Exploring the impact of argumentation on pre-service science teachers' conceptual understanding of chemical equilibrium
Mehmet
Aydeniz†
*a and
Alev
Dogan
b
aDepartment of Theory and Practice in Teacher Education, The University of Tennessee, USA
bDepartment of Secondary Science Education, College of Education, Gazi University, Turkey
First published on 26th October 2015
Abstract
This study examines the impact of argumentation on pre-service science teachers' (PST) conceptual understanding of chemical equilibrium. The sample consisted of 57 first-year PSTs enrolled in a teacher education program in Turkey. Thirty two of the 57 PSTs who participated in this study were in the experimental group and 25 in the control group. The experimental group students learned the concept of chemical equilibrium through argumentation; the control group students learned the same concepts through business as usual (i.e. lectures, supported by laboratory experiments). The intervention lasted for 12 instructional hours, of which 4 were spent in the laboratory. The chemical equilibrium concept test was administered to both groups of students one week after the intervention. The results show that the experimental group students performed significantly better than then control group students on the chemical equilibrium concept test. The mean difference between two groups is 14.026. This difference is statistically significant at (*p < 0.001). However, the control group students performed significantly better on the comprehensive course final exam.
Introduction
Numerous scholars have studied students' conceptual understanding of chemical equilibrium (Banerjee, 1995; Quilez-Pardo and Solaz-Portoles, 1995; Huddle and Pillay, 1996; Voska and Heikkinen, 2000; Bilgin, 2002). Chemical equilibrium has been shown to be one of the most difficult chemistry concepts for students to understand due to its complexity (Hackling and Garnett, 1985; Niaz, 1995; Tyson et al., 1999; Chiu et al., 2002; Park and Park, 2002; Maia and Justi, 2009). As a result, students at all levels of education hold misconceptions or alternative conceptions related to chemical equilibrium (Voska and Heikkinen, 2000; van Driel and Gräber, 2003; Locaylocay et al., 2005; Piquette and Heikkinen, 2005; Çakmakçı et al., 2006; Demircioglu et al., 2013).Demircioglu et al. (2013) investigated first year chemistry students' conceptual understanding of chemical equilibrium and found that students hold several misconceptions related to chemical equilibrium. Some of these misconceptions include: (1) when a catalyzer is added to a system in equilibrium, concentration of reactants and products increases, (2) at an equilibrium system, the forward reaction rate is not equal to the reverse reaction rate, (3) whether the reaction is endothermic or exothermic, a change in the temperature will not affect the equilibrium, (4) Le Chatelier's principle can be applied to all systems, including heterogeneous equilibrium systems, (5) at equilibrium, the concentration of reactants is equal to the concentration of products. Others report that (1) students often fail to understand the dynamic nature of a system in a state of chemical equilibrium. Instead, many hold a static conception (Gorodetsky and Gussarsky, 1986; Thomas and Schwenz, 1998). Ozmen (2008) has reported a comprehensive review of common misconceptions held by pre-service science teachers in Turkey. Others have also reported similar misconceptions among high school and university students in other countries (Park and Park, 2002; Piquette and Heikkinen, 2005; Maia and Justi, 2009). The prevalence and persistence of problems in the understanding of chemical equilibrium denotes limitations of traditional teaching methods and calls for testing the effects of new interventions informed by current learning and instructional theories.
Misconceptions are known to be well embedded in learners' cognitive ecology and resistant to change even after instruction (Locaylocay et al., 2005). Therefore, specific and effective instruction must be designed to address them. Because misconceptions related to chemical equilibrium continue to persist among students, science educators have implemented different instructional strategies to address students' misconceptions and help them to develop scientifically accurate conceptions about several aspects of chemical equilibrium.
Locaylocay et al. (2005) used several constructivist strategies such as analogies, small group discussions, and journal writing to address college students' misconceptions related to chemical equilibrium. Their results show that while the interventions used for instruction addressed some misconceptions held by the students, some continued to hold alternative conceptions even after these interventions. Maia and Justi (2009) explored the impact of model-based instruction on high school students' conceptual understanding of chemical equilibrium. The results of their study reveal a positive impact of model-based instruction on students' conceptual understanding of chemical equilibrium. Canpolat et al. (2006) conducted a study with 85 undergraduate students recruited from two introductory chemistry courses at a university in Turkey. The authors randomly assigned students in each class to either the “traditional instruction” intervention, or the “conceptual change instruction” intervention. While the students in the control group experienced instruction through lectures and problem solving assignments, students in the experimental group were taught through the conceptual change approach. This approach was designed based on Posner et al. (1982a, 1982b) model of conceptual change. The intervention consisted of: (1) conceptual change texts designed by the instructors to help students experience discontentment with their initial ideas, (2) models and demonstrations that took students' misconceptions into account and explanations that were specifically engineered to maximize the plausibility and intelligibility of the scientific concepts covered in the course. The results showed that the students in the experimental group performed significantly better than the control group. The average percent of correct responses of the experimental group was 70%, and that of the control group was 51%, after treatment. Similarly, the number of students who held misconceptions about chemical equilibrium after instruction was significantly higher among the students in the control group than those in the experimental group.
While science educators have explored the impact of various instructional strategies on students' conceptual understanding of chemical equilibrium, to our knowledge only one study (Kaya, 2013) has explored the impact of argumentation on pre-service science teachers' conceptual understanding of chemical equilibrium. Given the attention that argumentation has received as being one of the most effective instructional strategies from science educators in recent years (Jiménez-Aleixandre and Erduran, 2008; Lee et al., 2009), and its relevance to the practice of future teachers more such studies are needed. In this study, we attempted to address this gap in research. The question that guided our inquiry was:
What is the impact of argumentation on pre-service science teachers' conceptual understanding of chemical equilibrium?
Argumentation and conceptual understanding in chemistry
Argumentation has received significant attention from the science education community in recent years (Newton et al., 1999; Osborne et al., 2004; Duschl et al., 2007; Lee et al., 2009). Merriam Webster defines argumentation as, “the act or process of forming reasons and of drawing conclusions and applying them to a case in discussion”. In the context of science learning, argumentation refers to the discursive practice whereby learners attempt to construct, support, evaluate or validate a claim through evidence-based reasoned judgment (Jiménez-Aleixandre and Erduran, 2008). In order to engage in argumentation, learners are expected not only to develop arguments that consist of claims, evidence and reasoning but also engage in a critical discourse in which they try to persuade each other of the validity of their arguments.Duschl et al. (2007) maintain that argumentation can shift the focus of science classrooms from one of rote memorization to engaging students in a complex scientific practice in which they construct and justify knowledge claims (as cited in Berland and McNeill, 2009, p. 1). The assumption is that when argumentation is implemented in the classroom, students engage in the dialogic exploration of ideas in-depth and their justification through reasoned action and critical discourse (Ford, 2008; Jiménez-Aleixandre and Erduran, 2008; Szu and Osborne, 2011). More importantly, through this dialogical learning experience, reasoned discourse and norms of the “accountable talk” (Michaels et al., 2008) embedded within argumentation, students achieve greater learning outcomes (Zohar and Nemet, 2002; Venville and Dawson, 2010; Aydeniz et al., 2012; Sampson et al., 2013).
Science educators have explored the impact of argumentation on students' conceptual understanding (Zohar and Nemet, 2002; Cross et al., 2008; Venville and Dawson, 2010 and students discourse in K-12 settings (Jiménez-Aleixandre et al., 2000)) as well as college settings (Aydeniz et al., 2012; Walker et al., 2012; Kaya, 2013). Author and colleagues also explored the impact of argumentation on students' conceptual understanding of properties and behaviors of gases and reported positive impacts of argumentation on college students' conceptual understanding. The results of this study showed that not only did the intervention group students performed significantly better than the control group students on the post-test, but they also showed a greater increase in their performance between the pre-test and the post-test. In addition, the authors reported that 80% of the intervention group students abandoned their misconceptions related to 17 misconceptions. This percent was less than 50 for the control group students.
To our knowledge, only two studies (Rudd et al., 2007; Kaya, 2013) have examined the impact of argumentation on college students' conceptual understanding of chemical equilibrium. However, only one study (Kaya, 2013) has focused on the link between argumentation and pre-service science teachers' conceptual understanding of chemical equilibrium.
Rudd et al. (2007) explored the impact of The Science Writing Heuristic (SWH) model of instruction (Keys et al., 1999), a form of written argument development, on college students' conceptual understanding of chemical equilibrium. The SWH approach follows a structure similar to Toulmin's claim, evidence, warrant argument model and engages students in individual argument development and reflection as well and in negotiation of conceptual understanding with peers. The authors tested to see if the SWH format of instruction resulted in better student performance on explaining the concept of chemical equilibrium using students' mean explanation scores as the basis of their judgment. They found that students who experienced instruction through the SWH format performed significantly better than those who experienced instruction through traditional methods. The results of their analyses also revealed that SWH led to greater student success in the identification of the equilibrium points, providing an accurate equilibrium reaction equation. However, the authors did not observe a significant difference between two groups' understanding of Le Châtelier's principle.
Kaya (2013) explored the effects of argumentation on 51 pre-service science teachers' conceptual understanding related to chemical equilibrium through a quasi- experimental study. She conducted her study with 100 pre-service elementary science teachers; 49 in the control group and 51 in the experimental group, over four weeks. Students were first charged to develop written arguments based on specific equilibrium concepts (e.g. factors affecting the direction of equilibrium). Then, the course professor (the author) facilitated a follow-up whole class discussion for each argumentation task. Students' conceptual understanding was measured through a 47-item conceptual test composed of multiple choice and true-false type questions. The results of her study showed that students who experienced argumentation performed significantly better than the students who experienced traditional teaching on the chemical equilibrium concept test.
While these results are promising, considering the small number of participants in this study, more effort is needed to confirm the reported effects of this instructional strategy. To contribute to these efforts we investigated the impact of argumentation on first year pre-service science teachers' conceptual understanding of chemical equilibrium in a general chemistry course.
Methodology
This study compared the learning outcomes of two similar groups of students taking the same course under two different instructors; therefore, it is a quantitative study. While we used the post-test only method, we controlled for the characteristics of two groups of students. To control for students' overall performance in chemistry we used students' midterm and final course test results that are independent of the chemical equilibrium test that was used to measure the effects of argumentation.Context and participants
This study took place in a general chemistry course designed for pre-service science teachers. Participants consisted of students enrolled in two general chemistry courses. One chemistry course served as the control group and one as the experimental group. Because there are only two sections of the same course, we used the convenience-sampling method for making group assignments. The professor of the experimental group students, also the second author has been exposed to argumentation and designed the intervention together with the first author. Therefore, her class was chosen to be the experimental group. Both professors typically consult with each other about the content of the course. The instructor of the control group students was informed about the intent of the study. The two professors went over the content to be covered for the duration of the study. The instructional time was the same for both groups of the study.The intervention group professor has worked as a research chemist for 15 years and has been teaching general chemistry courses and laboratories for 27 years. The control group professor has been teaching general chemistry courses and laboratories for 35 years. Both of them are chemistry educators with years of experience.
The experimental group consisted of 32 students (29 females, 3 males), and the control group consisted of 25 students (24 females, 1 male). The course covers the traditional chemistry topics but taught by a chemistry educator. The specific goals set for students for this section of the course included: helping pre-service science teachers to develop the coherent conceptual understanding of chemical equilibrium through argumentation, supported by active engagement in hands-on laboratory investigations. Teaching in the experimental group classroom is typically limited to teacher lectures, followed up with the teacher demonstration of three or four quantitative chemistry problems related to the topic of interest and lab-based activities. The teacher sometimes calls on one student to share his/her solutions on the board if time allows and when appropriate, the teacher conducts in-class demonstrations of chemical reactions. The instruction in the control group classroom is limited to teacher lectures and teacher-led solution of 3–4 quantitative problems on the board with no active hands-on activities beyond labs.
After the permission from the department was received to conduct the study, the control group instructor was informed of the intent of the study and agreed to participate in the study. After the control group professors' participation in the study was ensured, the students in both classrooms were informed of the intent of the study, the activities they were asked to complete and data to be collected. All students agreed to participate in the study.
Intervention
The intervention used in this study is argumentation. Argumentation means different things to different people. Argumentation in the context of this study refers to the process of proposing, justifying and defending claims to knowledge using scientific evidence (Driver et al., 2000). The purpose of scientific argumentation is to build consensus about the validity of a claim to knowledge through critical discourse (Jiménez-Aleixandre and Erduran, 2008) or to compare the validity of two alternative claims to knowledge.The intervention lasted for three weeks. During these three weeks, 8 instructional hours were spent on theoretical knowledge related to chemical equilibrium through argumentation and four hours were spent in the laboratory. The control group students also engaged in the same labs for four hours under the guidance of their instructor. The concepts covered during these lessons included: chemical equilibrium and factors affecting chemical equilibrium, physical and chemical equilibrium, equilibrium constant, and the Le Chatelier principle.
The instructor developed a set of argumentation questions related to chemical equilibrium for each lesson over the course of three weeks. Students were placed in groups of four and asked to discuss answers to the questions posed by the course professor. The students were encouraged to ask each other and the course professor answers that confused them. The course professor then facilitated whole class argumentation sessions through guided instruction. The course professor restated important questions raised by members of a specific group for the whole class discussion and elicited responses from all students. If confusion was present the course professor helped the students to arrive at the scientifically accepted knowledge through guided questioning. During the instruction the course professor attempted to help her students to integrate their prior knowledge, constantly asked them to come up with new questions based on their learning, understand the process of science through engagement in laboratory activities, make predictions based on their experimental data.
The 10-item post-test focusing only on chemical equilibrium proved to be reliable (Cronbach's alpha = 0.815). The content validity of the test was achieved through the expert panel method (Yaghmaie, 2003). The expert panel consisted of three chemistry professors, one being the course professor. The course professor initially developed the post-test, the initial question set was then reviewed by two other general chemistry professors, who also taught similar students. The experts were asked to evaluate the questions based on the following criteria using a likert scale (1–5). The criteria included: relevancy, clarity, simplicity, ambiguity and scientific accuracy (Yaghmaie, 2003).
The initial test was revised based on the feedback received from the other two professors. The course professor is a chemist by training, she spent 15 years conducting scientific research in chemistry laboratories and has been teaching chemistry and science education courses for the last 12 years. She knows this particular group of students' prior knowledge and their capacity for learning fundamental chemical concepts based on her extensive experience with teaching the course. The chemical equilibrium test was administered to the students one week after the argumentation instruction was over.
Data were analyzed both quantitatively and qualitatively. Graduate teaching assistants (GTAs) conducted the exams. They were instructed to cover student information in both classes before turning the exams to us for evaluation. We used a numbering system to track data. Each exam paper was given a number by the GTAs so we could track which group the students belonged after our evaluation. Data analyses took place in three stages. First, two authors screened a set of students' answers in an effort to observe patterns in students' responses and to develop criteria for grading students' responses. Second, we randomly selected 10 students' responses and together went over students' responses to the conceptual test questions, one by one and assigned a score. We first graded students' responses to each question on the chemical equilibrium test on a scale of 0–4, with 0 being incorrect to 4 being the most sophisticated response a student at this level could provide. Thus, the maximum score that a student could receive for the post-test was 40. Then, the second author graded the rest of the papers. Finally, the two authors reviewed the scoring of the answers to ensure consistency across participants and questions. The changes were made if deemed necessary.
After calculating the post-test scores for each student, we conducted an independent samples t-test to see if there was a statistically significant difference between the two groups of students' post-test performance. Students who enter this program are selected based on their performance on a national university entrance exam. To test whether the students in two groups were similar or not, we conducted an independent samples t-test based on students' midterm exams and final exam scores. The results showed that these students are not significantly different from one another (t = 0.620) based on their midterm scores, however, the test showed that the control group students are significantly better than the experimental group students based on their traditional final exam scores (t = 0.004), with a mean difference of 17.8. This final exam is different from the chemical equilibrium concept test, the post-test used for measuring the impact of argumentation (Table 1).
| Midterm exam | Final exam | Chemical equilibrium test | |
|---|---|---|---|
| Experimental group | 60.06 | 55.32 | 25.9 |
| Control group | 57.72 | 73.12 | 11.8 |
Results
The quantitative results show that experimental group students performed significantly better than the control group students on the post-test focusing on chemical equilibrium. The mean difference between two groups is 14.026. This difference is statistically significant at (*p < 0.005) (Table 2).| Student group | N | Mean | STD |
|---|---|---|---|
| The chemical equilibrium concept test was an open-ended test. Therefore, students' responses were evaluated qualitatively. In our evaluation of students' responses, we used a 0–4 scale to measure the quality of students' responses for each question (see Appendix A). | |||
| Experimental | 32 | 25.91 | 4.679 |
| Control | 25 | 11.88 | 6.648 |
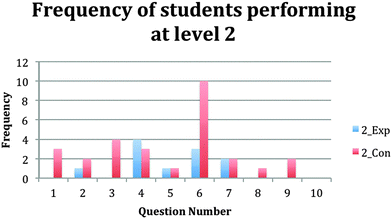
The results show that the experimental group students received more 4's and 3s than the control group students. (Fig. 1–5).
We provide exemplary responses to help the readers understand the quality difference between different answer levels in Tables 3 and 4.
| Question 4 | 4. Consider the following chemical reaction at equilibrium. |
| NH4Cl(s) ⇄ NH3(g) + HCl(g) | |
| If we add some NH3(g) how will the equilibrium get affected? Consider all factors that might affect the equilibrium in your answer and justify your rationale. | |
| Level 4 answer | When we add NH3 to the system equilibrium will change in favor of reactants, producing more NH4Cl. As a result, this will negatively affect the concentration level of HCl. However, because NH4Cl is a solid, its concentration will not change. There will just be more NH4Cl produced. The amount of products and reactants will no longer be the same, however, equilibrium constant Ka will remain the same. Because Ka only changes with temperature. |
| Level 2 answer | When we add NH3 to the system, the equilibrium will change, favoring the reactants. HCl concentration will be lower. |
| Quality level | |
|---|---|
| Question | |
| 7. Consider the following chemical reaction. | |
| N2O4(g) ⇄ 2NO2(g) ΔH = +14 kcal | |
| Colorless darker/brownish | |
| What will happen to the chemical system when we make the following changes to the system? Please justify your rationale. | |
| I. Increasing the temperature of the system | |
| II. Decreasing the volume of the system | |
| Level descriptors | |
| Level 4 | Because, ΔH = +14 kcal this reaction is endothermic. In endothermic reactions, an increase in temperature changes the direction of equilibrium in favor of products. An increase in temperature will move the direction of equilibrium towards products. This means, while the concentration of the products will increase, that of the reactants will decrease with time. The color of the mixture will look like brownish with time. Eventually, the system will reach the equilibrium but the equilibrium constant will now be different. If we reduce the volume of the system, the concentration of the ingredients will increase. A similar increase will be observed in the partial pressure of the ingredients in the gaseous phase. The equilibrium will react towards the less pressure side. The reaction will proceed towards the side that has fewer moles of the same ingredients. Therefore, the reaction will proceed towards the side that has fewer moles, (i.e., towards reactants). Therefore, the color of the gas mixture will be lighter. |
| Level descriptors | |
| Level 3 | This is an endothermic reaction, therefore, if we increase the temperature, the reaction will start to favor the products and the color of gas mixture will darken with time. The amount of products (in moles) is greater than the amount of reactants in this reaction. Therefore, if we decrease the volume of the system, the equilibrium will favor the reactants because this side has a less amount of substance. The amount of products will decrease and of reactants will increase. The color of the gas mixture will become less dark with time. |
| Level 2 | This is an endothermic reaction; therefore, an increase in temperature will result in equilibrium favoring the products. Therefore, the color of the gas mixture in the system will become darker with time. If we decrease the volume of the system, the reaction will favor the reactants and as a result, the color of the mixture will become lighter with time. |
| Level 1 | When we increase the temperature, equilibrium tends to favor the product side. If we decrease the volume, it will favor the reactants. |
| Level 0 | No answer. |
Conclusions and discussion
The purpose of this study was to explore the impact of argumentation on pre-service science teachers' conceptual understanding of chemical equilibrium. Results show that argumentation made a statistically significant contribution (*p < 0.005) to students' conceptual understanding of chemical equilibrium. The experimental group students performed significantly better on the chemical equilibrium test than the controlled group students did. This is despite the fact that control group students are relatively more successful in the course based on two groups' midterm and final course exams. The results are consistent with other research reporting the positive effect of argumentation on students' conceptual understanding (Aydeniz et al. 2012, Zohar and Nemet, 2002; Venville and Dawson, 2010; Sampson and Walker, 2012; Kaya, 2013). For instance, Kaya in her work with PST observed that argumentation practices that she employed in her study made positive contributions to PSTs' performance on the chemical equilibrium concept test. She also measured the effects of argumentation on the quality of arguments that her participants (PSTs) produced and found that the experimental group students produced arguments of higher quality than the control group students did. One weakness of this study is that she measured the quality of students' written arguments only through one task. One difference between her study and our study is that while the same instructor taught both the control group and the intervention group students in Kaya's study, each group was taught by different instructors in our study. Another difference between our study and her study is that while she did not observe a statistical difference in students' conceptual understanding prior to the intervention, the students in our study were different in terms of their overall performance in class, with the intervention students performing significantly lower than the control group students in all tasks but the chemical equilibrium test. Finally, while our intervention lasted only for three weeks, her intervention spanned over four weeks.Chemical equilibrium is a hard concept for students to learn because it is inherently linked to students' prior knowledge of oxidation and reduction, acid–base chemistry, solubility and stoichiometry (Rudd et al., 2007). In spite of the differences in our implementation and Kaya's (2013) implementation, argumentation resulted in better student performance in chemical equilibrium in both studies. This reported positive effect is not surprising given the theoretical justifications rooted in the assumptions of sociocultural theories of knowledge (Brown and Campione, 1994; Driver et al., 1994; Greeno, 1998; Mercer et al., 2004) and the importance of co-construction and critique in developing substantial learning (Alexander, 2005; Ford, 2008; Michaels et al., 2008). When students are asked to construct and defend their answers, as it is in the case of argumentation, learners are guided to think critically, and become metacognitive in their learning and achieve a higher level of abstraction (von Aufschnaiter et al., 2008). This in turn can help students in various ways: (1) to become more intentional in their learning, (2) to become aware of the gaps in their knowledge structure, (3) to become aware of their misconceptions (Cross et al., 2008), (4) to become deliberate in asking questions that can help address their confusion and or address the gaps they have observed in their knowledge structure (Hatano and Inagaki, 1991; Kuhn et al., 2006; Bricker and Bell, 2008; Nussbaum et al., 2008; Yackel, 2001). Similarly, because argumentation exposes students to a critical discourse and calls for justification of the validity of proposed knowledge claims, it helps them to develop more coherent and meaningful knowledge (Leitão, 2001; Ford, 2008). When students are asked to use this knowledge to solve conceptual and quantitative chemistry questions, they can successfully retrieve and use the relevant knowledge. These factors may be responsible for the reported positive effects of argumentation on students' conceptual understanding in chemistry (Aydeniz et al., 2012; Kaya, 2013) and other subjects such as biology (Zohar and Nemet, 2002; Venville and Dawson, 2010) across age groups (Ryu and Sandoval, 2012; Sampson et al., 2013; Zangori et al., 2013).
Increasingly more science educators are embracing and integrating argumentation in their courses. While this movement is impacting the quality of learning in few science classrooms in higher education (Aydeniz et al., 2012; Walker and Sampson, 2013), more work needs to be done to infuse argumentation into college science classrooms, especially science classrooms that are taken by pre-service science teachers. However, this can be challenging as the content courses for teachers are mostly offered through colleges of arts and sciences and not through colleges of education. To make a wider impact on the quality of learning that science teachers experience in college science classrooms, science educators should develop partnerships with college science professors and convince them of the power of reform-based instructional strategies such as argumentation and help them develop pedagogical capacity to teach chemistry, physics and biology through argumentation. One way to achieve this goal is to produce empirical research that can appeal to scientists willing to integrate current instructional theories such as argumentation into their instruction. We call science educators to invest more effort into designing and implementing controlled studies that explore the impact of reform-based instructional strategies such as argumentation in college science classrooms.
Limitations
Like any other studies conducted in education, this study is not immune to limitations. First, this study was conducted in a specific context with a specific group of students taught by two professors with significant years of experience. Second, while we used students' university entrance exam scores, midterm and final exam scores to ensure the comparability of the two groups, this alone may not be sufficient to ensure that the two groups are the same. Some students might have studied better than others, and the argumentation students might have learned to be more explicit and elaborate in their reasoning. This may have had an effect on the reported positive effects of augmentation on students' learning outcomes. We encourage our readers to keep these limitations in mind as they consider the implications of this study for their particular contexts.Appendix A
| Q# | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Quality of answers (0–4) | Frequency of answers for each knowledge level (0–4) | |||||||||
| Level 4_Exp | 20 | 7 | 13 | 13 | 19 | 16 | 25 | 5 | 28 | 4 |
| Level 4_Con | 2 | 0 | 3 | 1 | 4 | 1 | 4 | 0 | 2 | 1 |
| Level 3_Exp | 3 | 1 | 6 | 15 | 12 | 12 | 4 | 2 | 3 | 0 |
| Level 3_Con | 2 | 1 | 5 | 8 | 3 | 0 | 3 | 2 | 13 | 0 |
| Level 2_Exp | 0 | 0 | 0 | 4 | 1 | 3 | 2 | 0 | 0 | 0 |
| Level 2_Con | 3 | 1 | 4 | 3 | 1 | 10 | 2 | 1 | 2 | 0 |
| Level 1_Exp | 5 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 28 |
| Level 1_Con | 8 | 3 | 0 | 8 | 2 | 2 | 7 | 3 | 3 | 24 |
| Level 0_Exp | 4 | 23 | 13 | 0 | 0 | 0 | 1 | 25 | 1 | 0 |
| Level 0_Con | 10 | 20 | 13 | 5 | 15 | 12 | 9 | 19 | 5 | 0 |
References
- Aydeniz M., Pabuccu A., Cetin P. S. and Kaya E., (2012), Argumentation and students' conceptual understanding of properties and behaviors of gases, Int. J. Sci. Math. Educ., 10(6), 1303–1324.
- Alexander R., (2005), Towards dialogic teaching, York: Dialogos.
- Banerjee A. C., (1995), Teaching chemical equilibrium and thermodynamics in undergraduate general chemistry classes, J. Chem. Educ., 72, 879–881.
- Berland L. K. and McNeill K., (2009), Using a learning progression to inform scientific argumentation in talk and writing, paper presented at the Learning Progressions in Science (LeaPS) Conference, Iowa City, IA.
- Bilgin I., (2002), The effect of cooperative learning approach based on conceptual change conditions on students' understanding of chemical equilibrium (Unpublished Doctorate Thesis). The Middle East Technical University, Ankara.
- Bricker L. A. and Bell P., (2008), Conceptualizations of argumentation from science studies and the learning sciences and their implications for the practices of science education, Sci. Educ., 92(3), 473–498.
- Brown A. L. and Campione J. C., (1994), Guided discovery in a community of learners, in McGilly K. (ed.), Classroom lessons: integrating cognitive theory and classroom practice, pp. 229–270.
- Canpolat N., Pınarbaşı T., Bayrakçeken S. and Geban O., (2006) The conceptual change approach to teaching chemical equilibrium, Res. Sci. Technol. Educ., 24(2), 217–235, DOI: http://10.1080/02635140600811619.
- Çakmakçı G., Leach J. and Donnelly J., (2006), Students' ideas about reaction rate and its relationship with concentration or pressure, Int. J. Sci. Educ., 28(15), 1795–1815.
- Chiu M. H., Chou C. C. and Liu J. R., (2002), Dynamic processes of conceptual change: analysis of constructing mental models of chemical equilibrium, J. Res. Sci. Teach., 39(8), 688–712.
- Citation [Def. 2]. (n.d.), Merriam Webster Online, retrieved October 4th, 2015, from http://www.merriam-webster.com/dictionary/argumentation.
- Cross D., Taasoobshirazi G., Hendricks S. and Hickey D. T., (2008), Argumentation: a strategy for improving achievement and revealing scientific identities, Int. J. Sci. Educ., 30(6), 837–861.
- Demircioğlu G., Demircioğlu H. and Yadigaroglu M., (2013), An investigation of chemistry student teachers' understanding of chemical equilibrium, Int. J. New Trends Educ. Their Implic., 4(2), 192–199.
- Driver R., Asoko H., Leach J., Mortimer E. and Scott P., (1994), Constructing scientific knowledge in the classroom, Educ. Res., 23(7), 5–12.
- Driver R., Newton P. and Osborne J. F., (2000), Establishing the norms of scientific argumentation in classrooms, Sci. Educ., 84(3), 287–312.
- Duschl R., Schweingruber H. and Shouse A. (ed.), (2007), Taking science to school: learning and teaching science in grades K-8, Washington, DC: National Academies Press.
- Ford M. J., (2008), Disciplinary authority and accountability in scientific practice and learning, Sci. Educ., 92(3), 404–423.
- Gorodetsky M. and Gussarsky E., (1986), Misconceptualization of the chemical equilibrium concept as revealed by different evaluation methods, Eur. J. Sci. Educ., 8, 427–441.
- Greeno J. G., (1998), The Situativity of Knowing, Learning, and Research, Am. Psychol., 53(1), 5–26.
- Hackling M. W. and Garnett P. J., (1985), Misconceptions of chemical equilibrium, Eur. J. Sci. Educ., 7(2), 205–214.
- Hatano G. and Inagaki K., (1991), Sharing cognition through collective comprehension activity, in Resnick L. B., Levine J. M. and Teasley S. D. (ed.), Perspectives on socially shared cognition, Washington, DC: American Psychological Association, pp. 331–348.
- Huddle P. A. and Pillay A. E., (1996), An in-depth study of misconceptions in stoichiometry and chemical equilibrium at a South African university, J. Res. Sci. Teach., 33(1), 65–77.
- Jiménez-Aleixandre M. P. and Erduran S., (2008), Argumentation in science education: an overview. chapter, in Erduran S. and Jiménez-Aleixandre M. P. (ed.), Argumentation in science education: perspectives from classroom-based research, Dordrecht: Springer, pp. 3–27.
- Jiménez-Aleixandre M., Rodriguez A. and Duschl R., (2000), ‘Doing the lesson’ or ‘doing science’: argument in high school genetics, Sci. Educ., 84(6), 757–792.
- Kaya E., (2013), Argumentation practices in classroom: pre-service teachers' conceptual understanding of chemical equilibrium, Int. J. Sci. Educ., 35(7), 1139–1158, DOI: http://10.1080/09500693.2013.770935.
- Keys C. W., Hand B., Prain V. and Collins S., (1999), Using the Science Writing Heuristic as a tool for learning from laboratory investigations in secondary science, J. Res. Sci. Teach., 36, 1065–1081.
- Kuhn L., Kenyon L. O. and Reiser B. J., (2006), Fostering scientific argumentation by creating a need for students to attend to each other's claims and evidence, Paper presented at the International Conference of the Learning Sciences, Bloomington, IN.
- Lee M.-H., Wu Y.-T. and Tsai C.-C., (2009), Research Trends in Science Education from 2003 to 2007: a content analysis of publications in selected journals, Int. J. Sci. Educ., 31(15), 1999–2020.
- Leitão S., (2001), Analyzing changes in view during argumentation: a quest for method, Qualit. Soc. Res., 2(3), 1–19.
- Locaylocay J., Van Den Berg E. and Magno M., (2005), Changes in college students' conceptions of chemical equilibrium, in Boersma K., Goedhart M., De Jong O. and Eijkelhof H. (ed.), Research and the quality of science education, Dordrecht: Springer, pp. 459–470.
- Maia P. F. and Justi R., (2009), Learning of chemical equilibrium through modeling-based teaching, Int. J. Sci. Educ., 31(5), 603–630.
- Mercer N., Dawes L., Wegerif R. and Sams C., (2004), Reasoning as a scientist: ways of helping children to use language to learn science, Br. Educ. Res. J., 30(3), 359–377.
- Michaels S., O'Connor C. and Resnick L., (2008), Deliberative discourse idealized and realized: accountable talk in the classroom and in civic life, Stud. Philos. Educ., 27, 283–297.
- Newton P., Driver R. and Osborne J. F., (1999), The place of argumentation in the pedagogy of school science, Int. J. Sci. Educ., 21(5), 553–576.
- Niaz M., (1995), Relationship between student performance on conceptual and computational problems of chemical equilibrium, Int. J. Sci. Educ., 17, 343–355.
- Nussbaum M., Sinatra G. and Poliquin A., (2008), Role of the epistemic beliefs and scientific argumentation in science learning, Int. J. Sci. Educ., 30(15), 1977–1999.
- Osborne J. F., Erduran S. and Simon S., (2004), Enhancing the quality of argument in school science, J. Res. Sci. Teach., 41(10), 994–1020.
- Özmen H., (2008), Determination of students' alternative conceptions about chemical equilibrium: a review of research and the case of Turkey, Chem. Educ. Res. Pract., 9, 225–233.
- Park J. Y. and Park H. J., (2002), Identification of college student's and teacher's conceptions for chemical equilibrium and equilibrium shift, J. Korean Chem. Soc., 46(3), 265–278.
- Piquette J. S. and Heikkinen H. W., (2005), Strategies reported used by instructors to address student alternate conceptions in chemical equilibrium, J. Res. Sci. Teach., 42, 1112–1134.
- Posner G. J., Strike K. A., Hewson P. W. and Gertzog W. A., (1982a) Accommodation of a scientific conception: toward a theory of conceptual change, Sci. Educ., 66, 211–227.
- Posner G. J., Strike K. A., Hewson P. W. and Gertzog W. A., (1982b) Accommodation of a scientific conception: toward a theory of conceptual change, Sci. Educ., 66, 211–227.
- Quilez-Pardo J. and Solaz-Portolez J. J., (1995), Students' and teachers' misapplication of Le Chatelier's principle: Implications for the teaching of chemical equilibrium, J. Res. Sci. Teach., 32(9), 939–957.
- Rudd II, J. A., Greenbowe T. J. and Hand B. M., (2007), Using the Science Writing Heuristic to improve students' understanding of general equilibrium, J. Chem. Educ., 84(12), 2007–2012.
- Ryu S. and Sandoval W. A., (2012), Improvements to elementary children's epistemic understanding from sustained argumentation, Sci. Educ., 96(3), 488–526.
- Sampson V. and Walker J. P., (2012), Argument-driven inquiry as a way to help undergraduate students write to learn by learning to write in chemistry, Int. J. Sci. Educ., 34(10), 1443–1485.
- Sampson V., Enderle P., Grooms J. and Witte S., (2013), Writing to learn and learning to write during the school science laboratory: helping middle and high school students develop argumentative writing skills as they learn core ideas, Sci. Educ., 97(5), 643–670.
- Szu E. and Osborne J. F., (2011), Scientific reasoning and argumentation from a Bayesian perspective, in Khine M. S. (ed.), Perspectives on scientific argumentation, Dordrecht: Springer, pp. 55–71.
- Thomas P. L. and Schwenz R. W., (1998), College physical chemistry students' conceptions of equilibrium and fundamental thermodynamics, J. Res. Sci. Teach., 35, 1151–1160.
- Tyson L., Treagust D. F. and Bucat R. B., (1999), The complexity of teaching and learning chemical equilibrium, J. Chem. Educ., 76(4), 554–558.
- van Driel J. H. and Gräber W., (2003), The teaching and learning of chemical equilibrium, in Gilbert J. K., de Jong O., Justi R., Treagust D. F., van Driel J. H. (ed.), Chemical education: towards research-based practice, Dordrecht, The Netherlands: Kluwer Academic Publishers, pp. 271–292.
- Venville G. J. and Dawson V. M., (2010), The impact of a classroom intervention on grade 10 students' argumentation skills, informal reasoning, and conceptual understanding of science, J. Res. Sci. Teach., 47(8), 952–977.
- von Aufschnaiter C., Erduran S., Osborne J. and Simon S., (2008), Arguing to learn and learning to argue: case studies of how students' argumentation relates to their scientific knowledge, J. Res. Sci. Teach., 45(1), 101–131.
- Voska K. W. and Heikkinen H. W., (2000), Identification and analysis of student conceptions used to solve chemical equilibrium problems, J. Res. Sci. Teach., 37(2), 160–176.
- Walker J. P. and Sampson V., (2013), Learning to argue and arguing to learn: Argument-Driven Inquiry as a way to help undergraduate chemistry students learn how to construct arguments and engage in argumentation during a laboratory course, J. Res. Sci. Teach., 50(5), 561–596.
- Walker J. P., Sampson V., Grooms J., Anderson B. and Zimmerman C. O., (2012), Argument-Driven Inquiry in undergraduate chemistry labs: the impact on students' conceptual understanding, argument skills, and attitudes towards science, J. Coll. Sci. Teach., 41(4), 82–89.
- Yackel E., (2001), Explanation, justification and argumentation in mathematics classrooms, in Proceedings of the 25th Annual Meeting of the International Group for the Psychology of Mathematics Education, Utrecht, The Netherlands: The Freudenthal Institute, vol. 1, pp. 9–24.
- Yaghmaie F., (2003), Content validity and its estimation, J. Med. Educ., 3(1), 25–27.
- Zangori L., Forbes C. T. and Biggers M., (2013), Fostering student sense making in elementary science learning environments: elementary teachers' use of science curriculum materials to promote explanation construction, J. Res. Sci. Teach., 50(8), 989–1017.
- Zohar A. and Nemet F., (2002), Fostering students' knowledge and argumentation skills through dilemmas in human genetics, J. Res. Sci. Teach., 39(1), 35–62.
Footnote |
| † A 408 Jane & David Bailey Education Complex, Knoxville, TN 37996-3442. E-mail: maydeniz@utk.edu; Fax: +1-865-974-8718; Tel: +1-865-974-0885. |
| This journal is © The Royal Society of Chemistry 2016 |