implement their knowledge.Research participants and context of the study
This study took place in a senior high school in Nakorn Nayok, a central province in Thailand. The research school is a government school. It provides basic Thai education levels, both primary and secondary school levels. Currently there are over two thousand students from a pre-school to a high school and 24 science teachers, 14 of them having more than 20 year teaching experience. The school has several laboratories, e.g., chemistry laboratory, physics laboratory, and biology laboratory, but they do not have enough tools and materials to support teaching and learning. Unfortunately, most of the students' parents are farmers and general workers who cannot add financial support to the school. However, the school does have a library, a computer room, and internet access to facilitate learning.The research participants included 90 science students (34 male and 56 female), studying in grade-11 in second semester of the 2014 academic year. They were separated into two mixed gender classes of the science-math department: one had 30 girls and 17 boys, while another one had 26 girls and 17 boys. They were grouped since the beginning of grade-10. Both classes were taught by the first author using the same teaching strategy. The participants were purposively selected. The reasons why they were chosen were as follows. (1) Normally, the students are taught by a traditional teacher-dominated method, where a teacher holds the knowledge and disseminating it to the students who are accustomed to being receptive learners. (2) Most of the participants do not like chemistry. They think it is hard and it is not an important subject and cannot be used in real life activities. (3) Some participants said that chemistry is too hard to understand because it occurs at an atomic level and cannot be seen with the naked eye. Before starting the learning period, the students were informed about the new learning strategy.
Ethical issues of the study
We conducted all study activities in alignment with the Institutional Review Board (IRB). Prior to the study being conducted, the documentation required by the school principal was submitted, and permission was obtained to collect data in the research school. All participants were initially informed about the purposes of the research, the procedure to be followed using the new STEM teaching approach and the study. Then the authors promoted them to join the learning activities only if they agree to take part in the study. All participants voluntarily took part in the current study.Data collection and analysis
This study utilized both quantitative and qualitative methods in the collection and analysis of data. The quantitative research tools consisted of a 60 minute pre- and post-test to assess students' analytical thinking abilities. Dependent t tests were used to check whether progress between the pre- and post-tests was statistically significant. The collected data were analysed by means of computing arithmetic mean, standard deviation, and percentage. The qualitative data included students' work sheets, reflective journals, classroom observations, students' artefacts, and semi-structured interviews. A 15 minute student attitude test was also administered.The pre- and post-analytical thinking ability test was checked and ensured content validity and understandability of the questions by five science educators, four of them chemistry educators. The difficulty score and the classification score were of individual questions. After the pre- and post-analytical thinking ability tests were performed with a group of 50 grade-12 students who had studied stoichiometry before and the scores were arranged in descending order, the score result was classified into two groups, the high and low groups, using the 27% technique of Chung-The Fan (Fan, 1954). Regarding the criteria of the difficulty in the range of 0.20–0.80 and the classification in the range of 0.20–1.0, the final test had 30 questions having the difficulty score (p) and the classification score (r) in the range of 0.22–0.78 and 0.21–0.71, respectively. Besides, by using the KR-20 equation, the test had a confidence score of 0.71. The prescribed criterion used in this research was that not less than 70 percent of all students scored higher than 70 percent of the total score. The pre- and post-analytical thinking ability test are seen in Appendix A.
Likerts' rating scales were used as a framework for the test measuring students' attitudes towards science learning. There were five levels of attitudes: absolutely agree, agree, fair, disagree, and absolutely disagree. The students' responses to each item received weighted values from 5 (absolutely agree) to 1 (absolutely disagree). There were 30 items on the attitude test, see in Appendix B.
Qualitative data from students' work sheets, reflective journals, classroom observations, students' artefacts, and semi-structured interviews were analysed by the inductive process (Guba and Lincoln, 1994); identifying incidents, coding, and categorizing. These codes were compared for their consistencies and differences. The consistencies between codes confirmed tentative sub-categories. Then new incidents were considered through the process and sub-categories assigned accordingly. Finally, sub-categories and their properties were reduced, refined, and, where appropriate, links between sub-categories were constructed to make sense of the data.
Results and discussion
The results of this research are presented in the following sections.Students' analytical thinking
In order to examine the pre- and post-analytical thinking ability results, a dependent t-test was conducted. The mean scores, standard deviations, and t-tests are presented in Table 1.| Pre-test | Post-test | t | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of students | Mean | S.D. | Number of students | Mean | S.D. | ||||
| N | n | n/N (%) | n | n/N (%) | |||||
| n = The number of students who gained higher than the prescribed criterion of 70%. N = The number of sample. | |||||||||
| 90 | 0 | 0 | 6.83 | 3.12 | 71 | 79 | 22.93 | 3.31 | 49.95 |
The criterion of 70% of the full score 30 points, which was 21 points, was used for passing the test. Regarding the pre-analytical thinking ability test's score as shown in Appendix C, it was shown that everyone had the pre-test score lower than 21 points. No one passed the pre-analytical thinking ability test (n = 0). The mean pre-test score of 90 research students was 6.83 (out of 30.00), which indicated that students had little knowledge and understanding of stoichiometry. After experiencing the new learning via STEM integrated with PBL, students achieved much higher scores, around four times higher. Besides, 71 out of 90 students (79% of students) passed the prescribed criterion, getting scores higher than 70%. The post-test scores were statistically significantly higher than the pre-test scores at the significant level of 0.01 as seen in Table 1.
After learning by STEM education integrated with PBL strategies, students showed high average scores for all lessons (as seen in Table 2). Moreover, most of the students gained high scores, more than 70% of the full score (>7 out of 10 points), especially, for the chemical equation, 96% of students passed the prescribed criterion. As expected, the lesson dealing with the relationship between the quantities of substances in a chemical reaction had the lowest percentage of students who gained more than the prescribed criterion. This might be because this lesson mainly involved a complicated calculation.
| Lesson no. | Title | Number of students | Result | |||
|---|---|---|---|---|---|---|
| N | n | n/N (%) | Mean | S.D. | ||
| n = The number of students who gained higher than the prescribed criterion of 70%. N = The number of sample. | ||||||
| 1 | Chemical equation | 90 | 86 | 96 | 9.10 | 0.91 |
| 2 | Masses of substances in a chemical reaction | 90 | 63 | 70 | 8.14 | 1.25 |
| 3 | Volume of gases in a chemical reaction | 90 | 52 | 58 | 7.67 | 1.63 |
| 4 | Relationship between the quantities of substances in a chemical reaction | 90 | 44 | 49 | 7.52 | 1.49 |
| 5 | Limiting reagent and calculation from multi-chemical reactions | 90 | 50 | 56 | 7.72 | 1.42 |
| 6 | Percent yield | 90 | 66 | 73 | 8.03 | 1.20 |
Students' attitude towards science learning
Table 3 shows that the attitude towards science learning was also improved significantly at the level of 0.01. The majority of the students had a positive attitude towards science learning. The students organized and summarized all knowledge by themselves instead of listening to the teacher as in a traditional learning setting. In addition, the students had more opportunities to do various supplementary experiments that did not exist in the text books. Consequently, the students could understand the principles and theories profoundly, so that they were able to implement their knowledge effectively. All activities emphasized practice, thus, the students were more excited and interested in the lessons than they would have been in a more traditional lecture format. They could create some questions and could determine for themselves if the solutions were in accordance with relevant theories. The more opportunities the students had to apply their knowledge, the better they were at the scientific process. The learning atmosphere was very positive. The students looked forward to the coming lessons. They were more curious, keen to work, and more courageous in expressing their ideas than they had been before. One of the students noted that “Learning activity challenges my ideas and also help me to think scientifically.” (Student A's reflective journal). Even at lunch break, some students went to the laboratory room to finish their experiments to prove their hypotheses or to see their answers and outcomes. The students paid more attention during the lessons and always asked the teacher what the next lesson would be. Such behaviours clearly showed an increase in their positive attitude towards science after participating in STEM with PBL activities. This was consistent with the STEM research results of Lou et al. (2010), who found that the STEM and PBL activities helped students to work step by step until they reached the final goal of the activity, provided students meaningful experiences, and boosted students' attitudes towards science significantly.| Pre-test | Post-test | t | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of students | Mean | S.D. | Number of students | Mean | S.D. | ||||
| N | n | n/N (%) | n | n/N (%) | |||||
| n = The number of students who gained higher than the prescribed criterion of 70%. N = The number of sample. | |||||||||
| 90 | 14 | 16 | 98.37 | 6.43 | 83 | 92 | 123.89 | 10.10 | 22.57 |
Characteristics of STEM with PBL activities
STEM teaching and learning is a quite new pedagogical strategy in Thailand. It is a student-centred approach in order to achieve engagement with multi-disciplines. Encouraging more students to participate in the enabling sciences and technology requires teachers or facilitators who have a good understanding of integrated STEM instructional strategies, engineering practices, and its applications. The STEM education approach was gradually brought to the students from the first lesson, Chemical Equation. In groups of five, students worked on a chemical reaction experiment of lead(II) nitrate and potassium iodide by varying the concentration ratio. They noticed that the heights of a yellow precipitate of lead(II) iodide in test tubes were gradually increased until finally they reached at a constant value. After a discussion to get the fundamental knowledge of the chemical equation, the teacher asked them to find out the hazard of the lead(II) iodide product produced and ways to eliminate it. Instead of throwing it away, the teacher showed students the sparkling lead(II) iodide and asked students to apply this beautiful thing to create some useful object. Every group was very excited from the first time they saw the glittering lead(II) iodide. Consequently, they paid close attention on their work.The following is an example of the conversions that occurred while the students were asked to create a useful object using the glittering lead(II) iodide.
Student A: Should we mix it with resin and mold it to be tiles for a walking path during night time?
Student B: Or we [could] mix it with paraffin to make a candle to decorate our homes. It is also very beautiful.
Student C: I think it can make a golden lampshade.
Student D: I agree with the idea to make the tiles. It completely suits to make a walkway around a resort.
Student A: So, we are going to mould it with resin for making some tiles. Let's post our idea in a Facebook group before other groups get this idea.
To encourage students, the teacher set a Facebook group for students to post their ideas and not allow any other group to repeat the first idea. Therefore, they tried hard to be the first. At the end of this activity, every group showed their useful items (e.g. hourglass, whirling jar, sparkling tile, glittering umbrella, numbers on a clock, etc.) with an explanation of the processing steps. Everyone was proud of their work. Even though most of the students enjoyed and were engaged with this activity, saying, “today's activity made me to create thinking skill, for example observation skill, calculation skill, communication skill, and correlation skill”, some students did not get along with searching or discussing in their groups. The teacher had to assign additional work via asking for an individual report showing his/her responsibility in collaborative group activities. The assessment used to evaluate the objects produced is shown in Appendix D.
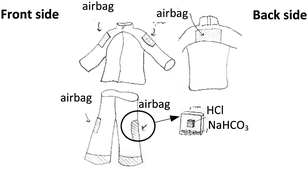
The second lesson was Masses of Substances in a Chemical Reaction. First, the teacher gave instructions regarding the reaction of hydrochloric acid (HCl) and sodium hydrogen carbonate (NaHCO3) to produce carbon dioxide gas (CO2). She assigned the students the task of designing an item related to the above reaction and combine it with their knowledge about the past chemical equation lesson. The assignment was very challenging for the students. They came up with a variety of work pieces (e.g. balloon, life suit, life buoy, balloon pet, etc.); one of them is shown in Fig. 1. The students integrated their knowledge in multi-disciplines to create the life suit as seen in Table 4. For the second assignment, students still expressed a sensation of discovery, amazement, and interest. Nevertheless, some group presenters were the same persons as in the first presentation. Thus, the teacher had to change the criteria for the third activity, allowing each person to present only once.
| Science (S) | The mass of substances in the chemical reaction and the relationship between the quantities of substances in the chemical equation |
| Technology (T) | Inventing mechanical parts of the life suit to make it practically and easily portable |
| Engineering (E) | Selecting substances to make a life suit and designing its shape |
| Mathematics (M) | Calculating a volume of gas produced, the appropriate quantities of substances used for the reaction, and calculating the volume of the life suit to ensure that it had the right proportion with the gas produced |
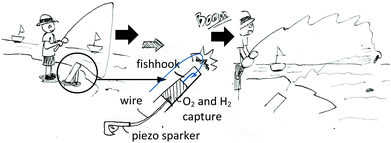
The third lesson was Volume of Gases in a Chemical Reaction. The teacher demonstrated a reaction of oxygen and hydrogen gases and allowed students to work on the experiment gaining knowledge of Gay-Lussac's and Avogadro's laws. After that, the teacher assigned a new task for each group to create a useful object using the reaction of oxygen and hydrogen gases. Fig. 2 shows an example of an implemented drawing of an automatic fishing tool.
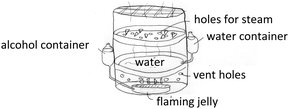
The fourth lesson was the Relationship between Quantities of Substances in a Chemical Reaction. At the beginning of the class, the teacher showed a video about the conversion of alcohol into flaming jelly using vinegar and antacid (http://www.youtube.com/watch?v=E356hphckDQ). After asking the students to write a balanced equation of this reaction, the teacher persuaded them to calculate the specific amount of the reactants used for producing five grams of flaming jelly. Then the teacher took them outdoors to prepare the flaming jelly since the reaction causes a strong odour. When students came back into the classroom, the atmosphere of learning was enjoyable. They showed great enthusiasm. Every group cooperated to express their ideas regarding their knowledge about the relationship between substances in the chemical reaction. Finally, the students were asked to utilize the flaming jelly. One of the designed tools is shown in Fig. 3.
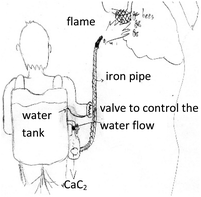
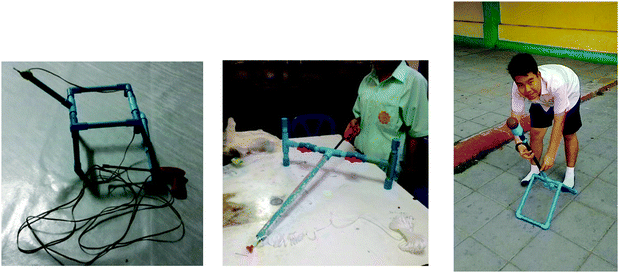
For the fifth lesson, Limiting Agent and Calculation from Multi-chemical Reactions, the teacher introduced a reaction to produce acetylene gas (C2H2) from calcium carbide (CaC2) and water. It was a two-step reaction. With the same teaching procedure, the teacher demonstrated the reaction and assigned the students the task of designing a tool made by using this reaction. The students created a variety of tools, for example, a stove for cooking, a lamp for use in the dark, a flambeau, a tool for chasing bees (Fig. 4), etc.
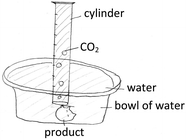
The last lesson was Percent Yield. The teaching started with the question, “How can we theoretically check the product?” by using the reaction of hydrochloric acid (HCl) and sodium hydrogen carbonate (NaHCO3) to produce carbon dioxide gas (CO2). After group brainstorming, the students came up with a water replacement method, as shown in Fig. 5. For the last assignment, the teacher asked every group to repeat their second item by recalculating the masses of substances used to compensate for the lost amount of carbon dioxide that took place in the real reaction.
Conclusions and discussion
Our study was set to develop the analytical thinking ability and positive attitudes towards science learning through STEM integrated with problem-based learning activities in the study of stoichiometry.1. Applying PBL strategies to the learning of STEM knowledge assisted students in analytical thinking, and establishing a positive attitude towards science learning.
Integrative STEM was used to improve high school students' STEM knowledge and higher-order thinking skills through the use of problem-based learning, which included lectures, demonstrations, and hands-on activities (Sanders, 2009; Bybee, 2013). Real-life based learning activities assisted students in realizing the importance of theories and fundamental science knowledge. Such activities allowed the students the opportunity to learn together and to exchange their ideas and experiences, and in turn, created an atmosphere that promoted thinking development among the students. In addition, in the presentation session, students' thinking and the creation of tools or useful things resulted in the development of their thinking process as interaction among students and between students and teacher. Consequently, this led to an expansion of the students' scope of thinking to become larger and more complex. Higher-order thinking skills require a high level of flexibility in understanding how to integrate and apply conceptual knowledge and procedural skills with a complex problem context. The process of thinking before acting is a critical stage for a well-planned design. They could use scientific methods in creating new innovation efficiently, consequently they could practice multiple skills. Moreover, they enjoyed the learning activities and became eager to learn with the result of making high learning achievement that met the prescribed goals.
2. Characteristics of the STEM integrated with problem-based learning activities.
The teacher should design opportunities for students with rigorous learning experiences that require higher-order cognition. Real-world task activities affected students' skills and attitudes related to STEM subjects (Burrows et al., 2014). The teacher should manage learning with an emphasis on analytical thinking. The learning management should allow the students to learn how to manage things, to cope with challenging situations, and to apply what they have learned to daily life activities. The teacher should also develop an assessment of STEM learning to evaluate not only what students know but also how they use their knowledge. Based on the implementation of all six activities in this study, the conclusions are as follows. (1) Initiative activities: students have undergone a series of hands-on processes, involving getting, searching and gathering, and connecting information. Thereafter, the teacher asked them to explore their knowledge by experiencing it themselves. Thus, they were able to create knowledge on their own and apply it efficiently in developing their assignment, in accordance with Kaberman and Dori (2009). (2) Situational problem: the product produced from exploring a chemical experiment will be used in efficiently creating a useful item. This may assist students in deeply understanding the theory concept, leading to the successful application of their knowledge to develop their items. (3) Product: to create a product by using STEM knowledge, the teacher must promptly guide, suggest, and support students both inside and outside of class. Most of all, the teacher should strengthen the role of a facilitator, thereby helping students become active learners who actively acquire necessary knowledge to resolve the problems appearing in the assignment.
Recommendations
In designing STEM based on problem-based learning activities, especially for high school students, teachers must allocate a good deal of time and effort explaining basic knowledge and skills, and provide numerous demonstrations and laboratories for students to practice their higher-order thinking skills and STEM knowledge. However, an explicit connection presented between the problem or task content and STEM knowledge is a crucial component (Hurst, 2015). Without a good connection, for example, the ones involving some meaningful topics in real-world issues (e.g. biodiesel production, climate change, and crisis pollution, etc.) (Burrows et al., 2014), students will lose interest in learning basic conceptual knowledge; consequently, they will hardly develop a feasible solution for their project. The tasks chosen should aid students in performing authentic opportunities to observe, acquire, apply, and consolidate ideas (Burke, 2009). The learning strategy designed by a cooperative group of educators from multi-disciplines would be perfect.Even though the ability to solve complex problems is one of the key competencies in science (Scherer and Tiemann, 2014), communication is also one of most important competencies for living in the 21st century (Kennedy and Odell, 2014). Collaborative group activity learning may encourage students to be more confident in debating and learning in the class. They should be provided with opportunities for working in pairs or groups to practice sharing their ideas while discussing and listening to the ideas of others. Teachers should set available sessions as many times as possible for giving students opportunities to present their thinking processes. These will reflect their conceptual knowledge and high-order thinking skills. Moreover, their communication skills will also improve.
Appendix A. Pre- and post-analytical thinking ability test in stoichiometry
The test has 30 questions. Read each question carefully and then choose the best answer by crossing X on the answer sheet.Acid rain
Acid rain can be identified by using a scale called the pH scale, a lower pH value means stronger acidity. Pure water has pH equal to 7, while acid rain has pH lower than 5.6. Acid rain affects plants, aquatic animals, and constructions such as a marble statue, consisting primarily of calcium carbonate. It was eroded by acid rain as shown in the figure below.http://www.nobelkepu.org.cn/07nbekpw/forume/warning/133458.shtml.html
Normal rain normally has little acidity since it dissolves a little bit of carbon dioxide gas. For acid rain, it has higher acidity because it is formed by a reaction between normal rain and some certain gases, e.g. carbon dioxide gas (CO2), sulfur dioxide gas (SO2) and oxides of nitrogen (NOx), forming compounds of carbonic acid, sulfuric acid and nitric acid, which are corrosive compounds.
1. Which chemical reaction, showing the formation reaction of acid rain, is not correct?
A. CO2(g) + H2O(l) → H2CO3(aq)
B. 2SO2(g) + O2 (g) → 2SO3(g)
SO3(g) + H2O(l) → H2SO4(aq)
C. 2NO(g) + O2(g) → 2NO2(g)
2NO2(g) + H2O(l) → HNO2(aq) + HNO3(aq)
D. 2NO3(g) + H2O(l) → 2HNO3(aq)
2. A scientist collected nitrogen gas releasing from different processes in four factories. Then he analyzed their components by examining the masses of N and O elements. The data received are shown as follows.
| Factory | Processing reaction | Result showing masses of elements composed | |
|---|---|---|---|
| Mass of N | Mass of O | ||
| A | 2NO + O2 → 2NO2 | 2.50 | 5.75 |
| B | 2N2O5 → 4NO2 + O2 | 3.20 | 7.36 |
| C | 2Pb(NO3)2 → 2PbO + 4NO2 + O2 | 1.80 | 4.14 |
| D | 4HNO3 + Sn → H2O + H2SnO3 + 4NO2 | 5.40 | 12.42 |
Regarding the data given, do these chemical reactions obey Law of Definite Proportions?
| Factory | Law of definite proportion | Mass ratio of N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O in NO2 O in NO2 |
|---|---|---|
| A | Not support | Not constant |
| B | Support | Constant at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.43 0.43 |
| C | Not support | Constant at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.00 2.00 |
| D | Support | Constant at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.30 2.30 |
Effect of acid rain on marble (CaCO3) can be imitated by placing a marble particulate into a beaker of vinegar (CH3COOH) and leaving it for a night. The vinegar and acid rain have a close acidity. A gas is produced while putting the marble particulate into the beaker of vinegar as the chemical reaction shown: CaCO3(s) + CH3COOH(aq) → Ca(CH3COOH)2(aq) + CO2(g) + H2O(l). Masses of the marble particulate and vinegar are 5.0 and 10.0 grams, respectively.
3. After the reaction is complete, it is found that the mass of the reminder is 12.8 grams. How many grams of carbon dioxide gas formed?
A. 1.5 grams B. 2.2 grams C. 10.0 grams D. 12.8 grams
4. Student who did the laboratory in question 3, he put a piece of marble particulate into a beaker of pure water (distilled water) and left it overnight. Why did the student also do this experiment step?
A. to prove if pure water has no acidity.
B. to study the porosity of the marble that affect absorption ability.
C. to exhibit that the marble particulate will react with acid well when it is left overnight.
D. to set a control to show that the marble particulate will be corroded only in the acid solution.
Sulfur dioxide gas (SO2) that reacted completely with oxygen gas (O2) forms sulfur trioxide gas (SO3). The stoichiometry ratio by volume is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
5. If there is 1.204 × 1025 molecules of oxygen gas, how many molecules of sulfur dioxide gas are needed to react completely?
A. 6.020 × 1023 molecules
B. 1.204 × 1023 molecules
C. 2.408 × 1025 molecules
D. 4.816 × 1025 molecules
6. From data in question 5, calculate the volume of sulfur dioxide that reacted completely with oxygen gas 150 dm3 at the same temperature and pressure.
A. 100 dm3 B. 150 dm3 C. 300 dm3 D. 450 dm3
7. A scientist collects a gas creating an acid rain from a place. The gas is the nitrogen oxide compound. A complete decomposition of 50 mL of this gas forms 50 mL of nitrogen gas (N2) and 25 mL of oxygen gas (O2) at STP. What is the molecular formula of this gas?
A. NO B. NO2 C. N2O D. N2O5
Bread flour
In making bread, a baker mixes yeast, a group of small fungi, into a mixture of flour, sugar, water, and salt. After mixing, the dough is kept for many hours to allow a fermentation reaction to occur as shown:8. Why does the baker have to add yeast for making the dough?
A. to make the bread blowing.
B. to make the bread sweet and has a good taste.
C. to make the bread brown and be tasty.
D. to make the bread easy to cook and use less time for baking.
9. When people eat some bread composed of yeast, why have not they got heady even through the fermentation of yeast produces an alcohol?
A. Alcohol has low boiling point.
B. An oven has high temperature.
C. Alcohol is changed to be energy for yeast.
D. Alcohol can penetrate out of the bread.
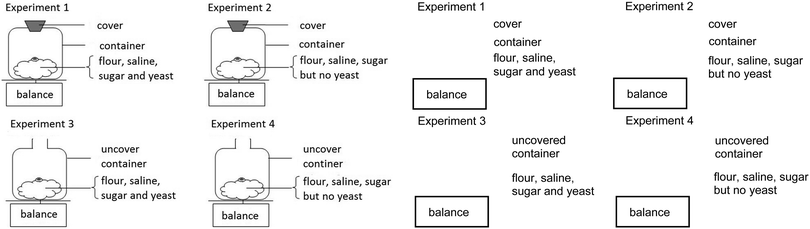
The baker weighs mass of flour used both before and after mixing with other ingredients. Then he keeps the dough for several hours. He notices that the weight of the dough is decreased.
10. Consider the following experiments. Which pair of experiments should the baker use to demonstrate that yeast is the reason why the mass of flour is decreased in terms of comparison, even though the starting mass of flour in every experiment is equal?
Yeast is an important ingredient for various bakeries even though some of the bakeries such as cake and muffin would be made by using baking soda instead of yeast. When baking soda is heated up, it will decompose and produce carbon dioxide gas and water as the chemical equation shown below:
| 2NaHCO3 → Na2CO3(aq) + CO2(g) + H2O(l) |
11. In terms of comparison with yeast, what is the pros of baking soda over yeast?
A. Easier to use and can be kept for a longer time than yeast.
B. The bakeries would be softer because of the water generated in the decomposition of the heated baking soda.
C. More carbon dioxide produced in the bakeries causing them to taste better.
D. More baking soda added in the bakeries causing them to be softer without any taste changing.
12. In the process of making cake, 2 grams of baking soda was added and allowed it to be completely decomposed. How many liters of carbon dioxide will be generated at STP? (H = 1, C = 12, O = 16, Na = 23)
A. 0.27 L B. 0.81 L C. 1.08 L D. 1.11 L
13. Which is not the reason of the reduction of cake dough's mass after the bakeries were baked?
A. Humid was eliminated due to heat.
B. The water produced was vaporized.
C. Flour and partial sugar in flour were disintegrated into carbon dioxide which was finally leaving off.
D. Carbon from baking soda decomposed to be carbon dioxide which was finally leaving off.
http://williamrwilson.hubpages.com/hub/CO2-Causes-Global-Warming-Heres-How-We-Know
Global warming is caused by overwhelming greenhouse gases in the atmosphere such as carbon dioxide. It is generated from fuel combustion that is directly caused by human in various activities, for example: methane (CH4) combustion in a car engine as the chemical equation shown below:
| CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) |
14. How does a carbon atom within a molecule of carbon dioxide come from?
A. It comes from a methane molecule.
B. It comes from an oxygen molecule.
C. It comes from a water vapor molecule in the air.
D. It comes from a combustion engine in a car.
5 kg of methane is filled in a car and allowed to react completely with 30 kg of oxygen in a car engine.
(H = 1, C = 12, O = 16)
15. Which substance is the limiting reagent to produce carbon dioxide?
A. Oxygen B. Lubricant oil in the car engine C. Water vapor in the air D. Methane
16. According to the information in question 15, after complete combustion which substance will remain and how much of it would be left?
A. 0.9 kg of methane left.
B. 1 kg of methane left.
C. 10 kg of oxygen left.
D. 25 kg of oxygen left.
17. According to the information in question 15, if the amount of methane filled in the car is used up, how many grams of carbon dioxide will be generated?
A. 1.82 kg B. 3.64 kg C. 13.75 kg D. 27.5 kg
18. How many times of mass of carbon dioxide produced, as compared with the mass of methane used in the car engine?
A. 0.5 times B. 1 time C. 2 times D. 3 times
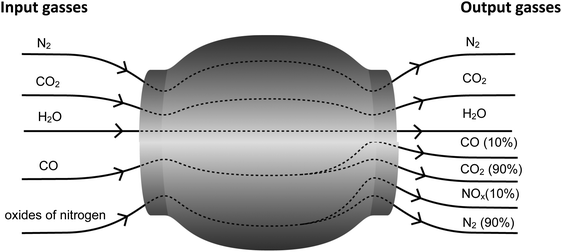
19. Most of modern cars would have the catalytic converter which converts toxic gases taking place in exhaust gas to less toxic pollutants to people and the environment, as shown in the picture below.
http://ngbr-pisa-team.blogspot.com/2012/06/blog-post_3278.html?m=1
Which of these is not the right demonstration showing how toxic gases are converted to less toxic gases by the catalytic converter?
A. Oxides of nitrogen are converted to nitrogen.
B. Carbon dioxide is converted to carbon monoxide.
C. The amounts of carbon monoxide and oxides of nitrogen are reduced.
D. The amounts of carbon dioxide and nitrogen are increased.
Besides, generated from petro combustion, carbon dioxide can be generated from various human activities such as coal combustion, the reaction of bathroom cleaning liquid (composing of HCl mixture) and calcium carbonate in the cement grout, during cleaning bathroom.
20. A scientist experimented and found the results as follows.
I. 2.4 grams of carbon reacted completely with 6.38 grams of oxygen and produced carbon dioxide.
II. The reaction of hydrochloric acid (HCl) and calcium carbonate (CaCO3) produced carbon dioxide (CO2). After analyzing, it was found that carbon dioxide is composed of 27.3% by mass of carbon.
Does the given information support the law of mass preservation?
| Item | Law of mass preservation | For the reason of the constant mass ratio between C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O in CO2 O in CO2 |
|---|---|---|
| A | Do not support | Does not remain constant |
| B | Support | Constant mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.33 1.33 |
| C | Do not support | Constant mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.00 2.00 |
| D | Support | Constant mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.66 2.66 |
21. Consider the following statements.
I. Duangjai burns garbage from her house.
II. Somchat burns rice stubble before plowing rice.
III. Anon rides a motorcycle to work every day.
IV. Chatchai smokes during his lunch time.
According to the given statements, which pair causes the most and the least global warming effects, respectively?
A. I and III B. I and IV C. II and III D. II and IV
22. Which is the key reason why all countries around the world make a campaign to reduce quantity of carbon dioxide generated into the atmosphere?
A. Carbon dioxide is the main cause of global warming.
B. Petro is running out and needed to be preserved in terms of a longer consumption period.
C. Carbon dioxide is a toxic gas causing a body's temperature to increase.
D. Carbon dioxide destroys ozone allowing a large amount of sun radiation to enter the Earth.
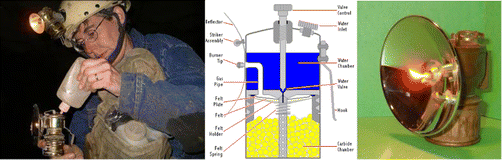
Carbide lamp
Carbide lamp or acetylene lamp is a tool that produces light used at night or in dark places. It is an important tool used for tapping rubber in the southern part of Thailand. It is also very useful for an explorer since it is made of brass or some materials that have not any electromagnetic properties causing no interruption to a compass. Besides, the chemical reaction producing light also releases heat to warm up the explorer's body.http://www.siamtakeang.com/webboard/index.php?topic=979.0
The principle of the carbide lamp is based on the reaction of water and calcium carbide (CaC2) liberating acetylene (C2H2). It is a hydrocarbon compound. The combustion reaction of C2H2 with oxygen in the air is shown as follows:
| CaC2(s) + H2O(l) → C2H2(g) + Ca(OH)2(s) |
| 2C2H2(g) + 5O2(g) → 4CO2(g) + 2H2O(l) |
23. Which principle do you think it controls the amount of fire produced as shown in the pictures above?
A. Control amount of O2 in the air.
B. Control amount of CO2 in the air.
C. Control flow rate of water to react with CaC2.
D. Control flow rate of C2H2 releasing off the tool.
24. A rubber farmer placing 10.0 grams of CaC2 into the lamp to react completely with water, how many milliliters of water needed? The density of water is 1 g mL−1. (H = 1, C = 12, O = 16, Ca = 40)
A. 5.63 mL B. 6.62 mL C. 8.22 mL D. 9.57 mL
25. According to question 24, how many grams of CO2, causing global warming, were produced?
A. 6.88 grams B. 13.75 grams C. 20.62 grams D. 61.88 grams
26. If 10.0 grams of CaC2 reacts completely with water and produces 3.66 grams of C2H2, what is the percent yield of the reaction? (H = 1, C = 12, O = 16, Ca = 40)
A. 65% B. 80% C. 85% D. 90%
Helium balloon
http://www.partyplus.com.au/files/GFCO2MWRSZ/helium%20balloons%20and%20streamers.jpg
In the past, various balloons were filled with hydrogen gas since it is lighter than air. Besides, it could be prepared using a very easy method by placing a piece of metal into an acid solution as shown below.
| Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g) |
27. If 5.6 liters of hydrogen is needed to fill a balloon by using 16.35 grams of zinc reacts completely with 12 mol dm−3 hydrochloric acid at STP, how many milliliters of hydrochloric acid is used? (H = 1, Zn = 65.4)
A. 12 cm3 B. 24 cm3 C. 32 cm3 D. 42 cm3
28. If 32.7 grams of a zinc plate reacts with an excess hydrochloric acid and produces only 80 percent of the theoretical yield, how many liters of hydrogen gas actually produced at STP? (Zn = 65.4)
A. 4.48 L B. 8.96 L C. 17.92 L D. 20.50 L
World No Tobacco Day Event was held on 31 May 1999. After the president using a knife cut a tobacco model made of metal, a spark accidently occurred and caused hydrogen balloons ignited and consequently exploded. The explosion caused many people dead and some were injuries in the accident on that day.
http://coursewares.mju.ac.th:81/e-learning47/ca512/chapter7/content7_3_2.htm
29. Which was the main reason of this accident?
A. There were some participants smoking near the hydrogen balloons.
B. There was a windy wind causing the balloons rubbed against each other.
C. The spark created during the knife hitting the tobacco model, consequently caused the explosion.
D. The air packed inside the cigarette model ignited due to a sword cut the model very strong.
30. Helium is a noble gas produced from petroleum industries. It is lighter than air and non-flammable. It is harder to penetrate from the balloon as compared with hydrogen, since the atomic size of helium is bigger and heavier than hydrogen. In many countries, Helium is used to fill up balloons instead of hydrogen, even though it costs higher price. Which is the main reason?
A. High safety.
B. It is easy to find.
C. Life long period.
D. It can make the balloon go higher than hydrogen does.
Appendix B. Attitude test
The test has 30 questions. Read the statement carefully and then give your opinion, choosing whether it is definitely agree, agree, not sure, disagree, or definitely disagree. There are no right or wrong answers. It does not affect your learning score. Choose the answer that is really true for you, and answer immediately. WORK RAPIDLY.| Item | Statement | Degree of your opinion | ||||
|---|---|---|---|---|---|---|
| Definitely agree | Agree | Not sure | Disagree | Definitely disagree | ||
| 1 | Scientific process promotes critical thinking abilities. | |||||
| 2 | New civilized culture is a knowledge society, therefore everyone need to improve their science knowledge. | |||||
| 3 | Science makes people to be reasonable persons and do not easily trust anyone. | |||||
| 4 | Development of science knowledge will make my country going forward to the worldwide level. | |||||
| 5 | I can live comfortably without having science knowledge. | |||||
| 6 | Science makes people improve their thinking process and have skills in search of their knowledge. | |||||
| 7 | Science learning stimulates creating a new idea. | |||||
| 8 | The progression in science education helps to solve problems in daily life more than the past. | |||||
| 9 | Learning in science subject makes me catch rapid progress of technology more than before. | |||||
| 10 | More improvement in science, more lazy people behave. | |||||
| 11 | I am always glad when I am going to do the laboratory. | |||||
| 12 | Data received from scientists are believable. | |||||
| 13 | Science subject is fun, exciting and interesting. | |||||
| 14 | Science thinking process cannot be applied with any other subjects. | |||||
| 15 | Contents of science subject are too difficult to understand. | |||||
| 16 | Learning science subject wastes budget and is wasteful. | |||||
| 17 | I feel very anxious when I am going to learn science subject. | |||||
| 18 | Teacher giving students opportunities to create creative projects based on science knowledge is a challenge. | |||||
| 19 | I like science more than other subjects. | |||||
| 20 | I am always pressured when teacher asks me to give an idea regarding scientific principles. | |||||
| 21 | Science activities boost me in discipline and moral, and having social responsibility. | |||||
| 22 | If I have a chance, I will persuade other people to learn the science program. | |||||
| 23 | If I find interesting science articles, I am going to keep it for further reading and studying. | |||||
| 24 | Science period is the one that I am waiting for. | |||||
| 25 | When teacher ask me to do the experiment, I do my best. | |||||
| 26 | During the science period, I always play with my mobile phone or read a comic book. | |||||
| 27 | If I have a chance, I always visit science exhibition. | |||||
| 28 | Whenever I do science experiment or create science project, I will continuously manage it until finishing. | |||||
| 29 | I concentrate my whole attention on TV showing about science or science documentary. | |||||
| 30 | I am pleased to transfer my science learning knowledge to other people. | |||||
Appendix C. Pre- and post-analytical thinking ability test's scores
The prescribed criterion of 70% of the full score (30 points) is 21 points, therefore the student who had the analytical thinking ability test's score equal to or more than 21 points is going to pass the test. The result scores of the pre- and post-analytical thinking ability test for students are shown in the table below.| Student no. | Pre-test score | Post-test score | Difference |
|---|---|---|---|
| Remarks: student no. 1–17 are boys, 18–47 are girls, 48–64 are boys, and 65–90 are girls. | |||
| 1 | 9 | 19 | 10 |
| 2 | 12 | 23 | 11 |
| 3 | 10 | 22 | 12 |
| 4 | 12 | 28 | 16 |
| 5 | 8 | 19 | 11 |
| 6 | 13 | 29 | 16 |
| 7 | 11 | 23 | 12 |
| 8 | 14 | 29 | 15 |
| 9 | 10 | 25 | 15 |
| 10 | 6 | 19 | 13 |
| 11 | 10 | 27 | 17 |
| 12 | 9 | 22 | 13 |
| 13 | 12 | 22 | 10 |
| 14 | 9 | 24 | 15 |
| 15 | 6 | 22 | 16 |
| 16 | 3 | 18 | 15 |
| 17 | 9 | 22 | 13 |
| 18 | 13 | 28 | 15 |
| 19 | 7 | 25 | 18 |
| 20 | 8 | 24 | 16 |
| 21 | 12 | 26 | 14 |
| 22 | 7 | 22 | 15 |
| 23 | 10 | 26 | 16 |
| 24 | 9 | 28 | 19 |
| 25 | 12 | 26 | 14 |
| 26 | 1 | 23 | 22 |
| 27 | 7 | 17 | 10 |
| 28 | 12 | 27 | 15 |
| 29 | 4 | 22 | 18 |
| 30 | 12 | 24 | 12 |
| 31 | 12 | 25 | 13 |
| 32 | 9 | 24 | 15 |
| 33 | 9 | 22 | 13 |
| 34 | 10 | 27 | 17 |
| 35 | 8 | 23 | 15 |
| 36 | 8 | 24 | 16 |
| 37 | 8 | 25 | 17 |
| 38 | 6 | 24 | 18 |
| 39 | 8 | 25 | 17 |
| 40 | 6 | 22 | 16 |
| 41 | 7 | 26 | 19 |
| 42 | 4 | 19 | 15 |
| 43 | 8 | 23 | 15 |
| 44 | 2 | 20 | 18 |
| 45 | 9 | 23 | 14 |
| 46 | 2 | 22 | 20 |
| 47 | 3 | 20 | 17 |
| 48 | 5 | 22 | 17 |
| 49 | 1 | 20 | 19 |
| 50 | 2 | 23 | 21 |
| 51 | 2 | 15 | 13 |
| 52 | 11 | 27 | 16 |
| 53 | 7 | 19 | 12 |
| 54 | 6 | 22 | 16 |
| 55 | 5 | 28 | 23 |
| 56 | 3 | 17 | 14 |
| 57 | 7 | 26 | 19 |
| 58 | 5 | 22 | 17 |
| 59 | 5 | 23 | 18 |
| 60 | 8 | 26 | 18 |
| 61 | 6 | 22 | 16 |
| 62 | 5 | 24 | 19 |
| 63 | 5 | 24 | 19 |
| 64 | 7 | 26 | 19 |
| 65 | 3 | 19 | 16 |
| 66 | 7 | 22 | 15 |
| 67 | 7 | 22 | 15 |
| 68 | 6 | 22 | 16 |
| 69 | 5 | 20 | 15 |
| 70 | 7 | 25 | 18 |
| 71 | 4 | 18 | 14 |
| 72 | 6 | 26 | 20 |
| 73 | 4 | 22 | 18 |
| 74 | 6 | 25 | 19 |
| 75 | 4 | 16 | 12 |
| 76 | 5 | 27 | 22 |
| 77 | 5 | 15 | 10 |
| 78 | 3 | 22 | 19 |
| 79 | 6 | 25 | 19 |
| 80 | 4 | 23 | 19 |
| 81 | 3 | 22 | 19 |
| 82 | 5 | 24 | 19 |
| 83 | 5 | 26 | 21 |
| 84 | 4 | 17 | 13 |
| 85 | 6 | 22 | 16 |
| 86 | 3 | 14 | 11 |
| 87 | 4 | 23 | 19 |
| 88 | 9 | 26 | 17 |
| 89 | 4 | 22 | 18 |
| 90 | 4 | 28 | 24 |
| Mean = 6.83 | Mean = 22.93 | ||
| S.D. = 3.12 | S.D. = 3.31 | ||
Appendix D. Assessment of project design
| Item | Issue | Very good (3) | Fair (2) | Needed improvement (1) | No point (0) |
|---|---|---|---|---|---|
| 1 | Creativity | ||||
| 2 | Integration with STEM | ||||
| 3 | Applied theoretical knowledge correctly | ||||
| 4 | Design technique | ||||
| 5 | Material selection | ||||
| 6 | Efficient work | ||||
| 7 | Value of work | ||||
| 8 | Safety in use | ||||
| 9 | Readiness in presentation | ||||
| 10 | Clear presentation | ||||
| Sum | |||||
| Evaluation result | |||||
| Criterion of evaluation | |
|---|---|
| Range of score | Level of quality |
| 21–30 | Good |
| 11–20 | Fair |
| 0–10 | Needed improvement |
Appendix E. Examples of work pieces and tools designed by students
The examples of work pieces and tools designed by students are as follows.Various types of life buoys. Students put some amount of NaHCO3 inside the life buoy with a zip lock bag of HCl. When they want to make the life buoys blowing up, they press the bag allowing NaHCO3 to react with HCl to produce CO2 gas.
Various shooting tool designs from the reaction of O2 and H2 gases and the real model of the automatic fishing tool.
Various applications by using the fuel jelly produced from the reaction of Ca(OH)2, CH3COOH and C2H5OH.
The experiment to verify the volume of gas produced from the reaction of NaHCO3 and HCl that is used in creating a useful thing in the second lesson.
Appendix F. Learning objectives and the brief STEM with PBL activities of each lesson
| Lesson's title | Learning objectives | Brief STEM with PBL activities |
|---|---|---|
| 1. Chemical equation | – Explain the meaning of a chemical reaction. | After doing the experiment of KI and Pb(NO3)2, students wrote the reaction took place. Teacher gave students the MSDS information of PbI2 and asked them to search for its toxicity. Then the teacher showed them the sparkling PbI2 precipitate and assigned them to create some useful things instead of throwing toxic PbI2 away. |
| – Explain the relationship between the reactants and products. | ||
| – Write and balance a chemical reaction. | ||
| – Draw the graph showing the relationship between the height of PbI2 precipitate formed and volume of KI solution used. | ||
| – Experiment to produce PbI2 and create some useful things from PbI2 produced. | ||
| 2. Masses of substances in a chemical reaction | – Explain the meaning of a system and the environment, and the state of a system. | Students compared total masses of all substances before and after the reaction of HCl(aq) + NaHCO3(s) → NaCl(aq) + H2O(l) + CO2(g). After brainstorming to get the knowledge of the mol ratio of the reaction, students were assigned to create some useful objects using CO2 gas produced from the reaction mentioned above. |
| – Explain the meaning of close and open system. | ||
| – Summarize the key concept of the law of conservation of mass and calculate the masses of substances in a chemical reaction. | ||
| – Summarize the key concept of the stoichiometry ratio and calculate the mole ratio of a compound. | ||
| – Calculate the masses of substances needed to create carbon dioxide gas. | ||
| – Create some useful things using CO2 gas produced from the reaction of NaHCO3 and HCl. | ||
| 3. Volume of gases in a chemical reaction | – Summarize the key concept of Guy-Lussac's law & Avogadro's principle. | Students studied the volume ratios of the reactions of 2H2O(l) → O2(g) + 2H2(g) and O2(g) + 2H2(g) → 2H2O(l). Besides, the reactions involve with a certain energy which could move the gas container forward. Then the students brainstormed to create some useful objects using the reactions mentioned and energy arising. |
| – Experiment to obtain an exact volume ratio of gases reacted completely. | ||
| – Calculate volumes of gases involved in the reaction and calculate the molecular formula when the volumes of involving gases are given. | ||
| – Experiment to receive the appropriate volume ratio of H2 and O2, and then create some useful objects by using the principle of the chemical reaction mentioned. | ||
| 4. Relationship between the quantities of substances in a chemical reaction | – Explain the relation of the amount of substances and the numbers used in balancing the chemical reaction. | Student studied the relation of a balanced reaction of Ca(OH)2(s) + 2CH3COOH(aq) → Ca(CH3COO)2(aq) + 2H2O(l). Its product could form fuel jelly when being mixed with alcohol. Student explored the relation of amounts of alcohol added and heat built up, finally converted to the degrees of temperature changing. Students were asked to design some useful products from fuel jelly. |
| – Calculate the mole, mass, or volume of a substance in a chemical reaction, when the quantity of another substance given. | ||
| – Experiment to produce fuel jelly from Ca(OH)2, CH3COOH and C2H5OH, then using the fuel jelly produced to create some useful products. | ||
| 5. Limiting reagent and calculation from multi-chemical equations | – Explain the relation of the limiting reagent and the product produced. | Students calculated the amounts of all substances needed for the reactions: CaC2(s) + 2H2O(l) → C2H2(g) + Ca(OH)2(s), and 2C2H5(g) + 5O2(g) → 4 CO2(g) + 2H2O(g). Then students were asked to design the useful tools giving light. They had to focus on weight, convenience, endurance to heat and safety. |
| – Explain the relation of substances in multi-chemical reaction. | ||
| – Identify the limiting agent in a reaction given. | ||
| – Calculate the product produced from the multi-chemical reaction. | ||
| – Create some useful objects giving light from the multi-reaction of CaC2, H2O and C2H2. | ||
| 6. Percent yield | – Explain the relation of percent yield, theoretical yield, and actual yield. | Student investigated the real volume of CO2 produced in the useful objects produced in the second lesson and calculated the percent yield. Then the teacher asked them to recalculate to compensate the loss. |
| – Calculate the percent yield. | ||
Acknowledgements
The success of this study was due to the support and cooperation of many people and organizations. The authors greatly appreciate the financial support provided by the Institute of Promotion of Science and Technology (IPST) and the Project for the Promotion of Talented Science and Mathematics Teachers (PSMT).References
- Barak M. and Dori Y. J., (2005), Enhancing undergraduate students' chemistry understanding through project-based learning in an IT environment, Sci. Educ., 89(1), 117–139.
- Barak M., Harward J., Kocur G. and Lerman S., (2007), Transforming an introductory programming course: from lectures to active learning to active learning via wireless laptops, J. Sci. Educ. Technol., 16(4), 325–336.
- Barak M. and Hussein-Farraj R., (2013), Integrating model-based learning and animations for enhancing students' understanding of proteins structure and function, Res. Sci. Educ., 43, 619–636.
- Becker K. and Park K., (2011), Effect of integrative approaches among science, technology, engineering, and mathematics (STEM) subjects on students' learning: a preliminary meta-analysis, J. STEM Educ., 12, 23–37.
- Breiner J. M., Johnson C. C., Harkness S.S. and Koehler C. M., (2012), What is STEM? A discussion about conceptions of STEM in education and Shelly Sheats Harkness Partnerships, Sch. Sci. Math., 112(1), 3–11.
- Burke K., (2009), How to assess authentic learning, Thousand Oaks, CA: Corwin.
- Burrows A. C., Breiner J. M., Keiner J. and Behm C., (2014), Biodiesel and integrated STEM: vertical alignment of high school biology/biochemistry and chemistry, J. Chem. Educ., 91, 1379–1389.
- Bybee R. W., (2013), The case for STEM education: Challenges and opportunities, Arlington, VA: NSTA Press.
- Caine R. N. and Caine G., (1990), Understanding a Brain-Based Approach to Learning and Teaching, Educational Leadership, 48(2), 66–70.
- Cantrell P., Pekcan G., Itani A. and Velasquez-Bryant N., (2006), The effects of engineering modules on student learning in middle school science classrooms, J. Eng. Educ., 95(4), 301–309.
- Chandrasegaran A. L., Treagust D. F. and Moccerino M., (2008), An evaluation of a teacher intervention to promote students' ability to use multiple levels of representation when describing and explaining chemical reactions, Res. Sci. Educ., 38(2), 237–248.
- Coll R. K., France B. and Taylor I., (2005), The role of models/and analogies in science education: implications from research, Int. J. Sci. Educ., 27(2), 183–198.
- Crawley F. E. and Black C. B., (1992), Causal modeling of secondary science students' intentions to enroll in physics, J. Res. Sci. Teach., 29(6), 585–599.
- Crow J. E., Kennedy T. J., Odell M. R. L., Ophus, J. D. and Abbitt J. T., (2013), Using just-in-time PD to technologically prepare high school STEM teachers, in Capraro M. M. Capraro R. M. and Lewis C. W. (ed.) Improving Urban Schools: Equity and Access in K-16 STEM Education, ch. 9, Information Age Publishing, pp. 143–157.
- Cullum J., Childress V., Dorward J., Hailey, C., Householder D. and Maurizio D., (2007), Infusing engineering design into the technology education curriculum professional development model, Unpublished internal research report, NCETE.
- Defarnette N. K., (2012), American's children: providing early exposure to STEM (Science, Technology, Engineering and Maths) initiatives, J. Educ., 133(1), 77–84.
- DeWitt J., Archer L. and Osborne J., (2014), Science-related aspirations across the primary-secondary divide: evidence from two surveys in England, Int. J. Sci. Educ., 36(10), 1609–1629.
- Dori Y. J. and Barak M., (2001), Virtual and physical molecular modelling: fostering model perception and spatial understanding, Educ. Technol. Soc., 4(1), 61–74.
- Dori Y. J. and Hameiri M., (2003), Multidimensional analysis system for quantitative chemistry problems-symbol, macro, micro and process aspects, J. Res. Sci. Teach., 40(3), 278–302.
- Dugger J. W. E., (1993), The relationship between technology, science, engineering, and mathematics, (ERIC No. ED 366795).
- Ejiwale J. A., (2012), Facilitating teaching and learning across STEM fields, J. STEM Educ., 13(3), 87–94.
- Ernest P., (1989), The knowledge, beliefs and attitudes of the mathematics teacher: a model, J. Educ. Teach., 15(1), 13–33.
- Fan C. T., (1954), Note on construction of an item analysis table for the high-low-27-percent group method, Psychometrika, 19(3), 231–238.
- Fan S. C. and Yu K. C., (2015), How an integrative STEM curriculum can benefit students in engineering design practices, Int. J. Technol. Des. Educ., DOI: http://10.1007/s10798-015-9328-x.
- Gonzalez H. B. and Kuenzi J. J., (2012), Congressional research service science, technology, engineering, and mathematics (STEM) education: a primer, Retrieved June 6, 2015, from http://www.stemedcoalition.org/wp-content/uploads/2010/05/STEM-Education-Primer.pdf.
- Guba E. G., and Lincoln Y. S., (1994), Competing paradigms in qualitative research, in Denzin N. K. and Lincoln Y. S. (ed.) Handbook of qualitative research, London: Sage, pp. 105–117.
- Halim L., Syed Abdullah S. I. S. and Mohd. Meerah T. S., (2014), Students' perceptions of their science teachers pedagogical content knowledge, J. Sci. Educ. Technol., 23(2), 227–237.
- Handal B. and Herrington A., (2003), Mathematical teachers' beliefs and curriculum reform, Math. Educ. Res. J., 15(1), 59–69.
- Hurst C., (2015), Thinking big about mathematics, science, and technology: effective teaching STEMs from big ideas, Int. J. Innov. Sci. Math. Educ., 23(3), 11–21.
- Hynes M. M. and Santos A. D., (2007), Effective teacher professional development: middle school engineering content, Int. J. Eng. Educ., 23(1), 24–29.
- Kaberman Z. and Dori Y. J., (2009), Question posing, inquiry, and modelling skills of chemistry students in the case-based computerized laboratory environment, Int. J. Sci. Math. Educ., 7, 597–625.
- Kahveci A., (2015), Assessing high school students' attitudes toward chemistry with a shortened semantic differential, Chem. Educ. Res. Pract., 16, 283–292.
- Kemmis S. and Mc Taggart R., (1988), The action research planer, 3rd edn, Victoria: Deakin University.
- Kennedy T. J. and Odell M. R. L., (2014), Engaging students in stem education, Sci. Educ. Int., 25(3), 246–258.
- Kuenzi J. J., (2008), Science, technology, engineering, and mathematics (STEM) education: background, federal policy and legislative action, Congressional research service. Retrieved June 6, 2015, from http://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1034&context=crsdocs.
- Lou S. J., Shih, R. C., Diez, R. C. and Tseng, K. H., (2010), The impact of problem-based learning strategies on STEM knowledge integration and attitudes: an exploratory study among female Taiwanese senior high school students, Int. J. Technol. Des. Educ., 21, 195–215.
- Meng C. C., Idris N. and Eu L. K., (2014), Secondary students' perceptions of assessments in science, technology, engineering, and mathematic (STEM), Eur. J. Math. Sci. Technol. Educ., 10(3), 219–227.
- Ministry of Education, (2006), A report on a synthesis of ideas and methods of learning management for the promotion of analytical thinking skill, Bangkok: The printing shop of Thailand National Agricultural Cooperative Group.
- Nadelson L. S., Seifert A., Moll A. J. and Coats B., (2012), i-SETM Summer Institute: an integrated approach to teacher professional development in STEM, J. STEM Educ., 13(2), 69–83.
- National Science Board, (2012), Science and engineering indicators 2012, Arlington, VA: National Science Board.
- Novak J., (2003), The promise of new ideas and new technology for improving teaching and learning, Cell Biol. Educ., 2(2), 122–132.
- OECD, (2012), PISA 2012 Results in focus: What 15-Year-Olds Know and What They Can Do with What They Know. Retrieved June 1, 2015, from http://www.oecd.org/pisa/keyfindings/pisa-2012-results-overview.pdf.
- Osborne J. and Dillon J., (2008), Science education in Europe: critical reflections (A Report to the Nuffield Foundation), London: King's College.
- Potvin P. and Hasni A., (2014), Interest, motivation and attitude towards science and technology at K-12 levels: a systematic review of 12 years of educational research, Stud. Sci. Educ., 50(1), 85–129.
- Reiss M. J., (2005), The importance of affect in science education, in Alsop S. (ed.) Beyond Cartesian dualism: encountering affect in the teaching and learning of science, Durdrecht: Springer, pp. 17–25.
- Sanders M., (2009), STEM, STEM education, STEMmania, Technol. Teach., 68(4), 20–26.
- Scherer R. and Tiemann R., (2014), Evidence on the effects of task interactivity and grade level on thinking skills involved in complex problem solving, Think. Skills Creat., 11, 48–64.
- Schnittka C. G. and Bell R. L., (2011), Engineering design and conceptual change in science: addressing thermal energy and heat transfer in eighth grade, Int. J. Sci. Educ., 33, 1861–1887.
- Shahali E. H. M., Halim L., Rasul S., Osman K., Ikhsan Z. and Rahim F., (2015), Bitara-STEM™ training of trainers' programme: impact on trainers' knowledge, beliefs, attitudes and efficacy towards integrated stem teaching, J. Baltic Sci. Educ., 14(1), 85–95.
- She H. C., (2004), Fostering radical conceptual change through dual-situated learning model, J. Res. Sci. Teach., 41, 142–164.
- Smith J. A., diSessa A. and Roschelle J., (1993), Misconceptions reconceived: a constructivist analysis of knowledge in transition, J. Learn. Sci., 3(2), 115–163.
- Stohlmann M. S., Moore T. J. and Cramer K., (2013), Pre-service elementary teachers' mathematical content knowledge from and integrated STEM modelling activity, J. Math. Appl., 1(8), 18–31.
- The Partnership for 21st Century Skills, (2011), Framework for 21st century learning, retrived March 1, 2013, from http://framework_2-paper.pdf.
- United Nations Educational, Scientific and Cultural Organization (UNESCO), (2010), Education for all, Global monitoring report, retrived from: http://www.unesco.org/new/en/education/themes/leading-the-international-agenda/efareport/reports/.
- Wayne C., (2012), What is S.T.E.M. and why do I need to know? STEM Magazine, August.
- Wendell K. B. and Rogers C. B., (2013), Engineering design-based science, science content performance, and science attitudes in elementary school, J. Eng. Educ., 102(4), 513–540.
- Wilkins J. L. M. and Ma X., (2003), Modelling change in student attitude toward and beliefs about mathematics, J. Educ. Res., 97(1), 52–63.
- Wiratchai N., Wongwanich S. and Ruangtrakul U., (2004), An evaluation of educational reform according to the national education act 1999, Bangkok, Thailand: Office of the secretary-general of education board, ministry of education.
| This journal is © The Royal Society of Chemistry 2016 |