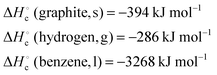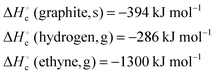Crossword puzzles for chemistry education: learning goals beyond vocabulary
Elizabeth
Yuriev
*,
Ben
Capuano
and
Jennifer L.
Short
Faculty of Pharmacy and Pharmaceutical Sciences, Monash University, 381 Royal Parade, Parkville VIC 3052, Australia. E-mail: elizabeth.yuriev@monash.edu; Tel: +61 3 9903 9611
First published on 30th March 2016
Abstract
Chemistry is a technical scientific discipline strongly underpinned by its own complex and diverse language. To be successful in the problem-solving aspects of chemistry, students must master the language of chemistry, and in particular, the definition of terms and concepts. To assist students in this challenging task, a variety of instructional techniques need to be explored. In this study we have developed crossword puzzles to aid students in mastering chemical terminology via meaningful learning as opposed to rote memorisation. We have evaluated this tool for its effectiveness in study and revision. Specifically, we asked: (i) are crosswords effective as in-class and out-of-class revision tools? (ii) Is crossword performance (as a measure of the command of scientific vocabulary) predictive of problem-solving ability? The results demonstrated that crossword puzzles improve the ability of students to solve problems and, when used systematically, contribute to increases in learning. The findings are discussed in the context of information processing and meaningful learning.
Introduction
The command of scientific vocabulary is commonly categorised as belonging to the “understanding” segment of Bloom's taxonomy (Bloom, 1956) and thus relegated to one of the Lower Order Cognitive Skills (LOCS). Yet, deep understanding/comprehension of terminology is crucial to effective scientific communication and problem solving and forms an indispensable foundation for higher levels of Bloom's taxonomy (such as analysis, evaluation, and synthesis) and for the development of the Higher Order Cognitive Skills (HOCS). In chemistry education, this translates into the goal of achieving broader, deeper and highly interconnected skills in scientific literacy, conceptual understanding, and application of knowledge in the context of chemical problems (Zoller and Pushkin, 2007).Customary introduction of students to chemical terms and concepts is through the presentation of definitions via lectures and/or directed textbook reading. The presentation of terms commonly precedes the discussion of concepts, followed by consideration of problems, cases, and/or scenarios (Middlecamp and Kean, 1988). While this sequence appears logical, students often find it daunting. New terms and concepts are introduced, often very quickly and conveyed through unfamiliar language. On average, 5000 words are spoken in a 50 min lecture, with students registering about 1500 (Johnstone and Al-Naeme, 1991). This results in the overload of working memory (Cassels and Johnstone, 1985) and ultimately may lead to the material being “poorly understood, poorly retained, and frequently confused” (Herron, 1996). In addition, precision in scientific terms may be lost when students use or substitute terms from everyday language, and this can impede the learning and association of scientific concepts. Often the vocabulary of the specific scientific discipline is very context dependent, and this can become lost when new terms are defined rapidly and without the explicit instruction as to their meaning and the limits of that meaning. Despite the importance of a tightly defined discipline-specific scientific language, instructors often consider the introduction of terminology to be an ‘easy’ stage of learning and thus race through it to get to the ‘real stuff’, without realising the difficulties it presents to learners and the importance of these difficulties as the source of misconceptions (Herron, 1996; Luxford and Bretz, 2013). Succinctly put, this “barrier to learning presented by language is worse because it is largely unrecognised” (Childs et al., 2015). Such lack of attention to language comprehension and to deep understanding of scientific terms is disturbing given it has been given considerable attention in educational research literature (Cassels and Johnstone, 1985; Herron, 1996; Gabel, 1999; Johnstone and Selepeng, 2001; Childs et al., 2015).
Language comprehension plays an important role in learners developing a deep understanding of chemical terms and definitions; many learning obstacles and misconceptions are caused when such understanding is not established (Cassels and Johnstone, 1985; Herron, 1996; Schmidt, 1997; Johnstone and Selepeng, 2001; Jasien, 2013; Childs et al., 2015). Briefly, the multifaceted language of chemistry is revealed in its unfamiliar, technical, and specialised nature. Furthermore, it is complicated by potential confusion with ‘everyday’ word usage or alternative meanings, by the use of symbols and Greek and Latin roots and prefixes, and by difficult spelling and pronunciation of words that are often very long. Yet in order to construct and interpret meaning, the language of chemistry must be precise and accurate (Childs et al., 2015).
While difficulties in learning chemistry are often manifested by students struggling with problem solving, their causes are associated with, but not limited to, poor learning of terminology or scientific vocabulary (Jasien, 2013). Thus, the task is clear: chemistry instructors should develop approaches for more efficient teaching of technical language (Herron, 1996; Pyburn et al., 2013; Childs et al., 2015). In the Australian context, such a requirement is actually included in the secondary and tertiary academic standards (ACARA, 2016; Lim, 2013).
Various techniques have been developed for teaching chemical definitions; among them, solving crossword puzzles has been shown to be a useful and engaging approach. In the following sections we will briefly review literature covering prior research on definitions and definitional problems in science teaching as well as the use of crosswords in education, with a focus on chemistry education.
Prior research on definitions in science teaching
Definitions represent an important philosophical and scientific concept (Feynman, 1998). Essentially, they are “explanations of the meanings of words or other bits of language” (Belnap, 1993). They commonly embed a significant amount of knowledge in a form that can be systematically unpacked (Wong et al., 2014). Yet, semantics and definitional problems may encumber effective learning.Ennis (1974) developed a taxonomy of definitions used in science teaching based on their form and/or function. By function, definitions are divided into the following categories: reported (stating how a term is used), stipulative (suggesting a meaning of the term), and programmatic (conveying a value of the term or a position of the definer). The difference between reported and stipulative definitions is subtle and depends on the context and student's level of knowledge of a particular subject matter. By form, definitions are divided into the following categories: classifications (labelling the terms), equivalent-expressions (describing context), synonyms or approximations, range and operational definitions (specifying characteristics and conditions, respectively, justifying the use of the term), and definitions by example. Out of all these forms, the classification, range, and operational definitions are particularly important in chemistry. The issues associated with these forms often lead to definitional problems as discussed below.
When introduced to definitions of chemical terms or concepts, students may experience a range of semantic challenges (Wong et al., 2014 and references therein). These could be associated with either alternative definitions, based on everyday life (e.g., the meaning of the word ‘spontaneous’ in thermodynamics vs. common usage (Hamori and Muldrey, 1984)) or specific discipline or sub-discipline (e.g., the meaning of the word ‘ligand’ for metal coordination vs. protein binding). These challenges represent problems of context. Important for quantitative areas of chemistry, such as its physical or analytical branches, is the problem of precision, which necessitates that technical terms should have not only verbal but also quantitative definitions (Williams, 1999). Other types of semantic problems are those of circularity, when definitions are self-referent, or of incompleteness. Thus inadequacies of definitions (either learnt or taught) could result in alternative conceptions or misconceptions (Wong et al., 2014). Wong and co-workers suggested that problem-based, rather than content-driven (i.e., by introduction of statements), learning of definitions could alleviate the development of alternative or erroneous conceptions.
Prior research on crossword puzzles in chemistry education
In education literature, solving crosswords is often referred to as an effective, engaging, and creative approach for learning terminology as well as an aid for the revision of concepts. Several studies have investigated the use of crosswords in scientific, medical, and humanities education, where they are usually used in a classroom setting or as a revision device (Table 1). In these studies crossword puzzles were found to be useful for (i) revision and reinforcement of concepts (Crossman and Crossman, 1983; Childers, 1996; Shah et al., 2010; Gaikwad and Tankhiwale, 2012); (ii) identifying important topics and gaps in learning (Childers, 1996; Franklin et al., 2003; Weisskirch, 2006; Shah et al., 2010); and (iii) as feedback devices (Shah et al., 2010). Crossword puzzles were also shown to promote and facilitate (iv) student engagement in the learning process (Franklin et al., 2003; Tifi, 2004; Weisskirch, 2006; Shah et al., 2010; Coticone, 2013); (v) collaborative work and development of teamwork skills (Sivagnanam et al., 2004; Weisskirch, 2006; Saxena et al., 2009; Shah et al., 2010); and (vi) critical thinking and association of concepts (Childers, 1996; Sivagnanam et al., 2004). Finally, they were suggested to (vii) provide alternative formative and summative assessment options (Childers, 1996; Franklin et al., 2003; Boyd, 2007) and (viii) ease the anxiety associated with summative assessments and boost student confidence (Crossman and Crossman, 1983; Childers, 1996).| Evaluation | Study design | Purpose | Number of puzzles | Findings | Ref. | |
|---|---|---|---|---|---|---|
| Learning gainb | Student perceptions | |||||
| a Blank cells (—) indicate that no relevant information was available in the cited work. b Yes, significant increase compared to the control group; No, no significant difference in post-test results. c Percentage increase is not reported. d Results were inconclusive. | ||||||
| Chemistry (incl. Medicinal) | ||||||
| Student perceptions (8 items) | — | Active learning activity during lectures | 3 | — |
Enhanced learning
Identification of important topics Good revision |
Shah et al., (2010) |
| Class averages (post-test) | Treatment and control groups (groups with similar central tendencies and dispersion) | Learning of the concept of periodicity via a process similar to solving a crossword puzzle | 1 | Yes | — | Joag, (2014) |
| Biology (incl. Pharmacology, Biochemistry, Pathology) | ||||||
| Student perceptions (8 items and thematic analysis of qualitative comments) | Random representative student groups | Revision tool in conjunction with laboratory classes | 3 | — |
Recall of definitions/terms
Good revision Fun |
Franklin et al., (2003) |
| Puzzle completion; student perceptions (5 items) | Random representative student groups | Revision tool | 2 | — | Enhanced learning | Sivagnanam et al., (2004) |
| Puzzle completion; student perceptions (7 items) | — | Tool to reinforce essential concepts and vocabulary | — | — |
Enhanced learning
Identification of key concepts and vocabulary Collaborative/competitive aspects Enthusiasm for crossword activities |
Saxena et al., (2009) |
| Remembering drug names (pre- and post-tests); student perceptions (10 items) | Treatment and control groups (randomised, two-arm intervention) | Self-learning tool | 2 | Yes |
Enhanced learning
Improved memory and recall Improved problem-solving skills Fun Effective self-learning |
Gaikwad and Tankhiwale, (2012) |
| Exam scores and class averages (post-test); student perceptions (8 items) | Treatment and control groups (previous year results used as control) | Self-made crosswords as an active learning method | 1 | No |
Enhanced learning
Better engagement |
Coticone, (2013) |
| Humanities (incl. Sociology, Psychology) | ||||||
| Student perceptions (14 items) | Random representative student group | Revision tool to reinforce pairing of concepts | 3 | Yesc |
Enhanced learning
Positive change in study habits Improved retention Increased confidence Increased motivation Fun |
Crossman and Crossman, (1983) |
| Student perceptions (open-ended comments) | — | Revision tool to reinforce essential concepts and vocabulary | 1 | — |
Enhanced learning
Identification of topics for further study Increased confidence Fun |
Childers, (1996) |
| Exam scores and class averages (post-test) | Treatment and control groups | Revision tool | 4 | Yes/Nod | — | Davis et al., (2009) |
| Student perceptions (9 items) | — | Revision tool | 2 | — |
Understanding of concepts
Increased confidence Preference for collaborative in-class puzzle solving |
Weisskirch, (2006) |
Crossword puzzles have been previously used in chemistry teaching and learning (such as Snead, 1975; Evans, 1985; Most, 1993; Lee and Tse, 1994; Sims, 2011; Cady, 2012). In addition to the reasons listed above, chemistry educators use crossword puzzles (as well as other educational games) to address the perception that chemistry is a dry subject and to make the material more interesting, while retaining rigour and improving learning outcomes (Russell, 1999; Boyd, 2007). However, to the best of our knowledge, only two studies have been carried out to evaluate the use of crosswords in chemistry education. Shah and co-workers investigated student perceptions and found that the majority of students reported enhanced learning after attempting medicinal chemistry crossword puzzles (Shah et al., 2010). Students also found that puzzles assisted in revision and pointed toward the important topics. Joag (2014) used a crossword puzzle-like approach to teach students about the periodicity concept and reported improved learning outcomes.
Researchers in all of the reviewed studies (Table 1) commented on crosswords being useful tools in their pedagogical arsenals. Some also reported positive student perceptions (Crossman and Crossman, 1983; Childers, 1996; Franklin et al., 2003; Sivagnanam et al., 2004; Weisskirch, 2006; Saxena et al., 2009; Shah et al., 2010; Gaikwad and Tankhiwale, 2012; Coticone, 2013). However, the quantitative findings relating to crossword effectiveness vary quite dramatically. Specifically, Coticone (2013) reported no appreciable effect (80.9 ± 0.5 vs. 79.4 ± 0.3 for treatment and control groups' examination results, respectively), while several studies described significant knowledge gain for treatment and control groups, respectively (33.9% vs. 18.6% (Gaikwad and Tankhiwale, 2012); 29% vs. 11% (percentage gain calculated by us, based on reported results) (Joag, 2014)). In the study published by Davis et al. (2009), some classes demonstrated learning gains after using crosswords, where others exhibited a negative effect.
Research questions
The above survey of the literature demonstrates that most studies have investigated student perceptions rather than the effectiveness of the crosswords (Table 1). Investigations into the use of crossword puzzles for effective revision are limited and somewhat contradictory, and the usefulness of crossword puzzles for improving students' performance of more challenging tasks (such as problem solving) has not been evaluated. In this study we investigated the use of crosswords as a revision tool for its potential not simply to improve students' learning of chemical terms but also their ability to solve chemical problems based on concepts associated with the terms used in the solved crosswords.For revision and self-assessment of knowledge of physical chemistry beyond the recitation of definitions, students were provided with crossword puzzles addressing terms and concepts. Importantly, it should be emphasised that, in the current study, crosswords were not used to introduce students to the relevant vocabulary of physical chemistry. These activities did not represent the initial encounter of the student group with these concepts and they were not intended as the primary/only learning approach. Instead, crossword puzzles were used for consolidation and revision. With this foundation, the findings we present here address the following questions:
(1) Are crosswords effective as in-class and out-of-class revision tools?
(2) Is crossword performance (as a measure of the command of scientific vocabulary) predictive of problem-solving ability?
The second question is related to the hypothesis that the deep understanding of chemical terms is critical for successful problem solving. Therefore, students should approach each problem by first checking that they know the exact meaning of all the terms used and consider the specific physical situation, which is referred to in the problem. There is a significant body of literature dealing with distinctions between an exercise and a problem. For the purpose of this paper, we are using the term problem to indicate either.
Theoretical framework
Issues relating to learning scientific terminology and using it effectively for problem solving could be rationalised using the Information Processing Theory (IPT) (Roberts and Rosnov, 2006; Proctor and Vu, 2012). The three main components of the IPT are sensory memory (or sensory filter), short-term or working memory, and long-term memory. Sensory and working memory act to manage limited amounts of incoming information during initial processing. The long-term memory acts as a permanent storage of knowledge, available for recall. IPT describes the main cognitive structures and processes (i.e., memory systems and strategies) of the learning process and thus is very useful for analysing, explaining, and rationalising the connections between the learning of terminology and problem solving.The term working memory is used to describe a temporary memory space, in which the following simultaneous processes are taking place: meaning-making, linking of elements of new information to each other and to the existing information, and inferring (Spillers et al., 2012). The most common model of working memory was developed by Baddeley (Baddeley and Hitch, 1974; Baddeley, 1992). Efficient cognitive processing in working memory is related to the limited nature of information processing, the ability to perform tasks quickly and efficiently as a result of repeated practice (i.e., automaticity), and selective processing (i.e., the intentional restricting of limited cognitive resources to the most relevant information) (Schraw and McCrudden, 2013). The working memory model has been adopted to explain learning in chemistry (Johnstone and Al-Naeme, 1991; Johnstone, 2000; Johnstone and Selepeng, 2001; Taber, 2003; Taber, 2013; Reid, 2014) and, specifically, for problem solving (Overton and Potter, 2008; St Clair-Thompson et al., 2010; Overton and Potter, 2011; St Clair-Thompson et al., 2012).
The IPT has important implications for instruction. Development of foundational skills (to which deep learning of terminology could be ascribed) is essential as it enables automaticity, which in turn frees available limited processing resources to be used for higher order tasks. These resources are then made available so that students can engage in self-regulation and comprehension monitoring. When encountering new information, students require guidance on linking it to their prior knowledge and creating highly organised knowledge. In order to develop automaticity, students should have multiple and regular opportunities for meaningful practice and support for developing effective learning strategies.
While the IPT and working memory model account for the cognitive processes involved in learning, Ausubel's and Novak's Assimilation Theory (Ausubel, 1978; Novak, 2009) offers an affective and behavioural viewpoint by considering the students' attitudes to and motivation for learning. Specifically, it proposes important conditions required for successful acquisition of deep learning: to be learnt meaningfully, new information should (i) be connected to what a learner already knows (prior knowledge), (ii) be relevant (concepts are significant), and (iii) be actively integrated into a learner's cognitive structure (learner's choice). Thus this theory emphasises the central role of the learner in knowledge construction (Grove and Bretz, 2012; Brandriet et al., 2013). In particular relevance to the present study, this theory makes a distinction between rote and meaningful learning.
Methods
Ethics approval
The study was designed to evaluate tools for scaffolding problem solving and was approved by the Monash University Human Research Ethics Committee. Students were briefed about the objectives of the study and were invited to participate. Students that decided to participate in the research signed written consent forms providing permission for their in-semester and final exam responses to questions to be used in the analysis. The online and in-class crossword solving constituted the in-semester responses. Data from 172 students was available for analysis.Students were also asked for permission to use their Australian Tertiary Admission Rank (ATAR) scores. ATAR is the main criterion for entry into most undergraduate-entry university degree programs in Australia.
Construction of crosswords
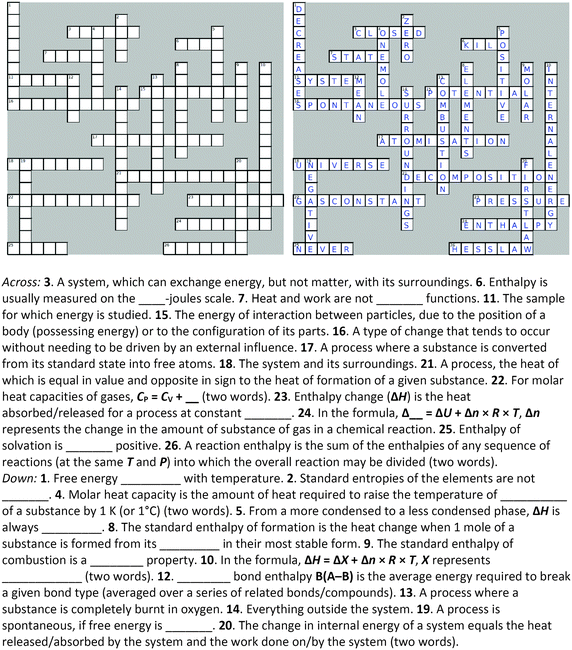
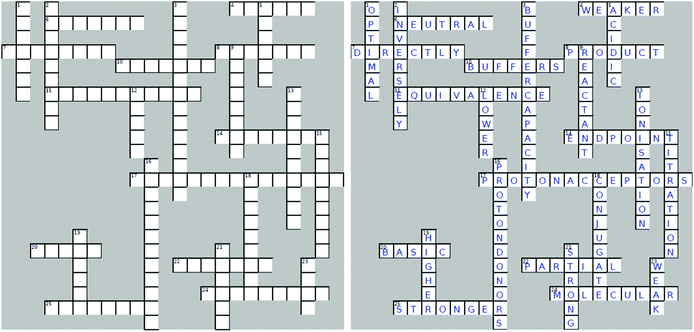
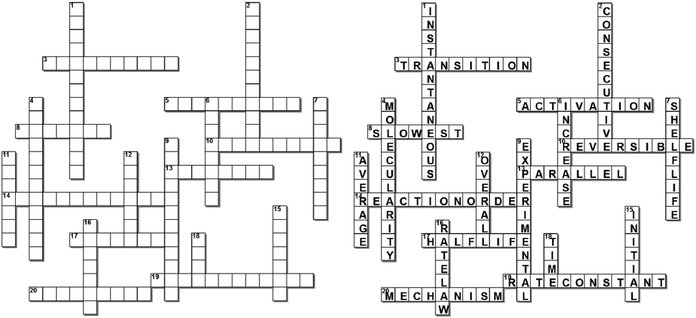
Four crossword puzzles were designed, one for each topic: Ionic Equilibria, Thermodynamics, Phase Equilibria, and Chemical Kinetics (Fig. 1 and Appendices 1–3) within a physical chemistry unit. The clues were constructed around words representing essential concepts and connections between them, and terms and definitions that are required to be easily recalled for use in problem solving. In composing clues, we sought to represent not only the classification category, but also used definitions that could be categorised as equivalent-expressions and operational definitions (by describing context and specifying characteristics and conditions) (Ennis, 1974). The clues varied in difficulty: some were straight definitions (i.e., the clue defines the term) while others represented deconstructed definitions (i.e., the clue is a sentence with a missing word). Some clues were designed to challenge students semantically and to provide an opportunity for students to learn the importance of context when using (scientific) terms (Appendices 4–7). Some of the clues were associated with alternative definitions, based on everyday life. For example, the meaning of the word ‘volatile’ in phase equilibria ((of a substance) easily evaporated at ambient temperature) vs. common usage ((of a person) liable to display rapid changes of emotion). Other terms contrasted the meanings specific for different sub-disciplines. For example, the meaning of the word ‘donor’ for acids and bases vs. coordinate bonding. For more examples of terms with multiple meanings, see Appendices 4–7.In several cases, the same concept was addressed more than once from different angles. For example, in the Thermodynamics crossword (Fig. 1), the clue 16 across alludes to the concept of spontaneity while the clue 19 down probes into the thermodynamic requirements for a spontaneous process. Such complementarity of clues is designed to reinforce the initial learning of terminology as well as facilitate a deeper comprehension of concepts. For more examples of terms with multiple relevant clues, see Appendices 4–7.
The puzzles contained 20–30 clues each. The puzzles were composed by one of the authors (E. Y.) and validated by another (B. C.). Table 2 exemplifies the types of clues and their relationship to problem solving for the Thermodynamics crossword puzzle (Fig. 1).
| Position | Clue | Term | Relevance for problem solving | Problem number from the evaluation sets |
|---|---|---|---|---|
| 22 across | For molar heat capacities of gases, CP = CV + __. | Gas constant | Relationship between heat capacity at constant volume and at constant pressure; concept of heat capacity is required for determining enthalpy. | 1 |
| 23 across | Enthalpy change (ΔH) is the heat absorbed/released for a process at constant ________. | Pressure | Definition of enthalpy; the operational condition (constant pressure) is very important when solving problems involving heat capacities. | 1 |
| 10 down | In the formula, ΔH = ΔX + Δn × R × T, X represents ____________. | Internal energy | Relationship between enthalpy and internal energy | 2 |
| 20 down | The change in internal energy of a system equals the heat released/absorbed by the system and the work done on/by the system. | First Law | Relationship between internal energy, heat, and work | 2 |
| 21 across | A process, the heat of which is equal in value and opposite in sign to the heat of formation of a given substance. | Decomposition | Relationship between decomposition and formation | 3 |
| 9 down | The standard enthalpy of combustion is a ________ property. | Molar | Recognition that enthalpy of combustion is defined as a molar property is required for correct problem solving | 4 |
Context, participants, and design
Students undertaking the Bachelor of Pharmacy and Bachelor of Pharmaceutical Science degrees took part in this study. Both degrees involve a core first year unit of physical chemistry. Note that the required level of conceptual learning in physical chemistry appropriate to these two degree programs is not as advanced as that for science students majoring in chemistry. The prescribed textbook is “Chemical Principles” (Atkins et al., 2013), and the content of the unit is limited to selected sections from the chapters on Thermodynamics, Physical equilibria, Acids and bases, Aqueous equilibria, Electrochemistry, and Chemical kinetics (chapters 8–10, 12–15). A typical fortnightly workload of students involves four one-hour lectures, a one hour problem-solving tutorial, and a two-hour workshop or a three-hour laboratory session. The lectures are delivered in a flipped format (McLaughlin et al., 2016) with regular active learning tasks (White et al., 2015; White et al., 2016).This study exploited a classroom-oriented quasi-experimental design and involved two stages. Initially, crosswords were evaluated for their usefulness as a revision tool and a study aid. Students were given hard copies of the Thermodynamics crossword puzzle (Fig. 1) to solve (20 minutes). The sessions took place two weeks after the thermodynamics lectures, and students were allowed to refer to their notes or textbooks while solving the puzzle. Pre- and post-crossword knowledge was assessed using two sets of four problems (Table 3); students were given 15 minutes for each set. The students did not see the post-crossword problems until after they had completed the crossword. The results of problem set one were not discussed before students undertook set two. Solutions to both problem sets were posted online after the class. Participation was voluntary and anonymous; 57 sets of responses were collected. The pre- and post-crossword evaluation problem sets were of equal difficulty; problems were different but addressed the same concepts. The solved problems were marked as follows: 0 – no attempt, 1 – incorrect, 2 – partially correct with some conceptual understanding, and 3 – completely correct. Problem-solving performance was treated as a dependent measure.
| Problem | Pre-test | Post-test |
|---|---|---|
| 1 |
A sample of nitrogen (112 g) is heated from a temperature of 298 K to 348 K in a sealed container. Calculate ΔH for this process.
C V (N2) = 20.95 J K−1 mol−1. |
Two moles of nitrogen are heated from a temperature of 298 K to 348 K in a sealed container. Calculate ΔH for this process. CV (N2) = 20.95 J K−1 mol−1. |
| 2 |
One step in the production of hydrogen as a fuel is the reaction of methane with water vapour under catalytic conditions (Ni):
CH4(g) + H2O(g) → CO2(g) + 3H2(g) ΔH = −191.7 kJ What is the change in internal energy for the production of 1.00 mol of hydrogen gas? |
Oxygen difluoride is a colourless, very poisonous gas that reacts rapidly with water vapour to produce O2 and HF:
OF2(g) + H2O(g) → O2(g) + 2HF(g) ΔH = −318 kJ What is the change in internal energy for the production of 1.00 mol of hydrogen fluoride? |
| 3 |
Calculate the standard enthalpy of decomposition of hydrogen chloride gas from the following data:
NH3(g) + HCl(g) → NH4Cl(s) ΔH° = −176.0 kJ N2(g) + 3H2(g) → 2NH3(g) ΔH° = −92.22 kJ N2(g) + 4H2(g) + Cl2(g) → 2NH4Cl(s) ΔH° = −628.86 kJ |
Calculate the standard enthalpy of decomposition of hydrogen bromide gas from the following data: NH3(g) + HBr(g) → NH4Br(s) ΔH° = −188.32 kJ N2(g) + 3H2(g) → 2NH3(g) ΔH° = −92.22 kJ N2(g) + 4H2(g) + Br2(l) → 2NH4Br(s) ΔH° = −541.66 kJ |
| 4 | Calculate the standard heat of formation of benzene given the following heats of combustion: | Calculate the standard heat of formation of ethyne given the following heats of combustion: |
After the initial pilot stage, crosswords were evaluated for their usefulness as an ongoing revision tool for self-directed study. All four crosswords (Fig. 1 and Appendices 1–3) were uploaded to the online Virtual Learning Environment website (Moodle) and unlimited access was provided. All students (i.e., including those that chose not to participate in the project) were encouraged to use crosswords for revision and formative self-evaluation outside of the formal scheduled teaching hours. Students were not required to submit completed crossword puzzles, and no credit toward the final mark was given for completion, nor the accuracy of completion.
After the examination period concluded, activity logs were generated for participating students. The logs contained information on how many times each student attempted the crosswords. Number of attempts was treated as a measure of student engagement with learning chemical terminology. The completion of the crosswords was not monitored, therefore each time a crossword was accessed was considered an attempt, although if a student accessed a given crossword more than once on the same date, these attempts were counted as one.
At this stage of the project, we have examined two aspects of crossword implementation: (i) the degree to which the students engaged with the tool and (ii) how efficient it was at assisting with the development of their problem-solving abilities. For the engagement aspect, the number of students undertaking a various number of attempts was collected. The level of engagement as a function of prior academic performance was also investigated. ATAR scores were used as a measure of prior academic performance. For the problem-solving aspect, final examination results were collected. The examination paper contained two sections: a 20-point multiple-choice section and an 80-point written-answer section (calculations [70 points] and a 10-term crossword puzzle with terms drawn from the thermodynamics material [10 points]). Both sections contained questions relating to the topics studied during the semester: ionic equilibria, thermodynamics, phase equilibria, and chemical kinetics. The scores for the crossword puzzle question were used as a measure of the command of scientific vocabulary. Examination results (for the calculation questions) were used as a measure of problem-solving proficiency (treated as an outcome measure).
Results
Evaluating crosswords as revision tools
Fifty seven students attempted two sets of four thermodynamics problems (Table 3), as pre- and post-tests before and after solving the Thermodynamics crossword puzzle (Fig. 1). Each test contained four different problems but addressed the same concepts: heat capacity (Q1), Hess law applied to provided reactions (Q3), first law of thermodynamics and the relationship between enthalpy and internal energy (Q2), and Hess law applied to reaction types (Q4). The results of this evaluation are shown in Fig. 2.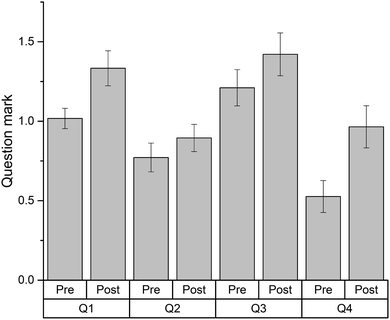 | ||
| Fig. 2 Problem-solving results for thermodynamics problems completed before and after solving the thermodynamics crossword puzzle (Fig. 1). Column heights represent the average mark for each question (n = 57). Highest mark possible is 3 points; error bars represent the standard error of the mean. Pair-sample t-tests for each question (with a Bonferroni's correction to adjust for multiple comparisons) revealed that students performed better in questions 1 and 4 after completing the crossword-based revision (p < 0.0125). | ||
As can be seen in Fig. 2, revising the material with the aid of the crossword effectively improved the students' ability to solve the problem sets. Interestingly, there was no significant gain for question 2, the only question that deals with internal energy. The other three questions deal with enthalpy in different scenarios. Also somewhat unexpected was significant gain for question 4, compared to question 3. In question 4, students had to apply the Hess law to the reactions defined only by their names (i.e. formation and combustion) while, in question 3, they had to apply the Hess law to the reactions they were given. While all these terms were alluded to by the crossword clues, it was surprising to see a better result for what was designed to be a more complex exercise.
The improvements observed may be ascribed to a practice effect, i.e., students doing better on problem set two after attempting set one. However, we would like to emphasise that the solutions to set one were not discussed in class and the answers were not confirmed as correct prior to attempting set two. Therefore, in this case it is more likely that the improvement is due to revision rather than a practice effect.
The common errors made by students when solving these problems are listed in Table 4. Significantly, the most common mistakes appear to be language-related rather than mathematical, even though the solutions to these problems are numeric. Language-related errors were mainly caused by misinterpretation of some words within the questions (Table 4). Some improvements, if marginal, are observed for errors of language-related origin.
| Error | Frequency | Cause | |
|---|---|---|---|
| Pre-test | Post-test | ||
| Question 1 | |||
| Using CV instead of CP | 31 | 27 | Language-related: not interpreting subscripts to signify words “constant volume” and “constant pressure” |
| Using a wrong formula | 10 | 10 | Mathematical |
| Forgetting that heat capacity given is a molar quantity (i.e., not including amount of substance n in the calculation) | 5 | 3 | Language-related: not interpreting the units to signify the word “molar” |
| Question 2 | |||
| Assuming ΔH = ΔU | 26 | 22 | Language-related: not interpreting the word “internal energy” correctly |
| Quoting wrong units in the answer (kJ instead of kJ mol−1) | 3 | 3 | Mathematical |
| Question 3 | |||
| Not calculating a molar property | 17 | 16 | Language-related: not interpreting the word “standard” to mean that 1 mole of a compound should be considered |
| Quoting wrong units in the answer (kJ instead of kJ mol−1) | 3 | 3 | Mathematical |
| Question 4 | |||
| Using a wrong formula | 3 | 1 | Mathematical: several students have applied the “products – reactants” concept using the provided heats of combustion. This concept is only applicable to heats of formation. |
Assessing uptake of crosswords as ongoing revision tools and study aids
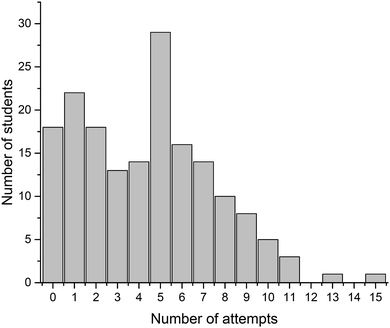
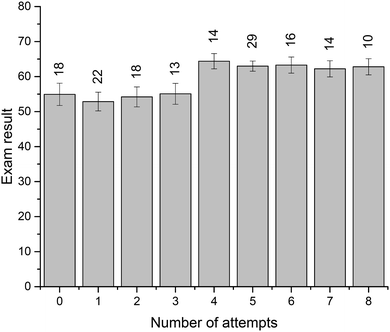
Four crossword puzzles (Ionic Equilibria, Thermodynamics, Phase Equilibria, and Chemical Kinetics) were made available to students on Moodle with unlimited access. Students were encouraged to use crosswords for revision and formative self-evaluation. Fig. 3 demonstrates the distribution of students undertaking a particular number of crossword attempts. These results demonstrate that a significant number of students attempted 1–4 crosswords (67 students, 39%) or 5–8 crosswords (69 students, 40%). Out of those that attempted 1–4 crosswords, most made none or one attempt of each crossword; out of those that attempted 5–8 crosswords, most made one or two attempts of each crossword (data per topic is not shown). Note that this data represents a total number of attempts, i.e., we have not examined when the attempts were made. However, it is reasonable to assume that when repeated attempts were made, these were done for self-assessment during preparation for final semester examinations. The number of students that made nine or more attempts was small (<10 for each number of attempts; 18 students in total) and this data was not analysed further.We were also interested to see whether the provision of crossword puzzles was received differentially by students of different levels of preparedness (i.e. prior academic performance). In order to analyse this, we have divided students into three groups, based on their ATAR scores. The ATAR is a percentile score (from <30 up to 99.95, in increments of 0.05), and represents a student's ranking relative to other students upon completion of their secondary education (TISC, 2014). For example, an ATAR of 95.00 means that the student performed better than 95% of other students.
There were 93 students that had ATAR values available and gave permission to access them: 33 with ATAR < 90, 29 with 90 ≤ ATAR ≤ 95, and 31 with ATAR > 95. We have analysed how many attempts were undertaken by students from each of these three groups (Fig. 4). Not unexpectedly, more students with higher ATAR scores attempted a greater number of crosswords. This relationship is most likely underpinned by their stronger motivation and more competent self-regulation skills, features strongly connected to the concept of meaningful learning as defined by Ausubel's and Novak's learning theory (Ausubel, 1978; Novak, 2009). The reasons explaining why some students only attempted a limited number of crosswords were not explored. Previously, Franklin et al. (2003) investigated this issue. In their study, students stated that they did not engage in crossword solving because they (i) had no time, (ii) were not aware of crossword availability, (iii) did not think crosswords would be useful.
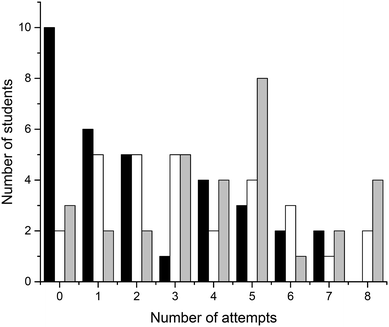 | ||
| Fig. 4 Distribution of students undertaking a particular number of crossword attempts grouped by ATAR (black, ATAR < 90; white, 90 ≤ ATAR ≤ 95; grey, ATAR > 95). | ||
Examining crossword performance as a predictor of problem-solving ability
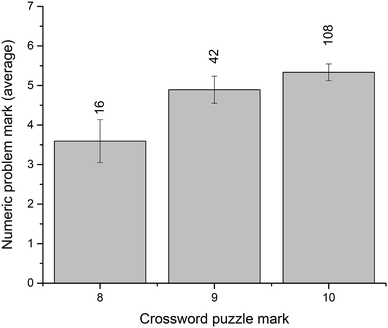
In the context of this study, crossword performance is considered a measure of the command of scientific vocabulary. We were interested to examine whether this command of terminology aligns appreciably with problem-solving ability. To assess this relationship, we have looked at students' exam performance. The total end-of-semester result (all written-answer questions, out of 80 points) vs. the number of in-semester crossword attempts (Fig. 5) shows that students who attempted each crossword more than once (i.e., 4–8 total attempts) had significantly better overall exam results. Specifically, the average result for these students was 63.1 ± 0.9 (n = 83) compared to 54.1 ± 1.4 (n = 71) for those with a smaller number of attempts (p = 0.000). Similar to the ATAR-related observations above, these results are likely related to overall student engagement and other factors, not only their use of crossword puzzles as a revision tool and study aid.To directly evaluate student crossword performance in the context of their problem solving, we have compared the examination results for the thermodynamics questions: crossword puzzle vs. numeric problems (both marked out of 10). The results (Fig. 6) reveal an encouraging relationship between vocabulary performance and problem-solving ability, although it should be noted that most students demonstrated a particularly good crossword performance on the exam, with the majority of the cohort achieving marks of 8, 9, or 10.
Discussion
An important component of the language of chemistry deals with definitions of technical terms and concepts. Chemistry students need to become familiar with a complex and multifaceted chemical vocabulary. This is an ongoing endeavour as the range of terms and concepts grows with each year level and specialisation. Furthermore, to be useful, chemical literacy needs to be active: students should more than just know the terms and concepts, they should be able to apply them in appropriate contexts.This study was guided by two research questions, addressing effectiveness of crossword puzzles as in-class and out-of-class revision tools and as aides for developing problem-solving skills. The study was underpinned by the working memory model and the concept of meaningful learning. In the following sections we will discuss our results within the framework of these theories as well as in the context of other findings in the field.
Importance of terminology for problem solving in chemistry
The delegation of knowledge of terminology, definitions, and classifications to lower level cognitive tasks is based on the supposition that this task only requires recalling of facts and thus is fully based on memorisation. This supposition may hold true if a task of defining is performed in isolation. However, if it is inextricably linked with the expectation that the vocabulary is to be used in the attainment of knowledge and is to form the basis of deeper learning of chemical concepts, then care needs to be taken that this segment of teaching is not dismissed as easy, unnecessary, or unimportant. When terminology and the use of a specific scientific vocabulary is invoked in the process of problem solving, such as in physical chemistry, its cognitive role becomes essential and is inseparably linked to the medium level skill of ‘comprehension’. In many physical sciences, problem solving requires deep understanding of the underlying concepts. Consider these famous quotes: “a problem well put is half solved” (John Dewey) or “if I had an hour to solve a problem I'd spend 55 minutes thinking about the problem and 5 minutes thinking about solutions” (Albert Einstein). Therefore, grasping a problem goes a long way towards finding a solution or solutions. And analysis of a problem requires understanding of all the involved terms and concepts, the context of their usage, and the precise definitions of these terms under problem-specific conditions. Thus, language is crucial for understanding (Childs et al., 2015). This highlights the importance of developing the language abilities of students and the role of instructors and students in understanding the need for exerting a tight control of vocabulary or the usage of the language of chemistry.When dealing with quantitative problems common to physical chemistry, the solutions are usually mathematical. Yet, before a solution is attempted the problem itself must be “translated” from words and symbols into mathematical statements (Hamby, 1990). The words have “mathematical meanings” and their interpretation and recognition of the connection between them (i.e., translation into mathematical expressions) requires a strong command of the appropriate vocabulary. Consider, for example, the pre-test problem 1 (Table 3). Solving this problem requires a skill of interpretation. First comes a conceptual understanding of enthalpy and heat capacity. Specifically, a learner should comprehend that enthalpy ΔH is a heat exchange at constant pressure, not just any heat exchange. This is a definition of the term ‘enthalpy’. Further, the data in this problem contains the molar heat capacity at constant volume CV, which is the amount of heat required to raise the temperature of one mole of a substance by 1 K (or 1 °C) (definition). To succeed in problem solving, a learner is expected to demonstrate understanding of these concepts by organising these main ideas and concluding that to find ΔH, s/he needs to know CP (comprehension). Ignoring the requirement for constant pressure leads to using CV instead of CP, a common error (Table 4). This error is an example of manifestation of the definitional issue of precision (Wong et al., 2014). Proceeding to the higher level task of applying then involves using a mathematical relationship between CP and CV followed by that between ΔH and CP (back to definitions and their mathematical representations). This particular problem is also made more complex by providing a problem solver with molar heat capacity (in units of J K−1 mol−1) and requiring him/her to use it to find ΔH for a sample of a given mass (in units of g [grams]). This complexity is ubiquitous in physical chemistry problems and is a common cause for errors in calculations. Approximately 10% of students (5 out of 57) have made that mistake in our exercise and approximately 5% (3 out of 57) corrected it after the crossword-based revision (Table 4). Understanding units is a low level skill but applying them correctly is certainly not, as this requires a higher level cognitive skill, synthesis.
The above example represents a type of problem that cannot be solved by relying on the use of ‘parroted’ definitions and memorised algorithms/formulas without appreciating the meaning of the underlying concepts. Thus, one of the crucial factors that contributes to the success of problem solving is the relevant knowledge of scientific terms and their definitions (Sumfleth, 1988), the ability to comprehend them in their depth and complexity rather than memorise them by rote, to apply them appropriately with a careful consideration of problem complexities and contributing factors, and to synthesise all the available and required information to propose a plan or a set of operations to lead towards a solution.
The issue of the processes underlying the learning of terminology and its effective application to problem solving is markedly acute in chemical education (Sumfleth, 1988; Lee et al., 2001). Pyburn et al. demonstrated robustly that language comprehension ability is significantly correlated with general chemistry performance (Pyburn et al., 2013; Pyburn et al., 2014). This finding is particularly noteworthy given the common focus on mathematical, rather than language, skills as the important factor in chemical problem-solving success, particularly in physical chemistry (Becker and Towns, 2012; Shallcross and Yates, 2014).
Solving crossword puzzles as active learning
According to Ausubel's and Novak's learning theory (Ausubel, 1978; Novak, 2009), one of the key requirements of effective learning is the learner's choice to learn meaningfully. Using crossword puzzles for learning and revision provides students with opportunities for self-regulation. They can study at the time, pace, and place of their choosing (Evans, 1985). When crosswords are provided online with unlimited access (as implemented in this study), they give students opportunities for regular and meaningful practice – a condition for achieving automaticity, as proposed in the working memory model (Baddeley and Hitch, 1974; Baddeley, 1992; Spillers et al., 2012). Solving crosswords is interactive and the formative feedback is immediate and dynamic. Immediacy is due to the self-correcting features of the puzzles (i.e., the correct solutions should fit into the space and correctly overlap with other solutions) (Saxena et al., 2009). The dynamic nature means that feedback comes in chunks as the problem is being solved, not just at the end. And finally, the activity lends itself to be done either individually or collaboratively (Shah et al., 2010). All these factors contribute to crossword solving being an excellent active learning task to be used either in or out of class (Franklin et al., 2003).There were two types of comments made by students concerning the crosswords available on Moodle. These comments were not captured and analysed quantitatively, but are worth mentioning here. Firstly, some students remarked that they found crossword puzzles (or at least some clues) difficult to solve completely and therefore felt challenged. As a result, they were determined to finish even if it took extra effort. Secondly, some students observed that they were surprised, and reassured, when they found out that they were able to complete the crosswords without consulting their notes or textbooks. In other words, “they knew more than they thought they knew” (Childers, 1996). Interestingly, this latter finding is similar to the comments we received from learners in the FutureLearn Massive Open Online Course (MOOC) “The Science of Medicines” (Galbraith et al., 2014). We have introduced crossword puzzles in that MOOC to provide learners with a way to review their learning.
Solving crossword puzzles as a revision technique
When evaluating the usefulness of crossword puzzles, Gaikwad and Tankhiwale (2012) concluded that crosswords are more effective for revision when compared to simply reviewing notes or reading a textbook. The authors postulated that this effect was due to the students' enjoyment of solving crossword puzzles which improves the likelihood of sustainable use of this learning tool and the associated increase in time-on-task. We agree that the sustainability of the crossword-aided revision is indeed stronger due to the recreational nature of the activity and the enhanced motivation of students to learn meaningfully (Ausubel, 1978; Novak, 2009). However, we consider the effectiveness of such revision to be due not only to these behavioural factors. Contemplating revision in the framework of the Information Processing Theory (Roberts and Rosnov, 2006; Proctor and Vu, 2012), the crossword-aided approach represents information processing which involves both sensory and working memory (see in more detail below), while note-reading uses primarily sensory memory and therefore is not as effective a strategy at promoting the transfer of information into long-term memory systems.In addition, there is evidence that suggests that the consolidation of word meanings is a process that requires time (and possibly sleep) before these meanings are integrated into semantic memory systems, and that the effectiveness of this acquisition may be enhanced when the information is presented in a written format (van der Ven et al., 2015). Humans forget information if they do not make any attempt to retain it (Ebbinghaus, 1885). This loss is exponential, which means that the memory of newly acquired knowledge is halved in a matter of days or weeks unless the learned material is consciously reviewed. Our data indicates that it is the regular revision practice with crosswords (i.e., distributed studying) that is associated with improved problem-solving outcomes. We are being cautious here so as not to claim causality. Firstly, there are many other factors and learning activities experienced by students in the course of a semester, both within and outside a given unit of study, that are designed to improve their problem-solving skills. Secondly, students that regularly use crosswords for self-directed revision are likely to be those with stronger motivation and general engagement, and therefore, with a higher likelihood to demonstrate better exam performance. Franklin and colleagues (2003) have also quantitatively investigated student use of non-compulsory crossword puzzles for first year biology. Based on their findings, they suggested that voluntary crossword puzzles may appeal to more motivated students as well as to students with particular learning styles. Thus, our findings that some of the students embrace optional crossword-solving activities, while others do not, are not uncommon (Franklin et al., 2003; O'Connor et al., 2005; Boyd, 2007). In the future, we are considering changing crossword activities from optional to compulsory with an allocated (albeit small) credit (similar to Boyd, 2007; Pyburn et al., 2014) or keeping them voluntary but attracting extra credit (similar to Childers, 1996). Since these activities would be low-stakes, we are going to follow Boyd's approach and not be concerned about collusion. In fact, if these assessments are going to encourage some form of informal and spontaneous group activities, the positive outcome will be the development of cooperative and critical-thinking skills, which will offset a potential skewing of the grades. In addition to providing additional motivation for students to engage with the material, ongoing assessment is known to produce the ‘testing effect’, i.e. lead to test-enhanced learning (Pyburn et al., 2014).
With the above discussion in mind, we suggest that our evaluation of crosswords is distinctive in that the puzzles were used (voluntarily) throughout the whole semester and covered all topics within a unit of study. Other studies, in which crossword usage was evaluated quantitatively, were restricted to one or a limited number of topics/class sessions (Table 1). The intermittent nature of crossword implementation in these studies may explain the widely varied findings with respect to crossword effect on learning gain. Interestingly, implementing crossword puzzles for all and each topic within a unit of study and a general desire for more crossword puzzle activities were common suggestions expressed by students in many of these investigations (Crossman and Crossman, 1983; Childers, 1996; Franklin et al., 2003; Sivagnanam et al., 2004; Weisskirch, 2006; Saxena et al., 2009; Gaikwad and Tankhiwale, 2012).
Solving crossword puzzles as a learning aid
When definitions are memorised by rote, students are likely to focus on words rather than their meaning (Johnstone and Selepeng, 2001; Grove and Bretz, 2012; Luxford and Bretz, 2013). Furthermore, they may commit to memory incorrect meanings resulting from a change in word order or replacement of some words with inappropriate substitutions (for example, definitions based on ‘everyday’ or common word usage). Thus rote memorisation is not only an unproductive way to learn but is potentially hazardous as it could lead to naïve understandings and misconceptions and potentially lead to confusions between embedded knowledge and newly-encountered definitions. There are many examples in the literature of chemical misconceptions/alternative conceptions that demonstrate that students often cling to ideas already in their cognitive structures and resist new ideas if they conflict with the existing (or, alternative) conceptual frameworks (Taber, 2003).In order to deal with these pitfalls, instructional materials should be designed so as to encourage and motivate students to move from rote memorisation to meaningful learning (Grove and Bretz, 2012). When students solve crossword puzzles, they do not attempt to memorise definitions by simply “regurgitating the answer to a question which they have already seen” (Franklin et al., 2003). Instead they reconstruct the definitions of terms and concepts, thus triggering the recall and embedding the correct meanings into their knowledge structure without rote memorisation. Such learning may seem effortless, however it is well rationalised if one considers it through the Information Processing Theory (Roberts and Rosnov, 2006; Proctor and Vu, 2012). Namely, consideration of the crossword clues engages sensory memory, which is activated when students are presented with interesting or attention catching information (Johnstone and Selepeng, 2001). Next, finding the words to include into the puzzles (“solutions”) is the processing of the information, which activates working memory (Baddeley and Hitch, 1974; Baddeley, 1992; Spillers et al., 2012). As a result, the meaning of a concept or a definition of a term are learnt, i.e. permanently stored into the long-term memory. The role of working memory in information processing is nicely captured by Johnstone and Al-Naeme (1991) who suggested that “the emphasis should perhaps be placed more often on ‘working’ and less on the ‘memory’”.
In addition, filling in a crossword puzzle involves a motor task of writing. Writing down information by hand while studying has been shown to be beneficial for learning (Kiewra, 1989; Mangen and Velay, 2010; Mueller and Oppenheimer, 2014; Smithrud and Pinhas, 2015). Thus, the attendant improvements in learning and memory formation occur when cognitive and motor tasks are performed concurrently.
Crossword-aided learning is also superior to rote memorisation by allowing students to identify gaps in knowledge and develop new connections between concepts. In our crosswords, such connections are reinforced by considering different aspects of the same concept (e.g., clues 16 across and 19 down for spontaneity or clues 18 across and 14 down for surroundings, Fig. 1).
Furthermore, similar to good distractors in multiple-choice questions, well-designed crossword clues provide opportunities to address misconceptions (Schmidt, 1997). For example, a common misconception relating to the strength of acids and bases is that strong acids produce weak conjugate bases, and that weak acids produce strong conjugate bases. This misconception is the result of misinterpreting the statement “the weaker the acid, the stronger its conjugate base”. This misinterpretation is rooted in the reduced language abilities of learners. In order to address this misconception, we introduced the following clue into the Ionic Equilibria crossword (Appendix 1):
The stronger the acid, the ______ its conjugate base.
This clue compelled students to pause and contemplate the relative nature of the strength of the base (i.e., weaker vs. weak).
Lastly, learning definitions meaningfully, rather than by rote, allows students to chunk information and thus make an efficient use of the limited capacity of working memory (Reid, 2014).
Implications for teaching
To be sustainable, educational interventions must be effective, accepted by students, and practical to implement. Crossword puzzles tick all the boxes. Their effectiveness has been demonstrated by us and others, even though it is clear that it depends on many factors such as frequency of use, level of difficulty, and the complexity of clues. The positive and eager acceptance by students of crossword puzzles as a learning and revision tool has been established in numerous studies, and was confirmed by the level of engagement demonstrated in our findings. Finally, the feasibility of implementing crossword puzzles is based on the availability of many free and commercial crossword making programs and online resources (Table 5) and their ease of use. The puzzles could be easily hosted on various virtual learning environment systems. Furthermore, the technical ease of generating crossword puzzles means it is possible to give students a crossword-creating rather than a crossword-solving task. Such a task would require students to deconstruct and thus analyse the definitions for all the important factors. The literature on crossword-making tasks is quite limited but it demonstrates that students often make conceptual errors when composing definitions (Tifi, 2004). Thus, provided students receive timely and constructive feedback on their crossword puzzles, such tasks offer excellent opportunities to address misconceptions.| Resource name | URL |
|---|---|
| Crossword puzzle maker | http://www.armoredpenguin.com/crossword/ |
| CrosswordLabs | http://https://crosswordlabs.com/ |
| Eclipse Crossword | http://www.eclipsecrossword.com/ |
| eduBakery.com | http://edubakery.com/ |
| Hot Potatoes™ | http://hotpot.uvic.ca/ |
| Instant online crossword puzzle maker | http://www.puzzle-maker.com/CW/ |
| Puzzlemaker | http://www.discoveryeducation.com/free-puzzlemaker |
Crossword puzzles should contain clues of varying levels of difficulty. Too many simple clues and students will consider them a waste of time; too many very difficult ones and they may disengage (Childers, 1996; Franklin et al., 2003; Saxena et al., 2009). The clues that are ‘straight’ definitions of terms are easier to solve as students ‘recognise’ the definition they have seen before or look it up (Table 2). While they are easy, such clues serve a purpose of knowledge reinforcement by virtue of practice and they reinstate the correct and precise definition of a term, potentially strengthening and adding weight to the cognitive systems that will promote accurate recall of this term in another context. More difficult are the clues that ‘deconstruct’ the definition or involve verbal or numeric reasoning to solve. The deconstructing/reasoning clues access higher level cognitive processes as students are required not only to recall/look up a definition but also to interpret the relationship between the wording of the clue and the ‘missing’ word (Table 2). Working through such clues allows students opportunities for higher order processing and therefore “turns definitions into models” (Luxford and Bretz, 2013) or concepts.
Knowing what errors students make when solving problems (e.g., Table 4) should also help to create clues/puzzles that proactively address common pitfalls. In order to make such crossword puzzles into more efficient learning tools, instructors may consider convening focus groups or think-aloud interviews to establish why students make these common mistakes. If it is confirmed that the issue arises from a gap in language comprehension then specific strategies could be developed to improve vocabulary and the precise use of language. Such strategies could include tailor-made crosswords or other language-related activities as discussed below.
In our study, the crossword puzzle was also used for assessment. The results (Fig. 6) demonstrated very good vocabulary performance by students which matched up to their problem-solving results. However, if crosswords are used as a learning and revision tool during the semester, we believe that the assessment puzzles could be made more complex to robustly test deep understanding of concepts and to differentiate performance outcomes more finely.
Finally, crossword puzzles are most effective when students can practise with them flexibly and repeatedly. An efficient way to achieve this is by making them available online for students to practise at the time of their choosing. Our results show that this approach leads to a reasonable uptake by students. In our study, 90% of the students (154 out of 172) attempted at least one crossword puzzle. This is in comparison to 77% (Franklin et al., 2003) and 62% (Weisskirch, 2006); in these studies crossword puzzles were provided to students in specific class sessions.
Having presented the use of crossword puzzles as a learning and revision tool, we must note that they should not be the only approach to aid language development of students. Other possible strategies include (i) the use of other tools similar to crosswords (e.g., glossary construction); (ii) multiple-choice questions designed to reinforce the precise use of language in chemistry; (iii) activities focused on accuracy in oral and written communication (abstract writing, presentations, essays, etc.); and (iv) language support classes.
Limitations of the study and future work
Our results indicate that crosswords are an efficient approach for learning and revision and are well adopted by students. However, several limitations of the study and associated caveats should be noted. Firstly, the ‘convenience’ sample was used in an authentic classroom setting, i.e. the cohort of students taught by one of the authors (E. Y.). Due to the vocational nature of the two degrees, the composition of the class may be different to a general chemistry unit. However, we are confident that the fundamental findings described in this study are generally applicable. Secondly, crossword puzzles were only tested for some topics in physical chemistry and only for one cohort of students in a given semester. Further research is needed to investigate the usability of the tool in other areas of chemistry (organic, analytical) as well as longitudinally. Thirdly, this was a field study, where independent variables (such as prior academic ability) were not manipulated. And finally, the learning outcomes and problem-solving abilities of students are likely to be affected by factors outside of the unit of study where crossword puzzles were implemented. Thus we could only observe possible relationships rather than making any claims about cause and effect.The crossword puzzles described here included two types of clues: definition of terms and missing words. These crosswords were designed for students at the very beginning of their university degrees and therefore the clues were deliberately low in complexity so as not to lead to cognitive overload. Designing crossword puzzles with cryptic features or anagrams for students more advanced in their studies would certainly be of interest, particularly after students have been exposed to more simple crosswords in their first semester.
From our work and that of other groups, it is clear that more research into the effectiveness of crossword puzzles is required in order to ascertain their optimal use. Specifically, the following questions should be addressed. What are the appropriate teaching formats: in or out of class, compulsory or optional, individually or cooperatively? What are the most effective durations and the number of practices required for optimal learning? Is solving crossword puzzles more effective than simply doing more numerical problems or revising by building concept maps? What type of crossword puzzles, or other language-related activities, could assist in overcoming persistent conceptual errors?
Our plans for future use of crossword puzzles include encouraging students to devise their own crossword puzzles or just developing crossword clues. Such activities will promote learning of concepts and active engagement with learning.
Conclusions
In this project we have designed a series of crosswords to scaffold study and revision, in order to assist first year Pharmacy/Pharmaceutical Science students in developing their vocabulary and knowledge of the terminology relating to physical chemistry. We have evaluated the usefulness of this strategy with pre-crossword and post-crossword problem-solving tests. This study advances the literature by demonstrating that, when used as a revision tool, crosswords improve students’ ability to analytically approach physical chemistry problems. We have shown that the use of crossword puzzles for ongoing revision contributes to increased learning gains and improved problem-solving skills. Finally, we have rationalised crossword effectiveness for learning and revision in terms of information processing and meaningful learning.Appendix 1: ionic equilibria crossword puzzle
Across
4. The stronger the acid, the ______ its conjugate base.6. Salts of strong bases and strong acids produce _______ solutions.
7. Base strength is _______ proportional to the basicity constant Kb.
8. Strong acid hydrolysis is _______ dominant.
10. Solutions that resist a change in pH when small amounts of strong acid or base are added.
11. The theoretical point in a titration, at which the amounts of the reactants are equivalent.
14. The experimental estimate of the equivalence point in a titration.
17. Bases are molecules or ions that are ____ ______. (two words)
20. Salts of weak acids and strong bases produce _______ solutions.
22. A buffer is prepared by ________ neutralisation, when a limiting amount of strong base is added to an excess amount of weak acid.
24. For good absorption, a substance needs to be in its _________ form to be able to cross cellular barriers.
25. Base A (pKb 3.5) is ________ than base B (pKb 5.2).
Down
1. Level of buffering occurring when the ratio b/a (amounts of base (b) and its conjugate acid (a)) equals 1.2. Acid strength is _______ proportional to pKa.
3. The measure of the amount of protons (or hydroxide ions) a buffer can absorb without a significant change in pH. (two words)
5. Salts of weak bases and strong acids produce _______ solutions.
9. Weak base hydrolysis is _______ dominant.
12. In the titration of a weak base with a strong acid, the pH of the stoichiometric point is _______ than 7.
13. For weak acids and bases, the more dilute the solution the greater its extent of __________.
15. Adding a titrant solution (with an accurately known concentration) from a burette to a flask containing the sample of an analyte.
16. Acids are molecules or ions that are ____ ______. (two words)
18. For a ______ acid/base pair: pKa + pKb = 14
19. A buffer with ________ concentrations of the constituents will have a greater buffer capacity.
21. Acids and bases that are effectively completely ionised in water.
23. Acids and bases that ionise in water to a slight extent (<5–10%).
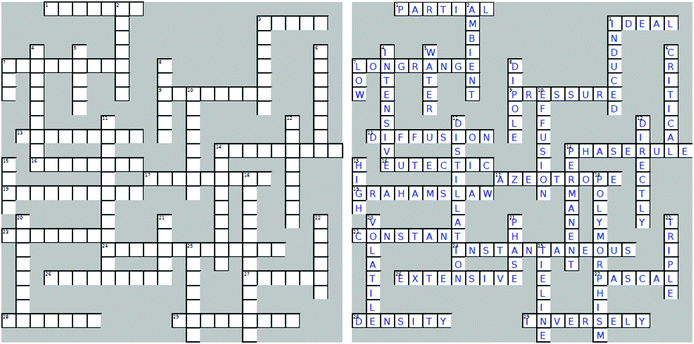
Appendix 2: phase equilibria crossword puzzle
Across
1. The pressure that the gas would exert if it occupied the container alone.2. A gas whose behaviour follows the relationship: PV = nRT.
7. Crystalline solids exhibit ____ ______ order. (two words)
9. Measure of force per unit area.
13. A gradual dispersal of one substance through another substance.
14. F = C − P + 2, where F = degrees of freedom, C = number of components, P = number of phases. (two words)
16. A mixture of two solids, which has a melting point lower than the melting point of either solid.
17. A mixture of compounds that boils with a constant composition.
19. At constant temperature, the rate of effusion of a gas is inversely proportional to the square root of its molar mass. (two words)
23. All systems prepared on a tie line separate into two phases of ________ compositions.
24. Non-polar compounds possess _____________ dipoles.
26. Volume is an _________ property.
27. SI unit of pressure.
28. A unique property of water that causes the negative slope of the solid/liquid boundary on its phase diagram.
29. The volume of a quantity of gas is __________ proportional to pressure at constant temperature.
Down
2. 25 degrees Celsius is a standard ________ temperature.3. A disruption of an electron cloud on a neighbouring molecule leads to _________ dipoles.
4. Temperature is an _________ property.
5. Solids are of greater density than liquids. Exception?
6. The point on a single component phase diagram, where there ceases to be any difference between liquid and gas phases.
7. Real gasses obey The Ideal Gas Law at ___ pressure.
8. Two partially charged particles (or parts of a structure) of opposite sign which are separated by a finite distance.
10. The process of a gas passing through a small hole into a vacuum.
11. The process of separating compounds with different boiling points.
12. The pressure of a fixed amount of gas is __________ proportional to its temperature at constant volume.
14. Polar molecules possess _____________ dipoles.
15. Real gasses behave in ideal manner at ___ temperature.
18. The existence of at least two different crystal structures for the same substance.
20. The greater the vapour pressure, the more __________ the compound and the weaker the intermolecular forces in the liquid.
21. A form of matter that is uniform throughout in both chemical composition and physical state.
22. The point on a single component phase diagram, where three phases can co-exist in dynamic equilibrium.
25. A horizontal line drawn across the region of two phases.
Appendix 3: chemical kinetics crossword puzzle
Across
3. __________ state – a combination of molecules, which is not a molecule in its own right.5. Chemical reactions must go over an energy barrier – __________ energy – before a reaction can take place.
8. Rate-determining step – the _________ elementary step of a multi-step process.
10. Any reaction that can exist at equilibrium is a __________ reaction.
13. A reaction where a reactant either forms a product via two different mechanistic pathways or forms two (or more) different products.
14. Exponents x in a rate law: rate = k[A]x (two words)
17. The time (from the beginning of a reaction) it takes for the concentration of a reactant to decrease by half of its original value.
19. Proportionality constant k in a rate law: rate = k[A]x (two words)
20. Sequence of steps by which a reaction occurs.
Down
1. Rate at a particular moment in time.2. A reaction, in which the product from the 1st step becomes the reactant for the 2nd step, and so on.
4. The number of molecules that come together in an elementary step.
6. Catalysts – species which ___________ the rate of chemical reactions by lowering the activation energy.
7. The time from the manufacture or preparation until the original potency or content of active ingredient has been reduced by 10%.
9. Rate Laws are derived from _____________ observations on the macroscopic level.
11. Rate over a certain time interval.
12. Order reflects the ________ change in going from reactants to products.
15. Rate at the beginning of the reaction.
16. The relationship between the reactant concentrations and the chemical rate. (two words)
18. Rate is a change (in a property) over _________.
Appendix 4: meaning of terms used in thermodynamicsa
| Termb | Everyday language/common usage | Context-specific scientific language | Clue(s) (Fig. 1) |
|---|---|---|---|
| a Definitions are from the Oxford Dictionary. Where scientific definitions are not available in the Oxford Dictionary, definitions from the prescribed textbook were used (Atkins P. W., Jones L. and Laverman L., (2013), Chemical principles: the quest for insight, New York: W H Freeman). bSimilar or identical terms with different technical meanings in different contexts are shown in bold. | |||
| Bond |
• A thing used to tie something or to fasten things together
• An agreement with legal force |
Bond is a strong force of attraction holding atoms together in a molecule or crystal, resulting from the sharing or transfer of electrons. | 12 down |
| Capacity |
• The maximum amount that something can contain or produce
• The ability or power to do or understand something • A specified role or position |
Heat capacity is the ratio of heat supplied to the rise in temperature produced. | 22 across |
| Condensed | • Made denser or more concise; compressed or concentrated | Condensed phase is a solid or liquid phase, not a gas. | 5 down |
| Decomposition | • The state or process of rotting; decay | Decomposition is reaction in which a substance is broken down into simpler substances. | 21 across |
| Element |
• An essential or characteristic part of something abstract
• Any of the four substances (earth, water, air, and fire) regarded as the fundamental constituents of the world in ancient and medieval philosophy • A person's or animal's natural or preferred environment • A part in an electric kettle, heater, or cooker which contains a wire through which an electric current is passed to provide heat |
Element is (i) a substance that cannot be separated into simpler components by chemical techniques; (ii) a substance consisting of atoms of the same atomic number. | 8 down |
| Formation |
• The action of forming or process of being formed
• A group of people or things in a particular arrangement or pattern |
Standard enthalpy of formation refers to the formation of a substance from its elements in their most stable form. |
21 across
8 down |
| Spontaneous |
• Performed or occurring as a result of a sudden impulse or inclination and without premeditation or external stimulus
• Having an open, natural, and uninhibited manner |
Occurring without needing to be driven by an external influence. |
16 across
19 down |
| State |
• A nation or territory considered as an organized political community under one government
• The civil government of a country |
State function is a property of a substance that is independent of how the sample was prepared. State of matter is the physical condition of a sample. | 7 across |
| Surroundings | • The things and conditions around a person or thing | Surroundings is the region outside a system, where observations are made. | 14 down |
| Universe |
• A particular sphere of activity or experience
• All existing matter and space considered as a whole |
Universe is the system and its surroundings. |
18 across
14 down |
Appendix 5: meanings of terms used in ionic equilibriaa
| Termb | Everyday language/common usage | Context-specific scientific language | Clue(s) (Appendix 1) |
|---|---|---|---|
| a Definitions are from the Oxford Dictionary. Where scientific definitions are not available in the Oxford Dictionary, definitions from the prescribed textbook were used (Atkins P. W., Jones L. and Laverman L., (2013), Chemical principles: the quest for insight, New York: W H Freeman). bSimilar or identical terms with different technical meanings in different contexts are shown in bold. | |||
| Acceptor |
• A person or thing that accepts or receives something
• An atom or molecule which is able to bind to or accept an electron or other species |
A base is a proton acceptor (Brønsted–Lowry theory). | 17 across |
| Capacity |
• The maximum amount that something can contain or produce
• The ability or power to do or understand something • A specified role or position |
Buffer capacity is an indication of the amount of acid or base that can be added before a buffer loses its ability to resist the change in pH. | 3 down |
| Dominant | • Having power and influence over others | Indicating a position of equilibrium: product-dominant (prevalence of product(s)), reactant-dominant (prevalence of reactant(s)). |
8 across
9 down |
| Donor |
• A person who donates something, especially money to charity
• A person who provides blood, an organ, or semen for transplantation, transfusion, etc. • An atom or molecule that provides a pair of electrons in forming a coordinate bond |
An acid is a proton donor (Brønsted–Lowry theory). | 16 down |
| Limit/limiting |
• A point or level beyond which something does not or may not extend or pass
• A restriction on the size or amount of something permissible or possible |
Limiting reagent is the reactant that governs the theoretical yield of product in a given reaction. | 22 across |
| Strength |
• The quality or state of being physically strong
• The capacity of an object or substance to withstand great force or pressure • The potency or degree of concentration of a drug, chemical, or drink • A good or beneficial quality or attribute of a person or thing |
Strong acids and bases are acids and bases that are fully deprotonated or protonated, respectively, in solution. |
4, 7, 8, 25 across
2, 9, 21, 23 down |
Appendix 6: meanings of terms used in phase equilibriaa
| Termb | Everyday language/common usage | Context-specific scientific language | Clue(s) (Appendix 2) |
|---|---|---|---|
| a Definitions are from the Oxford Dictionary. Where scientific definitions are not available in the Oxford Dictionary, definitions from the prescribed textbook were used (Atkins P. W., Jones L. and Laverman L., (2013), Chemical principles: the quest for insight, New York: W H Freeman). bSimilar or identical terms with different technical meanings in different contexts are shown in bold. | |||
| Critical |
• Expressing adverse or disapproving comments or judgements
• Expressing or involving an analysis of the merits and faults of a work of literature, music, or art • Having the potential to become disastrous; at a point of crisis |
Relating to or denoting a point of transition from one state to another. | 6 down |
| Dynamic |
• (Of a process or system) characterized by constant change, activity, or progress
• (Of a person) positive in attitude and full of energy and new ideas |
Dynamic equilibrium is the condition in which a forward process and its reverse are taking place simultaneously at equal rates. | 22 down |
| Extensive | • Covering or affecting a large area; large in amount or scale | Extensive (property) is a physical property that depends on the size of the sample. | 26 across |
| Ideal |
• Satisfying one's conception of what is perfect
• Existing only in the imagination; desirable or perfect but not likely to become a reality |
Ideal gas is a gas that satisfies the ideal gas law and is described by a kinetic model. | 2 across |
| Instantaneous | • Occurring or done instantly | Instantaneous dipole moment is a dipole moment that arises from a transient distribution of charge and is responsible for the London force. | 24 across |
| Intensive |
• Concentrated on a single subject or into a short time; very thorough or vigorous
• Achieving maximum production with limited resources • Concentrating on or making much use of a specified thing |
Intensive (property) is a physical property that is independent of the size of the sample. | 4 down |
| Phase | • A distinct period or stage in a process of change or forming part of something's development | Phase is a specific physical state of matter. In certain cases, some substances can exist in more than one solid or liquid phase. | 21 down |
| Tie |
• Attach or fasten with string or similar cord
• Restrict or limit (someone) to a particular situation or place • Connect; link • Achieve the same score or ranking as another competitor or team |
Tie line – a horizontal line drawn across the region of two phases. | 25 down |
| Volatile | • (Of a person) liable to display rapid changes of emotion | (Of a substance) easily evaporated at ambient temperature. | 20 down |
Appendix 7: meanings of terms used in chemical kineticsa
| Termb | Everyday language/common usage | Context-specific scientific language | Clue(s) (Appendix 3) |
|---|---|---|---|
| a Definitions are from the Oxford Dictionary. Where scientific definitions are not available in the Oxford Dictionary, definitions from the prescribed textbook were used (Atkins P. W., Jones L. and Laverman L., (2013), Chemical principles: the quest for insight, New York: W H Freeman). bSimilar or identical terms with different technical meanings in different contexts are shown in bold. | |||
| Elementary |
• Relating to the rudiments of a subject: an elementary astronomy course
• Straightforward and uncomplicated |
Elementary reaction/step is an individual step in a proposed reaction mechanism. | 8 across |
| Instantaneous | • Occurring or done instantly | Instantaneous rate is the slope (gradient) of the tangent of a graph of concentration against time. | 1 down |
| Order |
• The arrangement or disposition of people or things in relation to each other according to a particular sequence, pattern, or method
• An authoritative command or instruction • A particular social, political, or economic system • A society of monks or nuns living under the same religious, moral, and social regulations and discipline; an institution; a fraternity • The degree of complexity of an equation, expression, etc., as denoted by an ordinal number (mathematics) |
Order of a reaction is the power to which the concentration of a single substance is raised in a rate law. | 12 down |
| Rate |
• The speed with which something moves or happens
• A fixed price paid or charged for something • A tax on commercial land and buildings paid to a local authority (usually plural) |
Reaction rate is the unique rate of a chemical reaction calculated by dividing the change in concentration of a substance by the interval during which the change takes place and by taking into account the stoichiometric coefficient of the substance. | 1, 11, 15, 16, 18 down |
Acknowledgements
The authors are indebted to Kim Styles for insightful discussions.References
- ACARA, (2016), Australian Curriculum, Assessment and Reporting Authority, http://www.australiancurriculum.edu.au/seniorsecondary/science/chemistry/rationaleaims, (accessed January 11th, 2016).
- Atkins P. W., Jones L. and Laverman L., (2013), Chemical principles: the quest for insight, New York: W H Freeman.
- Ausubel D. P., (1978), Educational psychology: a cognitive view, New York: Holt, Rinehart and Winston.
- Baddeley A., (1992), Working memory, Science, 255, 556–559.
- Baddeley A. and Hitch G. J., (1974), Working memory, in Bower G. H. (ed.) The psychology of learning and motivation, New York: Academic, vol. 8, pp. 47–89.
- Becker N. and Towns M. H., (2012), Students' understanding of mathematical expressions in physical chemistry contexts: an analysis using Sherin's symbolic forms, Chem. Educ. Res. Pract., 13, 209–220.
- Belnap N., (1993), On rigorous definitions, Philos. Stud., 72, 115–146.
- Bloom B. S., (1956), Taxonomy of educational objectives: the classification of educational goals, New York: D. McKay Co.
- Boyd S. L., (2007), Puzzling through general chemistry: a light-hearted approach to engaging students with chemistry content, J. Chem. Educ., 84, 619–621.
- Brandriet A. R., Ward R. M. and Bretz S. L., (2013), Modeling meaningful learning in chemistry using structural equation modeling, Chem. Educ. Res. Pract., 14, 421–430.
- Cady S. G., (2012), Elements are everywhere: a crossword puzzle, J. Chem. Educ., 89, 524–525.
- Cassels J. R. T. and Johnstone A. H., (1985), Words that matter in science, London: RSC.
- Childers C. D., (1996), Using crossword puzzles as an aid to studying sociological concepts, Teach. Sociol., 24, 231–235.
- Childs P. E., Markic S. and Ryan M. C., (2015), The role of language in the teaching and learning of chemistry, in García-Martínez J. and Serrano-Torregrosa E., (ed.), Chemistry Education: Best Practices, Opportunities and Trends, Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, ch. 17, pp. 421–445.
- Coticone S. R., (2013), Utility of self-made crossword puzzles as an active learning method to study biochemistry in undergraduate education, J. Coll. Sci. Teach., 42, 33–39.
- Crossman E. K. and Crossman S. M., (1983), The crossword puzzle as a teaching tool, Teach. Psychol., 10, 98–99.
- Davis T. M., Shepherd B. and Zwiefelhofer T., (2009), Reviewing for exams: do crossword puzzles help in the success of student learning? Journal of Effective Teaching, 9, 4–10.
- Ebbinghaus H., (1885), Memory: a contribution to experimental psychology, New York: Columbia University.
- Ennis R. H., (1974), Definition in science teaching, Instr. Sci., 3, 285–298.
- Evans M. H., (1985), Puzzles for teaching descriptive chemistry, J. Chem. Educ., 62, 1103.
- Feynman R. P., (1998), The meaning of it all: thoughts of a citizen scientist, Reading, MA: Addison-Wesley.
- Franklin S., Peat M. and Lewis A., (2003), Non-traditional interventions to stimulate discussion: the use of games and puzzles, J. Biol. Educ., 37, 79–84.
- Gabel D., (1999), Improving teaching and learning through chemistry education research: a look to the future, J. Chem. Educ., 76, 548–554.
- Gaikwad N. and Tankhiwale S., (2012), Crossword puzzles: self-learning tool in pharmacology, Perspect. Med. Educ., 1, 237–248.
- Galbraith K., McDowell J., Malone D., Yuriev E., Manallack D. T., Sewell K. and Larson I., (2014), The Science of Medicines, http://https://www.futurelearn.com/courses/the-science-of-medicines, accessed January 10th, 2016.
- Grove N. P. and Bretz S. L., (2012), A continuum of learning: from rote memorization to meaningful learning in organic chemistry, Chem. Educ. Res. Pract., 13, 201–208.
- Hamby M., (1990), Understanding the Language – Problem-Solving and the 1st Law of Thermodynamics, J. Chem. Educ., 67, 923–924.
- Hamori E. and Muldrey J. E., (1984), Use of the word “eager” instead of “spontaneous” for the description of exergonic reactions, J. Chem. Educ., 61, 710.
- Herron J. D., (1996), The role of language in teaching of chemistry, in The chemistry classroom, Washington, DC: ACS, ch. 13, pp. 161–182.
- Jasien P. G., (2013), Roles of terminology, experience, and energy concepts in student conceptions of freezing and boiling, J. Chem. Educ., 90, 1609–1615.
- Joag S. D., (2014), An effective method of introducing the periodic table as a crossword puzzle at the high schooll level, J. Chem. Educ., 91, 864–867.
- Johnstone A. H., (2000), Teaching of chemistry – logical or psychological? Chem. Educ. Res. Pract., 1, 9–15.
- Johnstone A. H. and Al-Naeme F. F., (1991), Room for scientific thought? Int. J. Sci. Educ., 13, 187–192.
- Johnstone A. H. and Selepeng D., (2001), A language problem revisited, Chem. Educ. Res. Pract., 1, 19–29.
- Kiewra K. A., (1989), A review of note-taking: the encoding-storage paradigm and beyond, Educ. Psychol. Rev., 1, 147–172.
- Lee A. W. M. and Tse C. L., (1994), Learning name reactions and name apparatuses through crossword puzzles, J. Chem. Educ., 71, 1071–1072.
- Lee K.-W. L., Tang W.-U., Goh N.-K. and Chia L.-S., (2001), The predictive role of cognitive variables in problem solving in mole concept, Chem. Educ. Res. Pract., 2, 285–301.
- Lim K. F., (2013), Threshold learning outcomes, Chem. Aust., 2013, March, 35.
- Luxford C. J. and Bretz S. L., (2013), Moving beyond definitions: what student-generated models reveal about their understanding of covalent bonding and ionic bonding, Chem. Educ. Res. Pract., 14, 214–222.
- Mangen A. and Velay J.-L., (2010), Digitizing literacy: Reflections on the haptics of writing, in Zadeh M. H. (ed.) Advances in haptics, InTech, ch. 20, pp. 385–401.
- McLaughlin J. E., White P. J., Khanova J. and Yuriev E., (2016), Flipped classroom implementation: a case report of two higher education institutions in the United States and Australia, Comput. Schools, 33, 24–37.
- Middlecamp C. and Kean E., (1988), Problems and “that other stuff”: types of chemical content, J. Chem. Educ., 65, 53–56.
- Most C., (1993), General chemistry crossword puzzle, J. Chem. Educ., 70, 1039.
- Mueller P. A. and Oppenheimer D. M., (2014), The pen is mightier than the keyboard: advantages of longhand over laptop note taking, Psychol. Sci., 25, 1159–1168.
- Novak J. D., (2009), Ausubel's Assimilation Learning Theory, in Learning, creating, and using knowledge: concept maps as facilitative tools in schools and corporations, New York: Routledge, 2nd edn, ch. 5, pp. 56–89.
- O'Connor C., Mc Donnell C. and Seery M., (2005), Chemistry crosswords: Learning activities to support non-traditional students in third level education, presented in part at the 7th Irish Variety in Chemistry Teaching, Dublin Institute of Technology, March, 2005.
- Overton T. L. and Potter N., (2008), Solving open-ended problems, and the influence of cognitive factors on student success, Chem. Educ. Res. Pract., 9, 65–69.
- Overton T. L. and Potter N. M., (2011), Investigating students' success in solving and attitudes towards context-rich open-ended problems in chemistry, Chem. Educ. Res. Pract., 12, 294–302.
- Proctor R. W. and Vu K.-P. L., (2012), Human Information Processing, in Seel N. M. (ed.) Encyclopedia of the Sciences of Learning, New York: Springer, pp. 1458–1460.
- Pyburn D. T., Pazicni S., Benassi V. A. and Tappin E. E., (2013), Assessing the relation between language comprehension and performance in general chemistry, Chem. Educ. Res. Pract., 14, 524–541.
- Pyburn D. T., Pazicni S., Benassi V. A. and Tappin E. E., (2014), The testing effect: an intervention on behalf of low-skilled comprehenders in general chemistry, J. Chem. Educ., 91, 2045–2057.
- Reid N., (2014), The learning of chemistry: the key role of working memory, in Devetak I. and Glazar S. A. (ed.) Learning with understanding in the chemistry classroom, Dordrecht: Springer, ch. 5, pp. 77–101.
- Roberts M. C. and Rosnov D., (2006), Information Processing Theory, in Salkind N. J. and Margolis L. (ed.) Encyclopedia of Human Development, Thousand Oaks, CA: Sage Publications, vol. 2, pp. 713–715.
- Russell J. V., (1999), Using games to teach chemistry – an annotated bibliography, J. Chem. Educ., 76, 481–484.
- Saxena A., Nesbitt R., Pahwa P. and Mills S., (2009), Crossword puzzles: active learning in undergraduate pathology and medical education, Arch. Pathol. Lab. Med., 133, 1457–1462.
- Schmidt H.-J., (1997), Students' misconceptions – looking for a pattern, Sci. Educ., 81, 123–135.
- Schraw G. and McCrudden M., (2013), Information processing theory, http://www.education.com/reference/article/information-processing-theory/, accessed 2016, January 6th.
- Shah S., Lynch L. M. and Macias-Moriarity L. Z., (2010), Crossword puzzles as a tool to enhance learning about anti-ulcer agents, Am. J. Pharm. Educ., 74, 117.
- Shallcross D. and Yates P., (2014), Skills in Mathematics and Statistics in Chemistry and tackling transition, York, U.K.: The Higher Education Academy.
- Sims P. A., (2011), Amino acid crossword puzzle, J. Chem. Educ., 88, 434–436.
- Sivagnanam G., Rajasekaran M., Jayashree C., Sreepriya R. and Rajakannu R., (2004), Crossword puzzle: a novel teaching–learning method, Indian J. Pharmacol., 36, 179–180.
- Smithrud D., B. and Pinhas A. R., (2015), Pencil–paper learning should be combined with online homework software, J. Chem. Educ., 92, 1965–1970.
- Snead C. C., (1975), P-Chem crossword puzzle, J. Chem. Educ., 52, 158.
- Spillers G., Brewer G. and Unsworth N., (2012), Working Memory and Information Processing, in Seel N. M. (ed.) Encyclopedia of the Sciences of Learning, New York: Springer, pp. 3474–3476.
- St Clair-Thompson H., Overton T. L. and Botton C., (2010), Information processing: a review of implications of Johnstone's model for science education, Res. Sci. Technol. Educ., 28, 131–148.
- St Clair-Thompson H., Overton T. L. and Bugler M., (2012), Mental capacity and working memory in chemistry: algorithmic versus open-ended problem solving, Chem. Educ. Res. Pract., 13, 484–489.
- Sumfleth E., (1988), Knowledge of terms and problem-solving in chemistry, Int. J. Sci. Educ., 10, 45–60.
- Taber K. S., (2003), Lost without trace or not brought to mind? – a case study of remembering and forgetting of college science, Chem. Educ. Res. Pract., 3, 249–277.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14, 156–168.
- Tifi A., (2004), Crosswords supporting concept mapping learning, presented in part at the Concept Maps: Theory, Methodology, Technology, First International Conference on Concept Mapping, Pamplona, Spain, 2004.
- TISC, (2014), Australian Tertiary Admission Rank, http://www.tisc.edu.au/static/guide/atar-about.tisc?cid=851212, accessed 2016, January 6th.
- van der Ven F., Takashima A., Segers E. and Verhoeven L., (2015), Learning word meanings: overnight integration and study modality effects, PLoS One, 10, e0124926.
- Weisskirch R. S., (2006), An analysis of instructor-created crossword puzzles for student review, Coll. Teach., 198–201.
- White P. J., Larson I., Styles K., Yuriev E., Evans D. R., Short J. L., Rangachari P. K., Malone D. T., Davie B., Naidu S. and Eise N., (2015), Using active learning strategies to shift student attitudes and behaviours about learning and teaching in a research intensive educational context, Pharm. Educ., 15, 116–126.
- White P. J., Larson I., Styles K., Yuriev E., Evans D. R., Rangachari P. K., Short J. L., Exintaris B., Malone D. T., Davie B., Eise N., Naidu S. and McNamara K., (2016), Adopting an active learning approach to teaching in a research-intensive higher education context transformed staff teaching attitudes and behaviours, High. Educ. Res. Dev., DOI: http://10.1080/07294360.2015.1107887.
- Williams H. T., (1999), Semantics in teaching introductory physics, Am. J. Phys., 67, 670–680.
- Wong C. L., Chu H.-E. and Yap K. C., (2014), Developing a framework for analyzing definitions: a study of the Feynman lectures, Int. J. Sci. Educ., 36, 2481–2513.
- Zoller U. and Pushkin D., (2007), Matching Higher-Order Cognitive Skills (HOCS) promotion goals with problem-based laboratory practice in a freshman organic chemistry course, Chem. Educ. Res. Pract., 8, 153–171.
| This journal is © The Royal Society of Chemistry 2016 |