Investigating students' engagement in epistemic and narrative practices of chemistry in the context of a story on gas behavior
A.
Pabuccu
*a and
S.
Erduran
b
aDepartment of Secondary Science and Mathematics Education, Faculty of Education, Abant Izzet Baysal University, No: 306, Bolu, 14280, Turkey. E-mail: aybuke@ibu.edu.tr; Fax: +90-374-253-4641; Tel: +90-374-254-1000
bDepartment of Education and Professional Studies, Faculty of Education and Health Sciences, University of Limerick, Ireland
First published on 13th April 2016
Abstract
This study investigated secondary school students' engagement in epistemic and narrative practices of chemistry in the context of a chemistry story on gas behavior. Argumentation is an example of an epistemic practice in science and stories are one kind of narrative (Ricoeur, 1981). By using a chemistry story, the authors hoped to engage students in the argumentation processes by linking chemistry knowledge to everyday contexts (Erduran and Pabuccu, 2012). Student group discussions and written frames during the activity were used as data sources. Analysis of these student outcomes concentrated on (a) the nature of the students' discourse; (b) the quality of students' argumentation; and (c) students' conceptual understanding of gas behaviors. The authors categorized the nature of group discourse using five different codes, determined the quality of student argumentation by counting the number of rebuttals, and measured conceptual understanding through students' answers in the writing frames. The results of this study add to the literature seeking to understand how to develop students' engagement in the argumentation process, how to enhance the quality of students' argumentations, and how to improve their conceptual understanding of gas behaviors.
Introduction
Contemporary science education places strong emphasis on ‘scientific reasoning’ and ‘scientific literacy’. The broader research contexts for these issues are the notions of epistemic and narrative practices of science. Epistemic practices include the articulation and evaluation of knowledge, coordination of theory and evidence, making sense of patterns in data, and holding claims accountable to evidence and criteria (Sandoval et al., 2000; Erduran and Garcia-Mila, 2015). Argumentation is an example of an epistemic practice in science. Engaging pupils in argumentation positions the students to collect interpret and evaluate evidence for the claims that they can construct. In this respect, science learning becomes aligned with how scientists themselves “do” science. Science is not about cookbooks where procedures are replicated mindlessly (Erduran, 2007). Manipulation of variables for the sake of verifying already known outcomes is also not scientific in nature. Authentic scientific enquiries would allow for the generation of evidence and justification of scientific knowledge in the classroom. They would create room for pupils not only to generate and evaluate evidence but also to establish the criteria and standards by which to judge evidence in the social environment of the classroom. Authentic scientific enquiries would have argument at their core (Erduran, 2007; Erduran and Msimanga, 2014). Participation in argumentation develops students' communication skills, metacognitive awareness, and their understanding of science and scientific literacy (Cavagnetto, 2010). In this respect, argumentation as the coordination of theory and evidence through justifications and reasons (Erduran and Jimenez-Aleixandre, 2008) achieves the goals of contemporary science education (Driver, et al., 2000). Many researchers have investigated the features of learning environments that support argumentation (Duschl and Osborne, 2002; Jimenez-Aleixandre, 2008; Cetin, 2014; Erduran et al., 2015; Xolocotzin, et al., 2016; Erduran and Kaya, in press).Despite the wealth of research for argumentation in science classrooms (NRC, 2000; Kelly and Takao, 2002), designing learning environments to promote argumentation still presents challenges at the classroom level. To address some of these challenges, we designed a series of stories related to chemistry as the basis for student discussions. This study details how epistemic and narrative practices of chemistry can be integrated into chemical education in the context of a story.
Narrative is regarded as one of the most appropriate pedagogical approaches for the teaching and learning of science at all levels of education (Bruner, 1990). For instance, Bostrom (2008) used narrative as a tool in designing chemistry curriculum to make abstract chemistry more meaningful. The students in his study appreciated the use of narratives as meaning-making activities to help them grasp the abstract subject. Stories are a sub-set of narrative (Ricoeur, 1981). They are used every day as a way to make sense of and communicate events in the world. Everyday conversations are filled with the telling of stories and have a significant effect on influencing people's understandings (Schank, and Berman, 2002). Thus, many researchers have used the storytelling approach in science education (Strube, 1996; Folino, 2001; King et al., 2008). For instance, with funding from the United States National Science Foundation, thirty historical short stories were designed to teach science content and draw students' attention to the nature of science (Clough, 2011). Wiebe and Stinner (2010) used a story to help students' understand gas behavior. They found that using a story gave students an increased appreciation for the nature of science and helped them with conceptual understanding. Dinan (2005) designed case study-based laboratories offering an exciting alternative to traditional cookbook laboratories. The results of his study showed that using case study based laboratory instruction both improved the quality of that instruction and students' interest in the laboratory work.
Even though scientific reasoning and scientific literacy are fundamental in learning science, teachers and students face challenges trying to acquire and develop these skills. This paper examines the links between scientific reasoning and scientific literacy and offers a way of combining them for maximum effectiveness of teaching chemistry in the context of chemistry of gas behavior.
Research studies show that a significant number of college and high school students have difficulty in understanding the behavior of gases and they lacked appropriate daily life experience (Erduran and Pabuccu, 2015). However, there are not enough studies in the literature that focus on interventions to improve students' conceptual understanding of gas behaviors. There are only few studies that used argumentation pedagogy to promote students' conceptual understanding of the concept of gases (Aydeniz et al., 2012). In an attempt to make contribution to the existing literature, this study suggest that (1) argumentation could help students to develop a better understanding of the behavior of gases and (2) the use of the story format was useful to help students make connection between gases knowledge and the daily life. Moreover, this study fills the gap in the literature by exploring the impact of students' conceptual understanding of gas behavior on their engagement in the argumentation process.
Methodology
The study was guided by the following research questions:(1) What is the nature of students' discourse in the context of a chemistry story?
(2) What is the quality of student argumentation in the context of a chemistry story?
(3) How does students' conceptual understanding of gas behavior affect their engagement in the argumentation process?
Collectively, these questions address how secondary school students engage in epistemic and narrative practices of science in the context of a chemistry story.
Data sources
Verbal and written data were collected from two 9th grade high school classes led by the same teacher. The teacher was a chemistry graduate with 18 years of teaching experience. The school was a special public high school located in Europe, and had a student body population of 744. Prior to data collection, special permission for this research was obtained from the school principal and the teacher as well as the parents/guardians of the students. Students were provided with the information about the purpose, content, and expected duration of the activity before consenting to be involved in the study. They were assured about the confidentiality of all the records. The age range of the students in each class was from 15–16 years old. The first class had 25 students and the second class had 22 students. Students in each class were randomly assigned to four-and five-person groups (5 groups per class). Each group was asked to work through the same gas behavior activity for two class hours. Audiotape recorders were placed in the middle of each group to capture their talk and determine the nature of their argumentation. The teacher had not received any professional development or training on argumentation, and both the students and teacher were not generally familiar with argumentation as a strategy for science education.Classroom intervention
For the activity, students needed to have prior knowledge about air pressure and the behaviors of gases. These concepts had been covered as part of the regular curriculum in the chemistry course. The approach in the design of the activity was to motivate students by linking their chemistry knowledge to everyday contexts through the use of imaginative and creative stories (Erduran and Pabuccu, 2012). The story for this activity described strange incidents related to gas behavior that occurred when a group of teen-agers were having a party in a chalet during Halloween (see Appendix). While discussing this story, all of the ensuing group work audiotaped. The story was accompanied by a writing frame including discussion questions. Each group had a block of two class hours to complete the activity. Writing frame required students to evaluate the evidence presented on cards to construct their argument. The learning goals for the students were for them to evaluate evidence and arguments while providing justification for what they believe.Data analysis
Data analysis contains three major parts: (1) Characterizing the nature of students' discourse; (2) Assessing the quality of students' argumentation; and (3) Identifying students' conceptual understanding of gas behavior. The following paragraphs describe each of these analyses in more detail.The nature of students' discourse
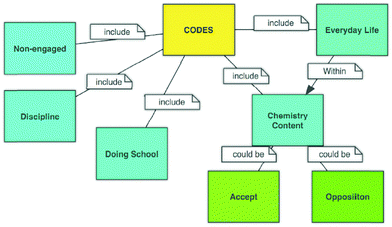
We used five codes to organize students' discourse patterns in the discussion groups: (1) “Non-engaged”, (2) “Discipline”, (3) “Doing school”, (4) “Chemistry content”, (5) “Everyday life” (see Fig. 1). The first three of the codes were pre-determined theoretical categories developed by other researchers (Jimenez-Aleixandre et al., 2000; Gillies and Khan, 2009). The remaining two codes (“Chemistry content” and “Everyday life”) were developed by the authors of the paper based on other researchers' accounts that utilized a grounded theory approach on iterative reading of transcripts of group discussions (Patricia and Barry, 1986). Each author coded the transcripts independently and then they compared their codes. The inter-rater reliability for the coding was 94% (Cohen's kappa = 0.93) and the remaining 6% of variance between the coders was resolved through discussion. Descriptions and examples of discourse for each code are provided below. Transcriptions have been translated to English for the purpose of publication.Non-engaged
This code was used when students were not engaged in conversations related to the activity. Topics included, but were not limited to, food, entertainment, and extra-curricular activities. For example:Student A: What time does the match start?
Student B: Around 6 am.
Discipline
This code was used when one group member disciplined another group member to focus attention during the activity (Gillies and Khan, 2009). Within groups, it is possible for members to be a source of aggravation to other members because they are distracted, off-topic, indifferent, or just personally disliked. It is important to consider how peers handle their frustrations (Salomon and Globerson, 1989; Alexopoulou and Driver, 1996). For example:Student C: Will you please shut up?
Student D: We still have two more questions to finish!
Doing school
This code was used when students signaled engagement in necessary classroom behaviors related to completing the activity or other aspects of the classroom environment. Classrooms are complex settings where any number of interaction dynamics takes place (Jimenez-Aleixandre et al., 2000). Jimenez-Aleixandre et al. (2000) developed this code while exploring the capacity of students to develop and assess arguments during a high school genetics instructional sequence. Their study distinguished discourse of doing science and doing school (Bloome et al., 1989) and reported which was more dominant in the classroom. Examples of “doing school” code are provided in Table 1.| Verbal data | Doing school |
|---|---|
| Everyone say their idea about the question | Classroom task |
| It is my turn to read | Classroom task |
| I think we don't write enough for this question | Rules for task |
| We have to write all of our thinking respectively | Rules for task |
Chemistry content
We interpret “Chemistry content” as accepting or opposing science claims proposed by other group members. We divided Chemistry content code accordingly into subcategories “Accept” and “Opposition” (see Fig. 1). Students tend to easily accept the claim of the first student who offers an answer without questioning the accuracy of their answer. Therefore, the presence of rebuttals, which requires students on both sides of the argument to justify and refute claims with evidence, can be a measure of more in-depth conversational engagement. In this study, we concentrated on how students argued with each other during the activity related to “Chemistry content”.The “Accept” code was modified from Barron's (2003) study. It includes agreeing with the first speaker's claim outright or reinforcing the claim with additional data or warrants. An example of this code is:
Student A: The lid of the oil can shot off when heated because the speed of the gas molecules increased.
Student B: Yeah.
Student C: Yes. I'm agreeing with you.
Another example for the “Accept” code is when students support the first claim by providing warrants. It also may be evidence of students' misconceptions.
Student D: I think the oilcan collapses on itself because the decrease in temperature decreases the volume of the gas molecules.
Student E: Yeah, it's right dudes.
Student F: When the temperature is increased, the volume increases.
Student G: Yeah, because that is the ideal gas law PxV = nxRxT.
For “Opposition” code, we searched the transcripts to find words such as ‘but’, I don't agree with you' and other signals of disagreement. In the following excerpt the original claim is opposed by a counter-claim without any warrant.
Student A: The oilcan crushed because there were no gas molecules inside.
Student B: That's stupid, you're wrong.
Student A: Why?
Student B: I don't know.
Everyday life
We developed the chemistry story since it is of relevance both to the curriculum and to the everyday experience of the students. For the “Everyday life” code, we counted the number of times students gave an example from daily life to support their claims (Erduran and Pabuccu, 2015).Student F: I think the oilcan would explode. Not the lid, the oilcan would burst. Therefore, I remember something… You know the mineral water in the glass bottle. My mom accidently placed it in a Deepfreeze. After a few days, we heard some noise in the kitchen and we saw the bottle burst. I still don't know the reason why it burst up to this moment I never thought about it before. For some reason, it does appear to be related to this question.
The quality of students' argumentation
Many studies have used Toulmin's Argumentation Pattern (TAP) for analyzing the components of arguments occurring in classroom discourse (Simon et al., 2006; Gray and Kang, 2014; Jun-Young, 2014; Erduran and Pabuccu, 2015). Erduran et al. (2004) found that main difficulty in the application of TAP was the distinction between data and warrants, or warrants and backings. Thus, this problem of ambiguity in TAP was overcome by concentrating on the quality of rebuttals only (Erduran et al., 2004). In this study, we accept the presence of a rebuttal as a significant indicator of the quality of argumentation. Accordingly, rebuttals make an argument sophisticated and complex (Simon et al., 2006). Thus, we focused on the numbers of rebuttals in the verbal transcripts and the written frames. Two researchers separately analyzed the same data for cross-comparison. The inter-rater reliability for the coding of rebuttal was 93% and the disagreements arising during the analysis were resolved later through discussion. An example of TAP analysis from Group 4 students' written frames is as follow:Claim: Oilcan crushed.
Data: Temperature decreases.
Warrant-1: When temperature decreases, the volume of gas molecules inside decreases.
Warrant-2: When temperature decreases, the speed of gas molecules inside changes.
Backing: The speed of the gas molecules changes the pressure inside the can.
Rebuttal: The shape of the can would not change if the number of molecules entering the can became equal to the number of molecules leaving the can.
Here, data was explicitly linked with the claim through warrants. In doing so, claim was made in the basic form of a scientific argument (Gray and Kang, 2014). Furthermore, the use of the statement “The shape of the can would not change if the number of molecules entering the can became equal to the number of molecules leaving the can” as a rebuttal creates an opposition to the justification used in the primary argument.
Students' Conceptual Understanding of Gas Behaviors Each group was asked to complete a writing frame together (see Appendix). The questions in the frame were related to explaining why the lid burst and why the oil was crushed by itself and predicting what would happen if the lid of the oilcan were not closed after it was removed from the oven. Given our interest in identifying students' conceptual understanding of the chemistry subject knowledge, we counted the number of correct and wrong evidences in the frames. We gave +1 point for each sentence piece of correct evidence and −1 point for each piece of wrong evidence. We then calculated the total scores of all the groups. Subsequently, we reported the students' misconceptions about the behaviors of gases.
Results
Table 6 displays the total scores of each group's written frames. Group 4 and Group 8 had the highest scores and Group 5 and Group10 had the lowest scores (see Table 7). For the sake of this analysis, the authors chose to compare three research questions only for these four groups to see if there were any patterns that may have affected their final performance.What is the nature of students' discourse in the context of a chemistry story?
For the first research question of this study, we analyzed how often groups initiated different codes. The entire stretch of discourse relating to a code was counted as one code regardless of the number of turns in that same thread. When the conversation switched to another code, that entire new exchange was counted as another code. We did not calculate the percentage of Everyday Life Code because it was counted as part of the Chemistry Content Code (see Fig. 1). Thus, Table 2 shows the percentages of four codes out of the total number of codes for all groups.| Codes | Nonengaged | Discipline | Doing school | Chemistry content |
|---|---|---|---|---|
| G1 | 0.0 | 5.0 | 55.6 | 39.4 |
| G2 | 1.0 | 4.0 | 70.0 | 25.0 |
| G3 | 2.2 | 1.1 | 52.2 | 44.5 |
| G4 | 0.0 | 0.0 | 60.0 | 40.0 |
| G5 | 8.3 | 13.3 | 54.9 | 23.5 |
| G6 | 10.7 | 10.7 | 55.7 | 22.9 |
| G7 | 3.8 | 3.8 | 54.4 | 38.0 |
| G8 | 0.0 | 1.7 | 49.9 | 48.3 |
| G9 | 0.8 | 12.6 | 46.5 | 40.1 |
| G10 | 18.2 | 1.6 | 55.5 | 24.7 |
Group 10 had the highest percentage (18.2%) of initiating the “Non-engaged” code compared to Group 4, which had no occurrences of that code (see Table 2). Group 4 remained on task the entire class period whereas Group 10 was distracted by topics such as entertainment, and extra-curricular activities. In this case, the reason for the low engagement of these groups could be result from group members' lower conceptual understanding of the gas behavior. Group 5 and 10 got the lowest scores from their written frames (see Table 6).
The “Discipline” code shows the existence of the tension in the groups. This code was very high for the Group 5 (see Table 2). In our study, the students did not receive any professional training on the argumentation and they were not familiar with the argumentation as a strategy for the science education. Thus, during the argumentation process, some students couldn't handle their disagreements with maturity and they tried to discipline another group member to focus attention on the activity and so on.
Almost all groups have higher percentages for “Doing School” code than that of the “Chemistry content” code (see Table 2). It means that students mostly spent their time on talking about how they should do the activity instead of focusing to do it. This result justifies the previous' studies' assumptions. For instance, Jimenez-Aleixandre et al., (2000) reported that students usually preferred to ‘doing school’ instead of ‘doing science’ in the classroom. They found that the students' dialogue mostly dominated by school culture during the argumentation process.
Group 8 had the highest scores (48.3) for the “Chemistry Content” code, (see Table 2). It means that members of Group 8 spent most of their time to talk about the gas behavior. However, it is difficult to say whether the talk was effective or not. For instance, when two people want to understand each other, they should contradict each other (Bachelard, 1940). So, we searched the transcripts if students contradict each other, and we classified the science talking as “Accept” and “Opposition”. We used “Accept” code if the students agreeing with the first speaker's claim outright and “Opposition” code if they were against to each other. As it is seen in the Table 3, all groups (except Group 4) had higher percentages for “Accept” code than that of “Opposition” code. That is, most students tended accept the first claim instead of opposing to it.
| Chemistry content code | Accept | Opposition |
|---|---|---|
| G1 | 82 | 18 |
| G2 | 77 | 23 |
| G3 | 80 | 20 |
| G4 | 40 | 60 |
| G5 | 88 | 12 |
| G6 | 69 | 31 |
| G7 | 80 | 20 |
| G8 | 70 | 30 |
| G9 | 69 | 31 |
| G10 | 74 | 26 |
Other data around this activity relate to the use of everyday life examples during students' discussions. Group 4 students gave the highest number of examples from their everyday life to justify their claims. However, most of the students rarely could link the gases knowledge to the everyday life. The fact that our activity managed to engage them to make links to their everyday lives is encouraging.
What is the quality of student argumentation?
For the second research question of this study, we accept the presence of a rebuttal as a significant indicator of the quality of argumentation. For the verbal data, we focused on the presence of the rebuttals in the episodes of opposition. As we mentioned before, most students tended accept the first claim instead of opposing to it (see Table 3). If we look at the episodes of opposition in detail, we can see that many claims were opposed by just a counter-claim without any rebuttal. Table 4 illustrates the percentages of the episodes with or without rebuttals for the episodes of opposition. For instance, for Group 1, Table 3 shows that 82% of Chemistry Content Code was classified as Accept Code and 18% as Opposition Code. Then, Table 4 presents that only 8% of the episodes of opposition at Group 1' discussion contained rebuttal and 10% of it contained just counter-claim without rebuttal.| Episodes | Without rebuttal | With rebuttal |
|---|---|---|
| G1 | 10 | 8 |
| G2 | 19 | 4 |
| G3 | 15 | 5 |
| G4 | 28 | 32 |
| G5 | 8 | 4 |
| G6 | 13 | 18 |
| G7 | 7 | 13 |
| G8 | 8 | 22 |
| G9 | 11 | 20 |
| G10 | 23 | 3 |
As seen in the Table 4, each group had different percentages of rebuttals. The highest percentages of rebuttals were observed for Group 4 and Group 8. The students in these groups also gave more examples from daily life to support their claims or disprove the others' claims (see Table 5). That is, we can say that these groups were highly engaged in the argumentation process. Similar to the other studies in the literature, we reported that there were fewer students' discussions contained rebuttals.
| Groups | Number of everyday life code |
|---|---|
| G1 | 1 |
| G2 | 0 |
| G3 | 3 |
| G4 | 10 |
| G5 | 1 |
| G6 | 2 |
| G7 | 0 |
| G8 | 5 |
| G9 | 2 |
| G10 | 1 |
For the written data, we found that Group 4 was the only group that had a rebuttal in the written frame (see Table 6). Also, with the exception of Group 4, we did not find any relationship between the quality of different groups' verbal argumentation and the quality of written argumentation. For instance, Group 8 had the second highest percentage for the episodes with rebuttal (22%) during their verbal argumentation (see Table 4). However, there was no rebuttal in the Group 8's written arguments. Indeed, usually we observed that the students did not write any rebuttals.
| Number of | Correct sentences | Wrong sentences | Rebuttals | Score |
|---|---|---|---|---|
| G1 | 9 | 10 | 0 | −1 |
| G2 | 10 | 10 | 0 | 0 |
| G3 | 10 | 8 | 0 | +2 |
| G4 | 11 | 1 | 1 | +10 |
| G5 | 8 | 13 | 0 | −5 |
| G6 | 8 | 10 | 0 | −2 |
| G7 | 8 | 9 | 0 | −1 |
| G8 | 12 | 6 | 0 | +6 |
| G9 | 10 | 11 | 0 | −1 |
| G10 | 4 | 13 | 0 | −9 |
How does students' conceptual understanding of gas behavior affect their engagement in the argumentation process?
For the third research question of this study, we counted the numbers of correct and wrong evidence in the frames. We gave +1 point for each of the correct evidence and −1 point for each wrong-evidence. The total scores of the groups were given at Table 6. Additionally, the classification of the groups in terms of their scores is presented at Table 7.| Groups | Score | |
|---|---|---|
| G4 | +10 | Successful |
| G8 | +6 | |
| G3 | +2 | Moderately successful |
| G2 | 0 | |
| G7 | −1 | |
| G9 | −1 | |
| G1 | −1 | |
| G6 | −2 | |
| G5 | −5 | Unsuccessful |
| G10 | −9 | |
Furthermore, we reported two misconceptions concerning gas in the written frames. They were the followings: “Gas particles expand as the temperature increases” and “the power of atmospheric pressure on the oilcan changes as the outside temperature increase” (see, Table 8). Unfortunately, almost all students (except Group 4 members) had these two misconceptions.
| Misconceptions | Gas particles expand as the temperature increases | Power of air pressure changes as heating the can |
|---|---|---|
| G1 | + | + |
| G2 | + | + |
| G3 | + | + |
| G4 | − | − |
| G5 | + | − |
| G6 | + | + |
| G7 | + | − |
| G8 | + | + |
| G9 | + | − |
| G10 | − | + |
We found that the numbers of the rebuttal in the more successful groups' transcripts were higher than that of the others' and vice versa. Moreover, the most successful group of the study was the only one has rebuttal in its written frame. Also, this group (Group 4) had not contained any misconception in its frame. Lastly, we recorded that the students from the more successful groups (Group 4, Group 8) gave more examples from daily life to support their claims (see Table 5). We found that students used the daily life examples to convince the other students who even had the scientifically correct idea. Although we determined 16 instances used scientifically incorrect statements to support claims, none of the Group 4 and Group 8 students misinterpreted the daily life examples to support their claim.
That is, we suggested that; (1) the students' level of understanding on gases was related with the quality of their verbal argumentation; (2) the students who could correctly link the chemistry knowledge to everyday life had more understanding about the concepts of gases than the others; and (3) there is no relationship between the groups' quality of the written argumentation and their level of understanding of gases concepts.
Conclusions
The conclusions focus on three themes in terms of how the results of this study add to the literature seeking to understand; (1) how to develop students' engagement in the argumentation process; (2) how to enhance the quality of students' argumentations, and (3) how to improve their conceptual understanding of gas behaviors.Scientific arguments are important because they expose the justification for belief in the scientific worldview (Osborne et al., 2004; Erduran and Pabuccu, 2012). However, it is harder to initiate argumentation and argument in a scientific context than in a socio-scientific context (Erduran et al., 2004). Actually, science educators have developed numerous ways to engage students in collaborative scientific argumentation (Sampson and Clark, 2009). However, grouping students and asking them to generate an argument or to evaluate the evidences for a given phenomenon will not always result in a beneficial outcome for students (Sampson and Clark, 2011). A major element important for engaging students in the argumentation processes is establishing effective conditions for argumentative discourse to take place. Thus, the activity presented here is critical in providing teachers with an example guideline for structuring the lessons in ways that support evidence-based reasoning in chemistry instruction.
The results of our study led us to conclude that the unsuccessful groups were lowly engaged in the argumentation activity. There is ongoing debate whether poor engagement in the argumentation process dependent on the lack of general competencies or whether it is results from the insufficient scientific understanding (Erduran and Pabuccu, 2012). Our results support the idea that it is inappropriate to ask students to engage in argumentation around scientific concepts when they lack any prior knowledge. Additionally, it is difficult for students to engage in argumentation unless they have some experience with the scientific statements (Norris and Phillips, 2003). Furthermore, most of the students had difficulty in understanding the behavior of gases and they lacked appropriate daily life experience (Erduran and Pabuccu, 2015). Thus, we suggest teachers to investigate whether or not their students are familiar with gas behavior before teaching argumentation-based activities.
Our approach in the design of the activity was to promote students' argumentation by linking chemistry knowledge to everyday contexts through the use of story (Erduran and Pabuccu, 2012). Fortunately, our activity managed to engage students to make links to their everyday lives. It is also encouraging to see that the group that had the highest score and the highest quality of argumentation was also able to make more correct link between chemistry and everyday knowledge during their argumentation. Hence, we suggest that if students are given the chance to make connections between chemistry knowledge and everyday life, their engagement in the argumentation and chemistry lessons could potentially improve.
The result of the study, consistent with some previous studies reported that the level of group members' commitment to accomplish a task (John-Steiner, 2000) and the ways group members interact with each other were important factors for the group outcomes (Gillies and Khan, 2009). Before this study, there was little interaction among the students in the classroom. Thus, during the argumentation process, some students couldn't handle their disagreements with maturity and they tried to discipline another group member. Thus, in order to promote students' engagement in the argumentation, we need to provide an opportunity for students to articulate their thinking and listen to others by working in small groups and to learn how to handle their disagreements. As such, these issues concern the establishment of particular social and cultural norms that surround effective implementation of argumentation. For instance, the ability to listen is an important one in engaging in counter-argumentation with others.
Our data indicate that students mostly spent their time on talking about how they should do the activity instead of focusing to do it. This result justifies other researchers' observations (Jimenez-Aleixandre et al., 2000). It's been pointed out that one of the obstacle to talking science in the science classrooms is the procedural displays, meaning students spend a great deal of time in managing procedures and not engaging in ideas. Thus, we should ask ourselves how to move classroom discourse away from the procedural displays that make up the routines and rituals of doing school (Jimenez-Aleixandre et al., 2000). The instruction in science education needs to clearly distinguish between doing school and doing science (Hodson, 1992). There is also the need to have a renewed focus in science education on how evidence is used in science for the construction of explanations and the development of an understanding of the criteria used in science to evaluate evidence and make explanations (Osborne et al., 2004).
Unfortunately, we noted that most students tended to accept the first students' claim outright without questioning the accuracy or challenging it with alternative solutions. This is probably because they were not used to questioning the arguments from the authority in the classroom. However, teaching should model to students that science often progresses through conflict and argumentation and this can be exemplified by rebutting any unjustified claims made by the teacher herself. Another observation was that the presence of rebuttals was accepted as a measure of conversational engagement. Thus, during the science talk, teachers should encourage the students to refute the others' claims with evidence (Barron, 2003).
In terms of quality of argumentation, we chose the presence of a rebuttal as a significant indicator of the quality of the argumentation in line with previous studies (Erduran et al., 2004; Erduran and Pabuccu, 2015). For the verbal data, we focused on the presence of the rebuttals in the episodes of opposition. Transcripts of group discussions were examined to determine the number of episodes of explicit opposition in student discourse. Opposition exists in many different forms. For instance, it may simply consist of a counterclaim. However, episodes with rebuttals had higher quality than those without (Erduran et al., 2004). In our study, there were significantly fewer students' discussions had contain rebuttals. The limited use of rebuttals by the students can be attributed to the fact that argumentation does not appear to be a common feature of the science classroom (Sampson and Clark, 2011). That is, teaching should foster the valuing of a certain sense of skepticism, not accepting a proposal outright, but questioning the accuracy of a proposal and challenging it with alternative solutions.
For the written data, we made TAP analysis to find out the number of rebuttals in the written frames. We observed that the students did not prefer to write the rebuttals. It is probably because they are not familiar with writing rebuttals when answering the questions. In addition, we reported that the students' quality of the verbal argumentation was not related with their quality of the written argumentation. However, future research needs to address this relationship. In addition, argumentation potentially enables students to make links between chemistry and everyday knowledge. The making of links could be an important factor for their performance on the argumentation process; and the students who lack any prior knowledge were not able to evaluate the evidence in terms of its relevance in the task.
The study provides some implications regarding the relationship between conceptual knowledge and argumentation skills. Many students have difficulty in understanding the gases concepts (Benson et al., 1993) because of the abstract nature of the concept and inappropriate daily life explanation (Cetin, 2009). Furthermore, we observed that almost all students participated in the study had misconceptions about the behavior of gases. We suggest that, argumentation could help students to address their misconceptions related to the behavior of gases and to develop a better understanding of the topic because in the argumentation process the students were able to apply their knowledge of science concepts to meaningful contexts (Venville and Dawson, 2010).
In making a contribution to the existing literature, we proposed that the use of the story format was useful to help students make connection between gases knowledge and the daily life. Using stories to support students' could be played a major role in bringing about improvements in students' learning. Also, the activity we present here provide guidelines for the incorporation of argumentation in everyday science classes.
In conclusion, this study is important because it will have distinct implications in terms of the development of instructional materials and strategies that would enable science teachers to prepare scientifically literate students who can make evidence-based and justified decisions within a science/technology context by drawing upon their rich scientific knowledge, including an understanding of the concepts, principles, theories, and processes of science (Abd-El-Khalick et al., 1998).
Appendix A
Halloween crush!
It was an unusually cold Halloween day. It was freezing and snow covered the mountains. A few teenagers had rented a chalet in the mountains to have a Halloween party. Because of the heavy snowstorm, they were stuck in the chalet. The teenagers did not care much about this unusual snowstorm because they had brought a lot of food and drinks with them. They started to prepare a big Halloween supper for themselves but after a while they realized that they had not brought any oil or butter with them. Because the chalet had not been used for a long time, there was little in the kitchen. After searching the chalet for a long time, they found one empty olive oilcan in the kitchen. They were upset and stopped preparing supper, eating just cold snacks and sandwiches in front of the fireplace. While they were telling each other horror stories, there was only Jane in the kitchen. Suddenly they heard Jane scream in the kitchen. The teenagers ran to the kitchen where they found Jane to be really scared. But a few seconds later when she saw her friends' fright, she burst into laughter and she cheerfully started to explain what happened. She said she realized there was some frozen olive oil at the bottom of the can, and in order to get rid of this frozen oil, she started to heat the can on the stove. However a few minutes later, because the lid of the can abruptly burst with a loud noise she got frightened and screamed. Another teenager, Eddie, held the oilcan with a piece of cloth and removed it from the stove. He attentively put the lid on the can and left it in the kitchen. After five minutes, while all the teenagers were in front of the fireplace continuing to tell horror stories, they heard some weird noises from the kitchen. But now, there was nobody in the kitchen. They got scared! At last when they plucked up the courage to go to the kitchen, they saw that there was nobody there, but the oilcan had crushed noisily by itself. Now they were really frightened and hugged each other in desperation. Sebastian tried to call them by saying that he knew what had happened!References
- Abd-El-Khalick F., Bell R. L. and Lederman N. G., (1998), The nature of science and instructional practice: making the unnatural natural, Sci. Educ., 82(4), 417–436.
- Alexopoulou E. and Driver R., (1996), Small-group discussion in physics: Peer interaction modes in pairs and fours, J. Res. Sci. Teach., 33(10), 1099–1114.
- Aydeniz M., Pabuccu A., Cetin P. S. and Kaya E., (2012), Argumentation and students' conceptual understanding of properties and behaviors of gases, Int. J. Sci. Math. Educ., 10(6), 1303–1324.
- Bachelard G., (1940), The philosophy of no, Paris: Paris University Press.
- Barron B., (2003), When smart groups fail, J. Learn. Sci., 12(3), 307–359.
- Benson D. L., Wittrock M. C. and Baur M. E., (1993), Students' preconceptions of the nature of gases, J. Res. Sci. Teach., 30, 587–597.
- Bloome D., Puro P. and Theodorou E., (1989), Procedural display and classroom lessons, Curriculum Inq., 19, 265–291.
- Bostrom A., (2008), Narratives as tools in designing the school chemistry curriculum, Interchange, 39(4), 391–423.
- Bruner J., (1990), Acts of Meaning, Cambridge, MA: Harvard University Press.
- Cavagnetto A. R., (2010), Argument to foster scientific literacy, Rev. Educ. Res., 80(3), 336–371.
- Cetin P. S., (2009), Effects of conceptual change oriented instruction on understanding of gases concepts, (Unpublished Doctorate Thesis), Ankara: The Middle East Technical University.
- Cetin P. S., (2014), Explicit argumentation instruction to facilitate conceptual understanding and argumentation skills, Research in Science & Technological Education, 32(1), 1–20, DOI: http://10.1080/02635143.2013.850071.
- Clough M. P., (2011), The story behind the science: bringing science and scientists to life in post-secondary science education, Sci. Educ., 20(7), 701–717.
- Dinan F. J., (2005), Laboratory based case studies: closer to the real world, J. Coll. Sci. Teach., 35(2), 27.
- Driver R., Newton P. and Osborne J., (2000), Establishing the norms of scientific argumentation in classrooms, Sci. Educ., 84(3), 287–312.
- Duschl R. A. and Osborne J., (2002), Supporting and promoting argumentation discourse in science education, Studies in Science Education, 38(1), 39–72.
- Erduran S., (2007), Special editorial: argument, discourse and interactivity, Sch. Sci. Rev., 88(324), 29–30.
- Erduran S. and Garcia-Mila M., (2015), Epistemic practices and thinking in science: fostering teachers' development in scientific argumentation, in Wegeriff R., Kaufman P. and Li L. (ed.) Routledge Handbook of Research on Teaching Thinking, London: Routledge, pp. 388–401.
- Erduran S. and Jimenez-Aleixandre M. P. (ed.), (2008), Argumentation in Science Education: Perspectives from Classroom-Based Research, Dordrecht: Springer, p. 285.
- Erduran S. and Kaya E., (in press), Scientific argumentation and democratic values: an incompatible mix in school science curricula? Theor. Pract.
- Erduran S. and Msimanga A., (2014), Science curriculum reform in South Africa: lessons for professional development from research on argumentation in science education, Education as Change, 18(S1), S33–S46.
- Erduran S. and Pabuccu A., (2012), Bonding chemistry and argument: supporting the teaching and learning of argumentation through chemistry stories. Teacher and Student Resource, retrieved October 4th, 2015, from http://www.bristol.ac.uk/education/news/2012/63.html.
- Erduran S. and Pabuccu A., (2015), Promoting argumentation in the context of chemistry stories, in Eilks I. and Hofstein A. (ed.) Book Chapter in Relevant Chemistry Education, Rotterdam: Sense Publishers, ISBN Hardcover: 9789463001748, pp. 143–161.
- Erduran S., Simon S. and Osborne J., (2004), TAPping into argumentation: developments in the application of Toulmin's argument pattern for studying science discourse, Sci. Educ., 88(6), 915–933.
- Erduran S., Ozdem Y. and Park J. Y., (2015), Research trends on argumentation in science education: A journal content analysis from 1998–2014, International Journal of STEM Education, 2(5), 1–12, DOI:http://10.1186/s40594-015-0020-1.
- Folino D. A., (2001), Stories and anecdotes in the chemistry classroom, J. Chem. Educ., 78(12), 1615–1618.
- Gillies R. M. and Khan A., (2009), Promoting reasoned argumentation, problem-solving and learning during small-group work, Cambridge Journal of Education, 39(1), 7–27.
- Gray R. and Kang N. H., (2014), The structure of scientific arguments by secondary science teachers: comparison of experimental and historical science topics, Int. J. Sci. Educ., 36(1), 46–65.
- Hodson D., (1992), Assessment of practical work, Int. J. Sci. Educ., 14(5), 541–562.
- Jimenez-Aleixandre M., (2008), Designing argumentation learning environments, in Erduran S. and Jimenez-Aleixandre M. (ed.) Argumentation in science education: perspectives from classroom-based research, New York, NY: Springer, pp. 91–116.
- Jimenez-Aleixandre M. P., Rodriguez A. B. and Duschl R. A., (2000), “Doing the Lesson” or “Doing Science”: Argument in high school genetics, Sci. Educ., 84(6), 757–792.
- John-Steiner V. (2000). Creative collaboration, New York: Oxford University Press.
- Jun-Young O., (2014), Understanding the alternative conceptions of pre-service secondary science teachers about tidal phenomena based on Toulmin's argumentation, Int. J. Sci. Math. Educ., 12(2), 353–370.
- Kelly G. J. and Takao A., (2002), Epistemic levels in argument: an analysis of university oceanography students' use of evidence in writing, Sci. Educ., 86, 314–342.
- King D., Bellocchi A. and Ritchie S. M., (2008), Making connections: learning and teaching chemistry in context, Research in Science Education, 38(3), 365–384.
- Norris S. and Phillips L., (2003), How literacy in its fundamental sense is central to scientific literacy, Sci. Educ., 87, 224–240.
- NRC, (2000), Inquiry and the National Science Education Standards, Washington DC: National Academy Press.
- Osborne J., Erduran S. and Simon S., (2004), Enhancing the quality of argumentation in school science, J. Res. Sci. Teach., 41(10), 994–1020.
- Patricia Y. M. and Barry A. T., (1986), Grounded theory and organizational research, J. Appl. Behav. Sci., 22(2), 141.
- Ricoeur P., (1981), Narrative time, in Mitchell W. J. T. (ed.) On Narrative, Chicago: Chicago University Press, pp. 165–186.
- Salomon G. and Globerson T., (1989), When teams do not function the way they ought to, Int. J. Educ. Res., 13(1), 89–99.
- Sampson V. and Clark D. B., (2009), The impact of collaboration on the outcomes of scientific argumentation, Sci. Educ., 93(3), 448–484.
- Sampson V. and Clark D. B., (2011), A comparison of the collaborative scientific argumentation practices of two high and two low performing groups, Research in Science Education, 41(1), 63–97.
- Sandoval W. A., Bell P., Coleman E., Enyedy N. and Suthers D., (2000), Designing knowledge representations for learning epistemic practices of science, Paper presented at American Educational Research Association Conference, New Orleans, LA.
- Schank R. C. and Berman T. R., (2002), The pervasive role of stories in knowledge and action, in Green M. C., Strange J. J. and Brock T. C. (ed.), Narrative Impact: Social and Cognitive Foundations, Mahwah, New Jersey: Lawrence Erlbaum Associates, 287–314.
- Simon S., Erduran S. and Osborne J., (2006), Learning to teach argumentation; research and development in the science classroom, Int. J. Sci. Educ., 28(2), 235–260.
- Strube P., (1996), Chemistry and narrative: Short stories and school chemistry, Research in Science Education, 26(2), 247–255.
- Venville G. J. and Dawson V. M., (2010), The impact of a classroom intervention on grade 10 students' argumentation skills, informal reasoning, and conceptual understanding of science, J. Res. Sci. Teach., 47, 952–977.
- Wiebe R. and Stinner A., (2010), Using story to help student understanding of gas behavior, Interchange, 41(4), 347–361.
- Xolocotzin U., Yeh S.-H. and Erduran S., (2016), Emotional modulation of perspective taking: implications for computer supported argumentation, in Tettegah S. and Espelage D. L. (ed.), Emotions, Technology and Behaviors, Communication of Feelings For, With and Through Media, Elsevier, pp. 3–20.
| This journal is © The Royal Society of Chemistry 2016 |