To what degree does handling concrete molecular models promote the ability to translate and coordinate between 2D and 3D molecular structure representations? A case study with Algerian students
Boukhechem
Mohamed-Salah
a and
Dumon
Alain
*b
aEcole Normale Supérieure de Kouba, Alger, Algérie
bEcole Supérieure du Professorat et de l'Education d'Aquitaine, Université de Bordeaux, France. E-mail: alain.dumon@u-bordeaux.fr
First published on 20th May 2016
Abstract
This study aims to assess whether the handling of concrete ball-and-stick molecular models promotes translation between diagrammatic representations and a concrete model (or vice versa) and the coordination of the different types of structural representations of a given molecular structure. Forty-one Algerian undergraduate students were requested to answer a pencil and paper questionnaire at the end of their training for a bachelor's degree in physical sciences to test their abilities to translate from Dash-Wedge or Newman representations to 3D ball-and-stick models (and vice versa) of two molecular structures and from one concrete 3D model to the Fischer projection of the molecule. Our results show that concrete molecular models have the potential to be an effective spatial tool to promote visualization, orientation and rotation abilities. However, the handling of the concrete model did not have the same impact on all students and this effectiveness in promoting the spatial abilities required to translate and coordinate between representations was dependent on the representations: it was greater for Dash-Wedge diagrams than for Newman, and was non-existent for the Fischer projection. An implication of our research is that it may be necessary to work with a model over an extensive period of time to improve the mechanisms by which one translates between various representations when the conventions of these representations are varied in nature.
Introduction
Understanding models is an important aspect of the understanding of science, since models and modeling are considered to be the basis of scientific reasoning (e.g.Gobert et al. 2011, Mendoça and Justi, 2014, Kênia et al., 2015). That is why their use to represent scientific information, explain and describe ideas, or provide means of visualising abstract scientific concepts is significant in science education (Gobert et al., 2011; Mendoça and Justi, 2014; Warfa et al. 2014). All over the world, national science standard documents (e.g. Québec, Ministère de l'Éducation, du Loisir et du Sport, 2009; France, Ministère de l'Education Nationale, 2011; USA, NRC, 2012) specifically call for students to be engaged in developing and using models, constructing explanations and participating in discussions. Scientists have developed different modes of representation of models with different degrees of abstraction (such as physical objects, photos, diagrams, graphs, texts) and different representational levels (such as macro, micro, submicro, and symbolic) (Kozma and Russell, 2005; Stull et al., 2012; Harlow et al., 2013; Treagust and Tsui, 2013; Roy and Hasni, 2014; Won et al., 2014). Having access to an assortment of representations helps connect various aspects of a phenomenon, build a more complete deeper understanding of science, and communicate scientific ideas more effectively (Ainsworth, et al., 2011). To be successful in science, students must therefore be able to understand, interpret, and readily translate among the different forms and types of representation, and also be able to choose the best representation for a given task (Gilbert, 2010; Kumi et al., 2013). From the work of different authors (Keig and Rubba, 1993; Kozma and Russell, 1997; Ainsworth, 2006; Schönborn and Bögeholz, 2009) we can define translation between representations as the ability to move across, interpret, and, in a multi directional manner, link between representations of an underlying scientific concept, principle or process at a particular level of organization. However novices often have difficulty mastering the use of multiple representations in scientific disciplines, such as biology (e.g.Ainsworth et al., 2011; Treagust and Tsui, 2013; Mulder et al., 2014; Won et al., 2014), physics (e.g.Harlow et al., 2013; Jong et al., 2015), chemistry (e.g.Gilbert and Treagust, 2009; Taber, 2013; Olimpo et al., 2015) and biochemistry (e.g.Schönborn and Anderson, 2010). Like Ainsworth (2006), Cook (2006) and de Jong et al. (1998, p. 32, cited by Won et al., 2014), we can consider that the ability to integrate and coordinate multiple representations is a characteristic of expertise. The coordination of representations was defined by Cook (2006, p. 1078) as “the creation of referential connections between corresponding features of different representations”. The coordination of representation is demonstrated, for example, by the capacity to understand and use the different types of structural representations of the same object reported in Fig. 1 interchangeably (Head et al., 2005; Khanfour-Armale and Le Marechal, 2009, Stull et al., 2012).The concept of models is omnipresent in chemistry teaching to represent abstract chemical ideas such as the nature of atomic and sub-atomic particles, molecular shapes, molecular polarity and other chemical concepts (e.g.Head et al., 2005; Chittleborough and Treagust, 2008; Jaber and Boujaoude, 2012). Multiple representations of models have been used, for example, to highlight relationships across macro, submicro and symbolic levels of model representations (e.g.Jaber and Boujaoude, 2012; Becker et al., 2015; Kênia et al., 2015) or, in organic chemistry courses, to visualize the spatial arrangement of atoms in molecules (e.g.Stull et al., 2012; Kumi et al., 2013; Olimpo et al., 2015). This arrangement determines the identity of compounds, each of which has its own spatial individuality and uniqueness (Seddon and Shubber, 1984; Habraken, 2004). To represent this arrangement, organic chemists use, for example, concrete physical models that provide a tangible representation of 3D spatial relationships between atoms in the molecule and 2D iconic representations using certain conventions that are supposed to represent the 3D relations concisely on paper (Pribyl and Bodner, 1987; Hegarty et al., 1991; Hoffman and Laszlo., 1991; Wu and Shah, 2004; Jones et al., 2005; Stull et al., 2012; Graulich, 2015). Such 2D representations have been created for specific purposes during the history of chemistry (Hoffman and Laszlo, 1991; Dumon and Luft, 2008; Goodwin, 2008). Some well-known examples are the Newman projection to illustrate the energy change of a molecule with rotation around the internal carbon–carbon σ bond (concept of conformation), the Dash-Wedge representation to depict the spatial arrangement of substituents within a molecule and the Fischer projection to highlight the different stereochemical relationships between members of the same carbohydrate family (Stull et al., 2012; Olimpo et al., 2015). The widespread use of these stereochemical representations in the teaching of organic chemistry requires students to acquire competence in building, identifying, interpreting and coordinating these different representations (Shepard, 1978; Pribyl and Bodner, 1987; Kozma and Russell, 1997; Wu and Shah, 2004; Cook, 2006; Stieff et al., 2010; Stull et al., 2012; Graulich, 2015; Olimpo et al., 2015).
These competences involve spatial reasoning abilities. Spatial ability is the over-arching concept that generally refers to skill in representing, transforming, generating, and recalling symbolic, nonlinguistic information (Linn and Petersen, 1985). Psychologists have conducted many studies on the subject (e.g.Michael et al., 1957; McGee, 1979; Linn and Petersen, 1985; Lohman, 1988; Carroll, 1993; Voyer et al., 1995). Three major factors representing different kinds of spatial abilities have emerged from these studies: spatial visualization, spatial orientation and spatial relation. Definitions of these terms vary depending on the researcher and the specific study. The following definitions, consistent with usage by previous workers, have been adopted by chemists (Tuckey and Selvaratnam, 1993; Coleman and Gotch, 1998; Barnea, 2000; Ferk et al., 2003; Gilbert, 2010; Harle and Towns, 2011; Carlisle et al., 2015): (1) spatial visualization: the ability to understand three-dimensional (3D) objects from their two-dimensional (2D) representations (and vice versa); (2) spatial orientation: the ability to imagine what a three-dimensional representation will look like from a different perspective; (3) spatial relations: the ability to visualize the effects of the operations of reflection, rotation or inversion, or to mentally manipulate objects.
So, interpreting how 2D diagrammatic conventions represent 3D space and providing the results of spatial transformations make a high cognitive demand on spatial working memory (Stull et al., 2012; Padalkar and Hegarty, 2014; Stull and Hegarty, 2015). Thus it is not surprising that understanding the spatial structure of organic molecules is a source of difficulties for many chemistry students (Dori and Barak, 2001; Lujan-Upton, 2001; Pellegrin et al., 2003; Jones et al., 2005; Kurbanoglu et al., 2006).
Students' understanding of the molecular structure representations: literature review
Many authors agree that students find it difficult to visualize the spatial structure of molecules from 2D iconic representations (e.g.Bodner and Domin, 2000; Wu et al., 2001; Ferk et al., 2003; Appling and Peake, 2004; Kuo et al., 2004; Wu and Shah, 2004; Head et al., 2005; Abraham et al., 2010; Kumi et al., 2013; Olimpo et al., 2015). Linking symbolic representations of molecules in two dimensions to the visualization of their three-dimensional aspect is a complex task that requires the spatial abilities defined previously (Kozma and Russell, 1997; Barnea, 2000; Wu and Shah, 2004; Jones et al., 2005; Graulich, 2015).Furthermore, to visualize the three-dimensional aspect of 2D representations, students must firstly understand and interpret the different graphic conventions used to translate the 3D reality into a planar representation (Habraken, 1996; Pellegrin et al., 2003; Kuo et al., 2004; Wu and Shah, 2004; Head et al., 2005; Jones et al., 2005; Bucat and Mocerino, 2009; Stull et al., 2010; Padalkar and Hegarty, 2014; Stull and Hegarty, 2015), conventions that are rather abstract and intangible in nature (Kuo et al., 2004; Olimpo et al., 2015). On the other hand, they must take the positioning of the observer relative to the observed molecular structure into account (Pellegrin et al., 2003; Head et al., 2005; Kumi et al., 2013; Carlisle et al., 2015), an activity termed “perspective taking” by Stieff et al. (2010) and Stull et al. (2010). The result is that students have difficulties translating between the different diagrammatic representations (Pribyl and Bodner, 1987; Wu and Shah, 2004; Boukhechem et al., 2011; Harle and Towns, 2011; Stull et al., 2010, 2012; Kumi et al., 2013; Koutalas et al., 2014; Carlisle et al., 2015; Graulich, 2015; Olimpo et al., 2015; Stull and Hegarty, 2015) and when they try to connect different representations, they often focus on surface-level features without being aware of the relevant underlying characteristics (Cook, 2006; Kumi et al., 2013; Olimpo et al., 2015).
To translate between the different diagrammatic representations, students can use various strategies (Stieff and Raje, 2010; Stieff et al., 2010; Stieff, 2011; Hegarty et al., 2013). One strategy can be named “imagistic”, as it involves creating mental models of diagrams and then carrying out internal spatial transformations (e.g. mental rotation, perspective taking, and the rule-based strategy). The other strategy, named “algorithmic–diagrammatic”, is used by manipulating the molecular diagram with heuristics or algorithms without invoking mental images (Stieff et al., 2010; Stieff, 2011). However, Stieff (2011) noted that students preferentially employ imagistic reasoning for translating between various molecular diagrammatic representations. For example to translate between the Dash-Wedge representation and the Newman projection of Fig. 1, students tended to compare the spatial information depicted in the two representations of the same molecule and then execute mental rotation of the group of substituents around the carbon atom C3 to adopt the conformation of the Newman projection.
Several authors have shown that many students find it difficult to view the atom positions after mental rotation of molecular structure (Tuckey et al., 1991; Head and Bucat, 2002; Stull et al., 2012.). Others report that it is the dynamic nature of the molecules that is forgotten when translating between the different diagrammatic forms (Grosslight et al., 1991; Stieff et al., 2005; Boukhechem et al., 2011; Kumi et al., 2013; Olimpo et al., 2015). This concerns the “spatial relation” ability, where the rotation is important but often not achieved. As a result, the students see the 2D diagrams in a fixed conformation and do not engage in the linking of different conformations of a molecular structure illustrated in a Dash-Wedge representation, a Newman projection, or the Fischer projection (Olimpo, 2013). For example, the translation from the Newman or Dash-wedge diagram to the Fischer projection of Fig. 1 is a complex task. It involves a high cognitive demand to interpret how all 2D diagrammatic conventions (Newman, Dash-Wedge and Fischer) represent 3D space, then requires use of spatial visualization (imagine the movement or displacement of parts of a spatial figure relative to other parts), spatial relation (mentally rotate Newman or Dash-Wedge representation to obtain the C2H5/CH3 pair of substituents in eclipsed conformation) and spatial orientation (imagine how the 3D object should be looked at to obtain the Fischer projection). The students can achieve these multiple transformations if they are able to coordinate the three diagrammatic representations. An illustration of the lack of such coordination of representations is that Fischer projections were always restricted to the simple projection, or “flattening”, of the representation in the plane (Boukhechem et al., 2011; Olimpo, 2013; Olimpo et al., 2015).
It is commonly accepted that handling concrete and/or virtual molecular models facilitates students' understanding of the three-dimensional structure of molecules and is a means to help them identify spatial relations so as to understand 2D representations (see for example the most recent studies: Ferk et al., 2003; Appling and Peake, 2004; Habraken, 2004; Kuo et al., 2004; Wu and Shah, 2004; Jones et al., 2005; Stieff et al., 2005; Cook, 2006; Kurbanoglu et al., 2006; Abraham et al., 2010; Kumi et al., 2013; Carlisle et al., 2015; Olimpo et al., 2015). By making it easier to visualize molecular structures from different viewing perspectives and/or to physically rotate the model around the carbon–carbon bond and observe the result rather than mentally rotating, these tools contribute to students' understanding of the different representations (Copolo and Hounshell, 1995; Wu et al., 2001; Cook, 2006; Stull et al., 2012, 2013; Al-Balushi and Al-Hajrib, 2014; Olimpo et al., 2015). They can serve as “catalysts” (or “cognitive scaffolds”, Stull and Hegarty, 2015) that enable students to make connections between 2D and 3D representations (Dori and Barak, 2001; Head and Bucat, 2002; Ferk et al., 2003; Stull et al., 2012). Some studies have shown that by reducing the cognitive load, since “the conventions of a diagram (for depicting the 3D structure of the molecule in the 2 dimensions of the page) do not have to be maintained in working memory” (Stull et al., 2012, p. 408), the handling of a concrete model improved students' performance in translating between different diagrams of molecules (Stull et al., 2010; Stull et al., 2012; Paddakar and Hegarty, 2014; Stull and Hegarty, 2015). However, it is important to note that placing the models in their hands did not have significant effects on their performance of spatial transformation tasks for all students (Stull et al., 2012; Kumi et al., 2013). For example, in a study by Stull et al. (2012), many students ignored the models and other studies have shown that some students have difficulties in building the molecular models from stereochemical representations (Ferk et al., 2003; Appling and Peake, 2004) or when they try to turn or rotate models while discerning structural properties (Copolo and Hounshell, 1995).
Research aims and methodology
In some studies conducted in the US on the use of concrete molecular models during the translation process between Newman, Dash-Wedge and Fischer diagrammatic representations, students can use or not models as help or feedback. The aim of these studies is to examine how students use or not concrete models. In a different institutional and cultural context, our study looked into the question of whether the effective handling of a concrete molecular model by students promotes translation between diagrammatic representations and the concrete model (or vice versa) and the coordination of the different representations of a given molecular structure. We make the hypothesis that the ability to coordinate 3D and 2D representations involves being able to translate both from the diagrammatic representations to the concrete model and from the concrete model to its representation in 2D diagrams (Head and Bucat, 2002; Al-Balushi and Al-Hajrib, 2014). The evaluation of students' ability to coordinate the different representations will therefore be based on an evaluation of three translation processes between representations of two molecular structures (Al-Balushi and Al-Hajrib, 2014):– Construct 3D concrete models from Dash-Wedge and Newman representations;
– Draw a Dash-Wedge and a Newman 2D representation of a 3D concrete molecular model after rotating it to a certain degree;
– Produce a Fischer projection of a molecule from a 3D concrete model or any other 2D drawings.
Methodology
The tasks of these questions were intended to evaluate students' abilities to coordinate the representations in translating from Dash-Wedge or Newman representations to 3D molecular models (and vice versa) for two molecular structures and, for one molecular structure, to translate from the 3D molecular model in one staggered conformation to the Fischer projection. In the questionnaire, there was nothing that could orient students towards identifying that the structure I Dash-Wedge diagram was one Dash-Wedge diagram of the concrete model of structure III and that the structure II Newman diagram was one Newman representation of the structure IV concrete model.
Abilities to translate from the Dash-Wedge representation of structure I (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) III) and the Newman representation of structure II (
III) and the Newman representation of structure II (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) IV) to their representation by concrete (ball-and-stick) models were evaluated by the tasks of the first question. In other words, did the students make use of a spatial visualization ability related to their knowledge of the conventions used for 2D representations? The tasks of the second question assessed their abilities to translate from the structure III (
IV) to their representation by concrete (ball-and-stick) models were evaluated by the tasks of the first question. In other words, did the students make use of a spatial visualization ability related to their knowledge of the conventions used for 2D representations? The tasks of the second question assessed their abilities to translate from the structure III (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) I) concrete model to these Dash-Wedge and Newman diagrammatic representations and thus concerned the abilities of visualization and spatial orientation, and a knowledge of the rules governing 2D representations (Dash-Wedge and Newman). The abilities evaluated with the tasks of the third question were: the ability to identify, by handling the 3D concrete model of structure IV (
I) concrete model to these Dash-Wedge and Newman diagrammatic representations and thus concerned the abilities of visualization and spatial orientation, and a knowledge of the rules governing 2D representations (Dash-Wedge and Newman). The abilities evaluated with the tasks of the third question were: the ability to identify, by handling the 3D concrete model of structure IV (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) II), the conformation for which interactions between substituents were minimal (spatial relationship ability); the ability to translate from this concrete model conformation to its Newman representation by specifying the position selected by the observer (visualization and spatial orientation abilities); the ability to represent the molecular structure and conformation of structure IV (
II), the conformation for which interactions between substituents were minimal (spatial relationship ability); the ability to translate from this concrete model conformation to its Newman representation by specifying the position selected by the observer (visualization and spatial orientation abilities); the ability to represent the molecular structure and conformation of structure IV (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) II) respecting the rules to obtain the Fischer projection using the concrete model and then to draw these Dash-Wedge and Fischer representations (abilities in visualization, orientation and spatial relation related to the knowledge of conventions).
II) respecting the rules to obtain the Fischer projection using the concrete model and then to draw these Dash-Wedge and Fischer representations (abilities in visualization, orientation and spatial relation related to the knowledge of conventions).
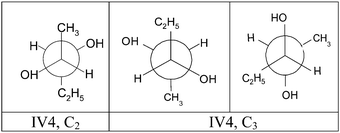
Our data did not raise any concerns about the validity of the items. First, they are ecologically valid because these tasks could be used in the real organic chemistry classroom (Stull et al., 2012; Reiss and Judd, 2014). Then several reasons are related to their construct validity: (1) they tested the degree to which students understood how the different representations depicted the same molecules: (2S,3S)-pentane-2,3-diol (structures I and III) and (2R,3S)-pentane-2,3-diol (structures II and IV); (2) asking students to translate between different representations of the same molecular structure was a good indicator of their coordination of molecular representations; (3) the choice of two stereoisomers, with the distribution of substituents around asymmetric carbons, symmetrical or not, and the order and wording of the questions allowed us to evaluate the spatial visualization ability related to the knowledge of conventions used for 2D diagrammatic representations, spatial orientation, spatial relationship abilities and the capacity to combine these spatial abilities. This choice also enabled such abilities to be successively compared for two molecular structures.
It should be noted that all the students had the opportunity to individually manipulate molecular models during their first academic year of general chemistry practical work and during one organic chemistry practical session in the third year to familiarize themselves with free rotation around a single bond, or breaking when a double bond was involved, and with the orientation of the substituents relative to the plane of a molecular structure. Nevertheless we assured ourselves that students were able to build and manipulate concrete models in two practical sessions concerning the spatial representation of molecular structures contained in the organic chemistry textbooks, prior to the assessment session.
Data analysis
For each question, an a priori analysis of the possible answers was carried out and the answers were encoded.To draw the Dash-Wedge diagram, students could orient the structure according to the axis C*2–C*3, or vice versa, looking at: the asymmetric carbon 2 or 3 from a position slightly shifted to the left (L) or to the right (R), the concrete model in the frontal position with respect to the C*–C* bond, in a position shifted slightly upward (U) or downward (D). Table 4 shows the coding of possible generic Dash-Wedge representations of staggered and eclipsed conformations of the molecular structures (limited to the orientation of the bonds, without indicating the nature of the substituents) according to the orientation of the structure and the position of the observer.
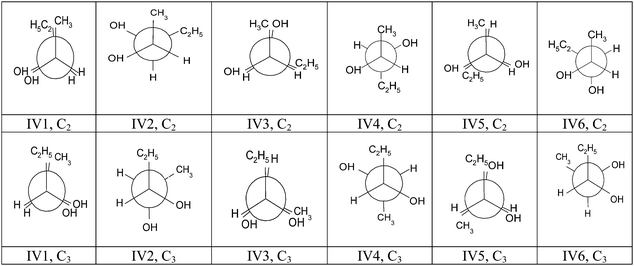
It should be noted that other drawings of these Newman or Dash-Wedge representations could be given if the observer rotated the entire concrete model to 120° or 240°. We indexed the representations as follows: structure number (III or IV), serial number in the energy – conformation diagram (1 to 6); carbon placed in front of the observer (C2 or C3), and the letter corresponding to the position adopted by the observer for Dash-Wedge representations (L, R, U or D). For example the conformation III1, C2, U (see Table 8) corresponds to the eclipsed conformation with the maximal interaction energy between substituents; the structure was oriented according to the axis C*2–C*3; the observer looked at the concrete model from a frontal position with respect to the C*–C* bond, in a position shifted slightly upward (U).
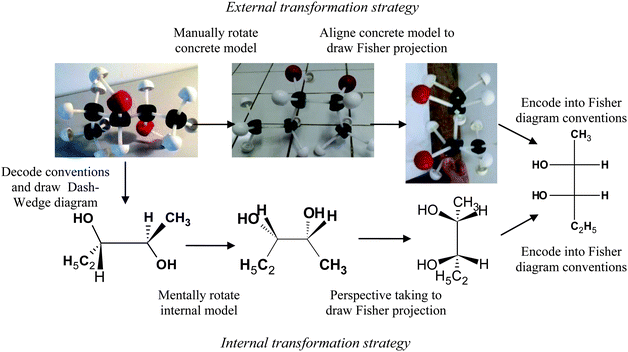 | ||
| Fig. 2 Illustration of translation strategies between the concrete model and the Fischer projection. | ||
Using the external strategy suggested by question 3b of the questionnaire, the student should first manually rotate the concrete model in staggered conformation IV4 to obtain the C2H5/CH3 pair of substituents in eclipsed conformation IV1. Second, s/he should select the observer position relative to the C*2–C*3 bond to observe this concrete model conformation (shifted slightly upward, coded U, or downward, coded D) and the model orientation in conformity with the Fischer representation. But various model photographs (and Dash-Wedge diagram) of the eclipsed conformation IV1 were possible depending on the position selected by the observer and the model orientation.
We have represented these different orientations in the Newman diagram of the conformation IV1, C3 of Fig. 3. The C2H5/CH3 pair can be set back (orientation 1) or forward (orientation 1′) of the observation plane as can pairs OH/OH (orientations 2 or 2′) and H/H (orientations 3 and 3′).
For the answer to each task, we also analyzed the different kinds of spatial reasoning abilities (spatial visualization, spatial orientation, and spatial relation) implemented by the students.
Results
We considered that students were able to translate between molecular structure representations of the same object if they correctly represented diagrammatic representations by their concrete models or drew correct (or acceptable) diagrammatic representations from the concrete models. Therefore, we will initially seek to identify, for the two proposed molecular structures, whether students knew the conventions used for different representations and whether they gave correct representations (concrete model, 2D diagrams) or not. If a student was able to represent or use the different types of structural representations of the same object correctly, we considered that s/he had the capacity to coordinate these different representations. To evaluate the students' spatial orientation and spatial relationship abilities we complemented the analysis of representations by watching the conformation adopted for the different representations of these structures and the position relative to the C*2–C*3 (or C*3–C*2) bond chosen by students to observe molecular structures.Translation of diagrammatic representations (Newman and Dash-wedge) ⇒ concrete models
| Quality of concrete model | N.I | N.II | |
|---|---|---|---|
| Correct model | Conformation ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) to diagram to diagram |
18 | 19 |
| Another conformation | 08 | 12 | |
| Incorrect model | Positioning error of the substituents on one or both C* | 14 | 8 |
| Other: model with 6 carbons | 01 | 0 | |
| No reply | 00 | 2 | |
| Total students | 41 | 41 | |
The majority of students (26 i.e. 63%) succeeded in building a correct concrete model from structure I (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) III) Dash-Wedge representation, either with conformation identical to the proposed molecular structure (18), or with another conformation obtained by free rotation around the C*–C* bond. The incorrect models built by other students mainly did not respect the position of the substituents, either on one of the asymmetric carbons (6 to C*2 and 5 to C*3) or on both (3).
III) Dash-Wedge representation, either with conformation identical to the proposed molecular structure (18), or with another conformation obtained by free rotation around the C*–C* bond. The incorrect models built by other students mainly did not respect the position of the substituents, either on one of the asymmetric carbons (6 to C*2 and 5 to C*3) or on both (3).
A slightly larger number of students (31 i.e. 76%) built a correct concrete model from a structure II (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) IV) Newman representation, either with an identical conformation to the proposed one (19) or with a different conformation (12). The incorrect concrete models built by students (8) showed an inversion of the arrangement of substituents on C*2.
IV) Newman representation, either with an identical conformation to the proposed one (19) or with a different conformation (12). The incorrect concrete models built by students (8) showed an inversion of the arrangement of substituents on C*2.
Twenty-three students (56%) built correct models from both representations and only 7 (17%) proposed incorrect models for two representations. Of the remaining students, 8 seemed to find it easier to build a concrete model from a Newman than from a Dash-Wedge representation, and 3 found the opposite.
The above results show that, in carrying out these tasks of translating 2D diagrams into 3D concrete molecular models, the majority of students used spatial visualization ability related to a knowledge of the conventions (76% Newman, 63% Dash-Wedge and 56% both). It should be noted, firstly, that the visualization of the position of functional groups in space, was favored by the Newman representation for some students, while some showed a spatial relation ability (rotation around the C*–C* bond) during the building of concrete molecular models.
Translation of the 3D ball-and-stick model ⇒ diagrammatic representations (Newman and Dash-wedge)
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e002.gif) I) (Q2).
I) (Q2).
Expected answers. The Dash-Wedge and Newman representations expected in answer to the second question, and corresponding to the conformation and orientation of the given concrete model are those drawn in Table 7.
To produce these drawings, the students had to place the observer in front of C*3 to achieve the Newman representation and in a position slightly shifted to the left to obtain the Dash-Wedge representation, respecting the sequence and orientation of the substituents and the conventions governing each representation.
Students' answers.
Dash-Wedge representations of structure III. Of the 41 representations of the concrete molecular model given to students (staggered conformation III2, C3), 34 (83%) were considered as acceptable: 18 were entirely correct and 16 approximately correct (sequencing of the substituents around each atom C* was correct, conventions for representing bonds were adopted, but the drawing was defective: the positioning of the bonds in space or bond angles was incorrect). For the other 7 representations, the sequencing and/or the conventions to represent bonds were not respected.
Table 8 reports the number of the different conformations identified in the 34 acceptable representations.
| Observer position in front of | Identified conformations | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eclipsed | Staggered | N | |||||||||
| III1 | III3 | III2 | III4 | III6 | |||||||
| C2 | C3 | C2 | C3 | C2 | C3 | C2 | C3 | C2 | C3 | ||
| Letters L, R, U, D correspond to the position adopted by the observer (see Table 4). | |||||||||||
| L | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 0 | 0 | 1 | 5 |
| R | 1 | 1 | 0 | 0 | 0 | 0 | 3 | 3 | 1 | 0 | 9 |
| U | 8 | 4 | 5 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 19 |
| D | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Total position | 10 | 5 | 5 | 2 | 1 | 0 | 6 | 3 | 1 | 1 | |
| Total conformation | 15 | 7 | 1 | 9 | 2 | 34 | |||||
We note that although the concrete model given to students presented a staggered conformation, many of them (22/34) chose to represent an eclipsed conformation, mainly conformation (III1), for which the interactions between the substituents are maximum. Conversely, conformation (III4), in which the interactions are weaker, predominated in the staggered conformations (9/12).
Note that no student represented the expected conformation (III2, C3) corresponding to the concrete model as it was presented to the students. While the concrete model was presented to them from left to right along the axis C*3–C*2, the majority of students (23/34) oriented the structure from left to right following the axis C*2–C*3 and observed it by placing themselves in front of this bond and in an upward shifted position (19/34). Note that the students worked standing up, which certainly affected their way of observing the model.
Newman representations of structure III. Table 9 reports the number of different conformations identified in the 40 students' Newman representations (correct or incorrect) of the concrete model given to them (staggered conformation III2, C3).
| Representation | Identified conformations | N | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eclipsed | Staggered | ||||||||||
| III1 | III3 | III2 | III4 | III6 | |||||||
| C2 | C3 | C2 | C3 | C2 | C3 | C2 | C3 | C2 | C3 | ||
| Correct | 7 | 2 | 5 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 17 |
| Incorrect | 4 | 1 | 0 | 1 | 0 | 0 | 7 | 5 | 0 | 5 | 23 |
| Total position | 11 | 3 | 5 | 1 | 1 | 1 | 8 | 5 | 0 | 5 | |
| Total conformation | 14 | 6 | 2 | 13 | 5 | 40 | |||||
The data in Table 9 show an equality of structure III concrete model representations in eclipsed or staggered conformations.
We note that only 17 students drew correct representations, primarily those in eclipsed conformations (14/17). For the others, a reversal of the position of substituents was found on one or (rarely) both asymmetric carbons. Concerning the conformation adopted we observe an equality of structure III concrete model representations in eclipsed or staggered conformations and, as for the Dash-Wedge representations, a preference for the eclipsed conformation (III1) and staggered conformation (III4). Finally, a majority of students (25/40) chose to position the observer in front of C*2 (25/40) to obtain Newman representations.
Results of translation of the structure III concrete model ⇒ Dash-Wedge and Newman representations. A significant proportion of students (31 i.e. 83%) gave an acceptable Dash-Wedge representation respecting substituent sequencing and conventions for representing bonds of the molecular structure III in and out of the plane of the sheet (spatial visualization ability and knowledge of conventions). To achieve these representations, the students all subjected the molecular structure to a rotation around the carbon–carbon bond, the majority (22, i.e. 54%) choosing the eclipsed conformation. A majority (23, i.e. 56%) oriented the structure in a direction different from that of the given model (C*2–C*3 instead of C*3–C*2), and 19 (46%) observed the structure from in front of the carbon–carbon bond in an upward shifted position. Orientation and spatial relation abilities were implemented.
The proportion of students giving a correct Newman representation respecting conventions and sequencing of substituents around the asymmetric carbon was significantly lower (17, i.e. 41%). Again the representation of the concrete model, whether correct or not, was performed after rotation around the carbon–carbon bond but with an equal choice of conformations between eclipsed (generally correct: 14/20) and staggered (generally incorrect: 17/20). The majority of students (25, i.e. 61%) chose to view the structure from in front of the C*2, and positioning errors of the substituents by students usually occurred on the C*3.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e002.gif) II) (Q3).
Table 10 shows a photograph of the concrete model as presented to the students with its Newman and Dash-Wedge representations.
II) (Q3).
Table 10 shows a photograph of the concrete model as presented to the students with its Newman and Dash-Wedge representations.
Translation of the structure IV concrete model ⇒ Newman representation of the most stable conformation
| Identified conformations | N | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Eclipsed | Staggered | ||||||||
| IV1 | IV5 | IV4 | IV6 | ||||||
| C2 | C3 | C2 | C3 | C2 | C3 | C2 | C3 | ||
| Structure IV acceptable Newman representations | 2 | 0 | 0 | 1 | 15 | 10 | 1 | 0 | 29 |
| Structure IV incorrect Newman representations | 0 | 0 | 0 | 5 | 4 | 0 | 0 | 9 | |
| Other representations, totally incorrect or incomplete | 3 | ||||||||
As regards the position of the observer relative to the concrete model for these representations, only 30 students gave an explicit response. We completed these responses using the data of Table 11. That is, whatever the nature of the response, the observer was positioned in front of carbon C*2 which was predominantly selected (24/41).
The data in Table 11 show that the majority of students (29, i.e. 71%) made use of their spatial relation and visualization abilities to draw an acceptable Newman representation of structure IV with respect to the sequencing and location of substituents around carbon atoms. Moreover, although 34 students (83%) represented structure IV in staggered conformation according to the most stable conformation (IV4), fewer of them (25, i.e. 61%) were able to draw the expected correct representation (15 IV4, C2 and 10 IV4, C3) for which interactions between substituents were minimal (spatial relation ability). It should be noted that only one student justified his correct representation of the most stable conformation by drawing the figure representing the different energy states of the molecule based on its conformation. For the other 9 representations, we noted an inversion of the positions of the substituents H and OH on one or two asymmetric carbons. Finally, a comparison of the relatively high non-response ratio for the position of the observer and the high proportion of acceptable conformations suggests that not all students felt the need to specify the observer's position when looking at the 3D molecular structure and projecting it onto a plane (spatial orientation ability); yet this is an important parameter for applying all elements of the rules governing the translation from one representation to another.
Translation of the structure IV concrete model ⇒ Dash-Wedge representation of conformation leading to the Fischer projection
| Representation | Conformation | ||||
|---|---|---|---|---|---|
| IV1, C2 ou C3, 1 ou 1′ | IV1, C2 ou C3, 2 ou 3 | IV4 | Other | N students | |
| Acceptable | 5 | 3 | 15 | 3 | 26 |
| Substituent inversion on a C* | 0 | 1 | 6 | 1 | 8 |
| Totally incorrect | 5 | ||||
| No answer | 2 | ||||
The data in Table 12 show that a majority of students (26 i.e. 63%) made use of their spatial visualization ability to provide an acceptable Dash-Wedge representation of structure IV. Although the expected response to the question corresponded to an eclipsed conformation of the structure, it was the staggered conformation of the original model given to students that was proposed by the majority (21 i.e. 51%). Only 9 students (22%) made use of their spatial relation (rotation) ability to obtain the C2H5/CH3 pair in eclipsed conformation and only 5 (12%) drew the expected representation (IV1, C2 or C3, 1 or 1′) correctly. The other students did not use their spatial relation ability, presumably because they forgot the rules leading to a Fischer projection. We can add that the majority of the identified representations (20/34) were, as in the case of structure III, oriented the direction C*2–C*3 with the position of the observer slightly shifted to the left.
Comparative study of translation of structure III and IV concrete models ⇒ Dash-Wedge and Newman representations
Table 13 records the number of students who gave an acceptable answer for the translation representation(s) → concrete model(s) and concrete model(s) → representation(s). Concerning the structure IV Dash-Wedge and Newman representations drawn by students according to the concrete model, it was the number of acceptable representations of this structure identified in students' answers that were counted, regardless of whether the representations agreed with the expected answers.| Concerte model building | Model → Dash-Wedge | Model → Newman | Model → Dash-Wedge and Newman | Coordination representations ↔ models | |
|---|---|---|---|---|---|
Structure III (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) I) I) |
26 (63%) (Dash-Wedge → model) | 34 (83%) | 17 (41%) | 16 (39%) | 22 (54%) |
Structure IV (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) II) II) |
31 (76%) (Newman → model) | 26 (63%) | 29(71%) | 18 (49%) | 21 (51%) |
| Structures III and IV | 23 (56%) (representations → models) | 22 (54%) | 10 (24%) | 5 (12%) | 12 (29%) |
The data in Table 13 show a difference between structures III and IV for translations between 3D models and 2D representations. For structure III, the percentage of students giving a correct Dash-Wedge representation (83%) was higher than for structure IV (63%). On the other hand, the opposite was true for the Newman representation: 41% for structure III and 71% for structure IV. It follows that globally the ability to translate from 3D models to 2D representations was better for structure IV: 49% against 39%. In addition, the percentage of students able to correctly translate the concrete models representing the two structures into 2D representations was larger for the Dash-Wedge representation (54%) than the Newman ones (24%). Thus the handling of concrete models seems to promote the mobilization of visualization, orientation and spatial relation abilities more when translating a 3D structure towards this Dash-Wedge representation than towards its Newman projection.
Translation of the structure IV concrete model ⇒ Fischer projection
The translation from the model presented to students to its Fischer projection is a complex task that requires spatial visualization, spatial rotation and spatial orientation abilities (see Fig. 2).The students took photographs of the model by adopting the different conformations shown in Table 14.
| Conformation represented | N |
|---|---|
| Eclipsed conformation IV1, C2 or C3, 1 or 1′ | 9 |
| Initial model conformation (IV4) | 23 |
| Other eclipsed conformations | 7 |
| No answer | 2 |
| Total students | 41 |
Only 9 students manually rotated the concrete model around the C*–C* bond in order to obtain a conformation where the C2H5/CH3 pair was in an eclipsed position. Other students either contented themselves with photographing the model in its initial conformation (23 students), sometimes by placing the model in a vertical position, or executed rotations leading to a variety of other eclipsed conformations.
| Categories of representations | N | ||
|---|---|---|---|
| Correct Fischer projection of conformation IV1, C3, 1 | 4 | ||
| Flattening of other IV1 conformations | With main carbon chain vertically but perspective taking incorrect | CH3 at the top | 2 |
| C2H5 at the top | 8 | ||
| Other | 2 | ||
| Flattening of IV4 conformations | With main carbon chain vertically | CH3 at the top | 12 |
| C2H5 at the top | 4 | ||
| Other | 5 | ||
| Flattening of other conformations | 3 | ||
| Totally incorrect | 1 | ||
| Total number of representations | 41 | ||
Only 4 students drew a correct Fischer representation. The analysis of the strategies used to obtain these representations showed that one student used the external strategy of Fig. 2 and another student used the internal strategy of Fig. 2 by representing the Dash-Wedge diagram of the initial model (conformation IV4) then mentally rotating the structure around the C*–C* bond to obtain conformation IV1 (without diagram) before projection. For the other two students, the strategy was mixed: after manually rotating the concrete model, they represented the Newman projection and used this representation to obtain the Fischer projection.
We note that the great majority of students (36/41, i.e. 88%) simply drew the projection (or “flattening”) onto the plane of molecular structure in various conformations, depending on their observer position relative to the model. We can also say that the rules leading to a Fischer projection were only partially known by our students. Although a significant proportion of students (30/41, i.e. 73%) remembered that the main carbon chain defined in the nomenclature should be upright, only 18 (44%) placed the carbon having the smallest index in the carbon chain at the top of the vertical axis and 16 (31%) knew that the molecular structure must be in a particular eclipsed conformation to obtain a Fischer projection. Finally, only a few students remembered the “perspective taking” necessary to obtain the Fischer projection of one molecular structure.
Discussion
Our results show that during the translation process of Dash-Wedge and Newman diagrams to concrete ball-and-stick models (and vice versa) the majority of students made use of their spatial visualization ability related to knowledge of the conventions used for representing the 3D configuration of a molecular structure in 2D. Even if students did not feel the need to specify the observer's position when looking at the 3D molecular structure, the spatial orientation ability was identified in the majority of students' answers with the modification of the direction of giving the molecular structure (C*2–C*3 instead of C*3–C*2). Finally, spatial relation ability (rotation around the C*–C* bond) was implemented by numerous students.So, it seems that handling a concrete molecular model promotes the translation process. But the manipulation of a concrete model seems more favorable to the mobilization of visualization, orientation and spatial relation abilities when translating a 3D structure to a Dash-Wedge representation than into a Newman projection. This finding can be linked to the work of Stull et al. (2012) and Olimpo et al. (2015) that showed that students encountered difficulties in translating Dash-Wedge to the Newman representations. Olimpo et al. (2015) believed these difficulties can be attributed to a lack of clear understanding of what a Newman projection represents in three-dimensional space and/or a failure to recognize the dynamic nature of the molecules. However, it is apparent from the analysis of our results that this was not the case for a high proportion of students in our sample. So, how should this difference in performance in the translation concrete model-Dash-Wedge representation and concrete model-Newman representation be interpreted? First of all, it can be attributed to the fact that the Dash-Wedge representation is itself a very explicit 3D representation that can easily be identified with the 3D concrete model (Kumi et al., 2013; Olimpo et al., 2015): visualization, orientation and spatial relation abilities are made easier. Then, it can be attributed to a greater difficulty with spatial relations consisting of mentally manipulating a 3D object to represent a Newman diagram in 2D. A frequently encountered error was an inversion of the position of the H and OH substituents on one (or rarely two) asymmetric carbons. According to Stull et al. (2012, p. 425) we think that this common error “…in which the molecular substituents were configured correctly on one side of the molecule but not on the other side is suggestive of a piecemeal strategy in which the same transformation was not applied consistently”. The fact that the proportion of correct Newman representations was higher for structure IV may be explained by a symmetrical configuration of substituents around the two asymmetric carbons, which promotes a uniform application of the transformation process to both sides of the representation.
In the case of structure III, where translation did not require changing conformations, like Stull et al. (2012) we note that reconfiguring the models by rotating substituents around bonds within the models was observed more often when translating to a Dash-Wedge diagram than to a Newman projection. From a staggered conformation of the model, such reconfiguring led to an eclipsed conformation. When translation began with the Fischer projection, Stull et al. (2012) observed the inverse: eclipsed → staggered. In the case of translation from the structure IV concrete model in staggered conformation to the Fischer projection that required adopting a conformation where the C2H5/CH3 pair of substituents was in eclipsed conformation, we noted that in contrast to the observation by Stull et al. (2012), few students changed conformation: they kept the staggered conformation of the original model. In addition, they misaligned the observer with respect to the substituents and the great majority adopted the “flattening” strategy of the model representation of the molecular structure, the strategy identified in the case of diagrammatic translations (Boukhechem et al., 2011; Kumi et al. 2013; Olimpo, 2013; Olimpo et al., 2015). Like Olimpo et al. (2015) we believe that this inappropriate combination of representational skills utilized by students indicates that students do not appreciate the conventions represented by the horizontal and vertical lines in the Fischer projection. They focus on surface-level features without being aware of the relevant underlying characteristics (Cook, 2006; Kumi et al., 2013; Olimpo et al., 2015). From this we can conclude that the manipulation of a concrete model does not favor the mobilization of visualization, orientation and spatial relation abilities during the translation from the model presented to students to its Fischer projection.
Finally, does handling a concrete molecular model promote the coordination of the different representations of a given molecular structure? Our results show that the coordination of each Dash-Wedge and Newman representation with their 3D structure was achieved by a majority of students. However, the students who coordinated these two representations with models were not the same in both cases, probably because of the difference in substituent distribution in the two structures. The result was that only a minority of our students showed spatial reasoning abilities allowing them to coordinate diagrammatic representations in 2D (Dash-Wedge and Newman) of the two molecular structures with their 3D concrete model. This can be explained by the difficulties encountered by students in respecting the atom positions after mental rotation of the molecular structure (Tuckey et al., 1991; Head and Bucat, 2002; Stull et al., 2012). Concerning the coordination of the concrete model with Dash-Wedge and Fischer representations of structure IV, the high degree of difficulty the students had in understanding the conventions of the Fischer projection (Olimpo et al., 2015) led to the result that no students coordinated these representations after handling the concrete molecular model.
Conclusion
Our results show that during the translation tasks, concrete molecular models have the potential to be an effective spatial tool to promote visualization, orientation and rotation abilities. However, their effectiveness is different for the different representations. These abilities are implemented more for Dash-Wedge than for Newman diagrams, and not at all for Fischer projections. These differences can be explained by the fact that during organic chemistry teaching, teachers place more emphasis on the Dash-Wedge representation than the Newman or Fischer projections. To echo Stull et al.: “The imbalance of familiarity by students may have had an influence over the results” (Stull et al., 2010, p. 343). Furthermore, although the spatial visualization ability related to the knowledge of the conventions used for 2D representations (Dash-Wedge and Newman) was used by a majority of students to build a concrete model, effective use of the model required them to do more than establish the correspondence between the diagrams and concrete models. The manipulation of a 3D molecular structure did not have the same impact of promoting the visualization of the substituents' distribution around asymmetric carbons for all students. This impact appears to vary according to the conformations (greater for the eclipsed than for the staggered conformation) and the distribution of substituents around asymmetric carbons (greater for symmetrical distribution).This research implies that working with concrete models should be effectively encouraged in the teaching of organic chemistry. To help students visualize the relationship between multiple representations of the same molecular structure, particularly when the conventions of these representations are varied in nature, considerable teaching time should be devoted to an explicit discussion of these diagrams and the mechanisms by which one translates between representations (Stull et al., 2012; Kumi et al., 2013; Olimpo et al., 2015). The teacher can:
– include examples of molecules depicted in various conformations and different examples of perspective-taking during classroom instruction, offering students extensive opportunities to practice working with each of these representations of a molecule, so that they can gain a better understanding of the relationship between diagrams (Olimpo et al., 2015);
– give opportunities for students to draw and describe 2D diagrammatic representations using a concrete Ball-and-Stick model and vice versa (Head and Bucat, 2002; Harle and Towns, 2011; Stull et al., 2012, 2013; Al-Balushi and Al-Hajrib, 2014; Olimpo et al., 2015; Stull and Hegarty, 2015);
– propose translation tasks with the opportunity to generate self-feedback using concrete models (Padalkar and Hegarty, 2014): “using models as feedback is a particularly effective way of inducing students to engage with models and experience their benefits” (Stull and Hegarty, 2015, p. 15).
References
- Abraham M., Vargese V. and Tang H., (2010), Using molecular representations to aid student understanding of stereochemical concepts, J. Chem. Educ., 87, 1425–1429.
- Ainsworth S., (2006), DeFT: a conceptual framework for considering learning with multiple representations, Learn. Instr., 16, 183–198.
- Ainsworth S., Prain V. and Tytler R., (2011), Drawing to learn in science, Science, 333, 1096–1097.
- Al-Balushi S. M. and Al-Hajrib S. H., (2014), Associating animations with concrete models to enhance students' comprehension of different visual representations in organic chemistry, Chem. Educ. Res. Pract., 15, 47–58.
- Appling J. R. and Peake L C., (2004), Instructional and molecular visualization, J. Sci. Educ. Technol., 13, 361–365.
- Barnea N., (2000), Teaching and learning about chemistry and modelling with a computer managed modelling system, in Gilbert J. K. and Boulter C. J. (ed.) Developing models in science education, Dordrecht: Kluwer Academic, pp. 307–323.
- Becker N., Stanford C., Towns M. and Cole R., (2015), Translating across macroscopic, submicroscopic, and symbolic levels: the role of instructor facilitation in an inquiry-oriented physical chemistry class, Chem. Educ. Res. Pract., 16, 769–785.
- Bodner G. M. and Domin D. S., (2000), Mental models: the role of representations in problem solving in chemistry, Univ. Chem. Educ., 4, 24–30.
- Boukhechem M. S., Dumon A. and Zouikri M., (2011), The appropriation of stereochemical knowledge by Algerian students intending to teach physical sciences, Chem. Educ. Res. Pract., 12, 331–343.
- Bucat B. and Mocerino M., (2009), Learning at the sub-micro level: structural representations, in Gilbert J. K. and Treagust D. (ed.) Multiple representations in chemical education: models and modeling in science education, Dordrecht: Springer, pp. 11–29.
- Carlisle D., Tyson J and Nieswandt M, (2015), Fostering spatial skill acquisition by general chemistry students, Chem. Educ. Res. Pract., 478–517.
- Carroll J. B. (1993). Human Cognitive Abilities: A Survey of Factor-Analytic Studies, Cambridge, England: Cambridge University Press, pp. 304–363.
- Chittleborough G. and Treagust D., (2008), Correct interpretation of chemical diagrams requires transforming from one level of representation to another, Res. Sci. Educ., 38, 463–482.
- Coleman S. L. and Gotch A. J., (1998), Spatial perception skills of chemistry students, J. Chem. Educ., 75, 206–209.
- Cook M. P., (2006), Visual representations in science education: the influence of prior knowledge and cognitive load theory on instructional design principles, Sci. Educ., 90, 1073–1091.
- Copolo C. F. and Hounshell P. B., (1995), Using three-dimensional models to teach molecular structures in high school chemistry, J. Sci. Educ. Technol., 4, 295–305.
- de Jong T., Ainsworth S., Dobson M., van der Hulst A., Levonen J., Reimann P., et al. (1998), Acquiring knowledge in science and mathematics: the use of multiple representations in technology-based learning environments, in van Someren M. W., Reimann P., Boshuizen H. P. A. and de Jong T. (ed.) Learning with multiple representations, Oxford, England: Pergamon, pp. 9–40.
- Dori Y. J. and Barak M., (2001), Virtual and physical molecular modeling: fostering model perception and spatial understanding, Educ. Technol. Soc., 4, 61–74.
- Dumon A. and Luft R., (2008), Naissance de la chimie structurale, Paris: EDP Sciences.
- Ferk V., Vrtacnik M., Blejec A. and Gril A., (2003), Students' understanding of molecular structure representations, Int. J. Sci. Educ., 25, 1227–1245.
- Gilbert J. K., (2010), The role of visual representations in the learning and teaching of sciences: an introduction, in Asia-Pacific forum on science learning and teaching, 11, Issue 1, 22 pages, http://www.ied.edu.hk/apfslt/.
- Gilbert J. K. and Treagust D. F. (ed.), (2009), Multiple representations in chemical education, Dordrecht, Netherlands: Springer.
- Gobert J. D., O'Dwyer L., Horwitz P., Buckley B. C., Levy S. T. and Wilensky U., (2011), Examining the relationship between students' understanding of the nature of models and conceptual learning in biology, physics, and chemistry, Int. J. Sci. Educ., 33, 653–684.
- Goodwin W. M., (2008), Structural formulas and explanation in organic chemistry, Found. Chem., 10, 117–127.
- Graulich N., (2015), The tip of the iceberg in organic chemistry classes: how do students deal with the invisible? Chem. Educ. Res. Pract., 16, 9–21.
- Grosslight L., Unger C., Jay E. and Smith C., (1991), Understanding models and their use in science: conceptions of middle and high school students and experts, J. Res. Sci. Teach., 28, 799–822.
- Habraken C. L., (1996), Perceptions of chemistry: Why is the common perception of chemistry, the most visual of sciences, so distorted? J. Sci. Educ. Technol., 5, 193–201.
- Habraken C. L., (2004), Integrating into chemistry teaching today's student's visuospatial talents and skills, and the teaching of today's chemistry's graphical language, J. Sci. Educ. Technol., 13, 89–94.
- Harle M. and Towns M., (2011), A review of spatial ability literature, its connection to chemistry, and implications for instruction, J. Chem. Educ., 88, 351–360.
- Harlow D. B., Bianchini J. A., Swanson L. H. and Dwyer H. A., (2013), Potential teachers' appropriate and inappropriate application of pedagogical resources in a model-based physics course: a “knowledge in pieces” perspective on teacher learning, J. Res. Sci. Teach., 50, 1098–1126.
- Head J. and Bucat R., (2002), Visualisation and mental manipulation of molecular structures, Aust. J. Educ. Chem., 59, 25–29.
- Head J., Bucat R., Mocerino M. and Treagust D., (2005), Exploring students' abilities to use two different styles of structural representation in organic chemistry, Can. J. Sci. Math. Tech. Educ., 133–152.
- Hegarty M., Carpenter P. A. and Just M. A., (1991), Diagrams in the comprehension of scientific text, in Barr R., Kamil M. L., Mosenthal P. B. and Pearson P. D. (ed.) Handbook of reading research, New York: Longman, pp. 641–668.
- Hegarty M., Stieff M. and Dixon B. L., (2013), Cognitive change in mental models with experience in the domain of organic chemistry, J. Cogn. Psycol., 25, 220–228.
- Hoffman R. and Laszlo P., (1991), Representation in chemistry, Angew. Chem., Int. Ed. Engl.30, 1–16.
- Jaber L. Z. and Boujaoude S., (2012), A macro–micro–symbolic teaching to promote relational understanding of chemical reactions, Int. J. Sci. Educ., 34, 973–998.
- Jones L. L., Jordan K. D. and Stillings N. A., (2005), Molecular visualization in chemistry education: the role of multidisciplinary collaboration, Chem. Educ. Res. Pract., 6, 136–149.
- Jong J. P., Chiu M. H. and Chung S. L., (2015), The use of modeling-based text to improve students' modeling competencies, Sci. Educ., 99, 986–1018.
- Keig P. F. and Rubba P. A., (1993), Translation of representations of the structure of matter and its relationship to reasoning, gender, spatial reasoning, and specific prior knowledge, J. Res. Sci. Teach., 30, 883–903.
- Kênia R. B. S. Oliveiraa, Justi R. and Mendonça P. C. C., (2015), The use of representations and argumentative and explanatory situations, Int. J. Sci. Educ., 37, 1402–1435.
- Khanfour-Armalé R. and Le Maréchal J. F., (2009), Représentations moléculaires et systèmes sémiotiques, Aster, 48, 63–88.
- Koutalas V. G., Antonoglou D., Charistos N. D. and Sigalas M. P., (2014), Investigation of students' ability to transform and translate 2D molecular diagrammatic representations and its relationship to spatial ability and prior chemistry knowledge, Procedia – Social and Behavioral Sciences, 152, 698–703, available online at http://www.sciencedirect.com.
- Kozma R. B. and Russell J., (1997), Multimedia and understanding: expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 34, 949–968.
- Kozma R. B. and Russell J., (2005), Students becoming chemists: developing representational competence, in Gilbert J. K. (ed.) Visualization in science education: models and modeling in science education, Dordrecht, The Netherlands: Springer, pp. 93–120.
- Kumi B. C., Olimpo J. T., Bartletta F. and Dixon B. L., (2013), Evaluating the effectiveness of organic chemistry textbooks in promoting representational fluency and understanding of 2D–3D diagrammatic relationships, Chem. Educ. Res. Pract., 14, 177–187.
- Kuo M. T., Jones L. L., Pulos S. M. and Hyslop R. M., (2004), The Relationship of Molecular Representations, Complexity, and Orientation to the Difficulty of Stereochemistry Problems, Chem. Educ., 9, 321–327.
- Kurbanoglu N. I., Taskesenligil Y. and Sozbilir M., (2006), Programmed instruction revisited: a study on teaching stereochemistry, Chem. Educ. Res. Pract., 7, 13–21.
- Linn M. C. and Petersen A. C., (1985), Emergence and characterization of sex differences in spatial ability: a meta-analysis, Child Dev., 56, 1479–1498.
- Lohman D. F., (1988), Spatial abilities as traits, processes, and knowledge, in Sternberg R. J (ed.) Advances in the Psychology of Human Intelligence, Hillsdale: Lawrence Erlbaum Associates, pp. 181–248.
- Lujan-Upton H. (2001), Introducing stereochemistry to non-science majors, J. Chem. Educ., 78, 475–477.
- McGee M. G., (1979), Human spatial abilities: sources of sex differences, New York: Praeger.
- Mendonça P. C. C. and Justi R., (2014), An instrument for analyzing arguments produced in modeling-based chemistry lesson, J. Res. Sci. Teach., 51, 192–218.
- Michael W. B., Guilford J. P., Fruchter B. and Zimmerman W. S., (1957), The description of spatial-visualization abilities, Educ. Psychol. Meas., 17, 185–199.
- Ministère de l'Éducation, du Loisir et du Sport, (2009), Programme de physique, enseignement secondaire, 2e cycle, Québec, QC: Gouvernement du Québec.
- Ministère de l'Education nationale, (2011), Programme de l'enseignement spécifique et de spécialité de physique-chimie: classe terminale de la série scientifique, B.O.E.N. spécial, 8, 13 October 2011, France: Ministère de l'Education nationale.
- Mulder Y. G., Lazonder A. W. and de Jong T., (2014), Using heuristic worked examples to promote inquiry-based learning. Learn. Instr., 29, 56–64.
- National Research Council, (2012), A framework for K-12 science education: practices, crosscutting concepts, and core ideas, Washington, DC: National Academy Press.
- Olimpo J. T., (2013), Making sense of 2D diagrams: examining how models and modeling impact novice students' development of representational competence in organic chemistry, Doctoral dissertation, University of Maryland, College park.
- Olimpo J. T., Kumi B. C., Wroblewski R. and Dixon B. L., (2015), Examining the relationship between 2D diagrammatic conventions and students' success on representational translation tasks in organic chemistry, Chem. Educ. Res. Pract., 16, 133–142.
- Padalkar S. and Hegarty M., (2014), Models as feedback: developing representational competence in chemistry, J. Educ. Psychol., 107, 451–467.
- Pellegrin V., Sivade A. and Barlet R., (2003), Représentation spatiale des molécules organiques, confrontation entre les représentations graphiques et les représentations des étudiants à l'université, Bull. Union Physiciens, 97, 909–931.
- Pribyl J. R. and Bodner G. M., (1987), Spatial ability and its role in organic chemistry: a study of four organic courses, J. Res. Sci. Teach., 24, 229–240.
- Reiss H. T. and Judd M., (2014), The Handbook of Research Methods in Social and Personality Psychology, 2nd edn, Cambridge University Press.
- Roy P and Hasni A. (2014), Les modèles et la modélisation vus par des enseignants de sciences et technologies du secondaire au Québec, Revue des sciences de l'éducation de McGill, 49, 349–371.
- Schönborn K. J. and Anderson T. R., (2010), Bridging the Educational Research-Teaching Practice Gap, Biochem. Mol. Biol. Educ., 38, 347–354.
- Schönborn K. J. and Bögeholz S., (2009), Knowledge transfer in biology and translation across external representations: experts' views and challenges for learning, Int. J. Sci. Math. Educ., 7, 931–955.
- Seddon G. M. and Shubber S. M., (1984), The effects of presentation mode and colour in teaching the visualisation of rotation in diagrams of molecular structures, Res. Sci. Tech. Educ., 2, 167–176.
- Shepard R. N., (1978), The mental image, Am. Psycol., 2, 125–137.
- Stieff M., (2011), When Is a Molecule Three Dimensional? A Task-Specific Role for Imagistic Reasoning in Advanced Chemistry, Sci. Educ., 92, 310–336.
- Stieff M. and Raje S., (2010), Expertise algorithmic and imagistic problem solving strategies in advanced chemistry, Spat. Cogn. Comp., 10, 53–81.
- Stieff M., Bateman R. and Uttal D., (2005), Teaching and learning with three-dimensional representations, in Gilbert J. K. (ed.) Visualization in science education, Dordrecht, Netherlands: Springer, pp. 93–120.
- Stieff M., Hegarty M. and Dixon B. L., (2010), Alternative strategies for spatial reasoning with diagrams, in Goel A. K., Jamnik M. and Narayanan N. H. (ed.) Diagrammatic representation and inference, Berlin: Springer, pp. 115–127.
- Stull A. T. and Hegarty M., (2015), Model manipulation and learning: fostering representational competence with virtual and concrete models, J. Educ. Psychol., advance online publication: http://dx.doi.org/10.1037/edu0000077.
- Stull A. T., Hegarty M., Stieff M. and Dixon B., (2010), Does Manipulating Molecular Models Promote Representation Translation of Diagrams in Chemistry? in Goel A. K., Jamnik M. and Narayanan N. H. (ed.) Diagrammatic representation and inference, Berlin: Springer, pp. 338–344.
- Stull A. T., Hegarty M., Dixon B. and Stieff M., (2012), Representational translation with concrete models in organic chemistry, Cogn. Instruc., 30, 404–434.
- Stull A. T., Barrett T. and Hegarty M., (2013), Usability of concrete and virtual models in chemistry instruction, Comput. Hum. Behav., 29, 2546–2556.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14, 156–168.
- Treagust D. F. and Tsui C.-Y. (ed.), (2013), Multiple representations in biological education, Dordrecht, Netherlands: Springer.
- Tuckey H. and Selvaratnam M., (1993), Studies involving three-dimensional visualization skills in chemistry: A review, Stud. Sci. Educ., 21, 99–121.
- Tuckey H., Selvaratnam M. and Bradey J. D., (1991), Identification and rectification of students difficulties concerning three-dimensional structures, rotation and reflection, J. Chem. Educ., 68, 460–464.
- Voyer D., Voyer S. and Bryden M. P. (1995), Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables, Psychol. Bull., 117, 250–270.
- Warfa A. M., Roehrig G., Schneider J. L. and Nyachwaya J., (2014), Collaborative discourse and the modeling of solution chemistry with magnetic 3D physical models – impact and characterization, Chem. Educ. Res. Pract., 15, 835–848.
- Won M., Yoon H and Treagust D. F., (2014), Students' Learning Strategies With Multiple Representations: Explanations of the Human Breathing Mechanism, Sci. Educ., 98, 840–866.
- Wu H.-K. and Shah P., (2004), Exploring visuospatial thinking in chemistry learning, Sci. Educ., 88, 465–492.
- Wu H., Krajcik J. S. and Soloway E., (2001), Promoting conceptual understanding of chemical representations: students' use of a visualization tool in the classroom, J. Res. Sci. Teach., 38, 821–842.
| This journal is © The Royal Society of Chemistry 2016 |