A laboratory-scale continuous flow chlorine generator for organic synthesis†
Franz J.
Strauss
a,
David
Cantillo
*ab,
Javier
Guerra
 c and
C. Oliver
Kappe
*ab
c and
C. Oliver
Kappe
*ab
aInstitute of Chemistry, University of Graz, NAWI Graz, Heinrichstrasse 28, 8010, Graz, Austria. E-mail: david.cantillo@uni-graz.at; oliver.kappe@uni-graz.at
bResearch Center Pharmaceutical Engineering GmbH (RCPE), Inffeldgasse 13, 8010 Graz, Austria
cCrystal Pharma, Gadea Pharmaceutical Group, A Division of AMRI, Parque Tecnológico de Boecillo, Valladolid, Spain
First published on 17th August 2016
Abstract
A simple continuous flow setup for the generation and use of elemental chlorine for organic synthesis has been developed. The chlorine generator is based on the reaction of HCl with NaOCl, generating NaCl and H2O as the only side products. As a proof-of-concept, the reactor has been applied for a variety of chlorinations and oxidations of organic compounds.
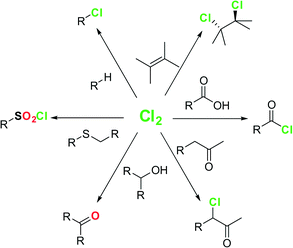
Elemental chlorine (Cl2) is a powerful and one of the cheapest oxidizing agents available, as well as an atom-economic chlorinating agent for organic compounds.1,2 Most of the Cl2 produced worldwide is used for the production of polyvinyl chloride (PVC) and other polymers.1 In addition, Cl2 can be used for the direct introduction of chlorine in organic compounds (Fig. 1). Chlorinated organic compounds are useful building blocks in organic synthesis, and a large number of agrochemicals and pharmaceuticals contain one or more chlorine atoms.3
Despite the importance of Cl2 as an oxidizing and chlorinating agent in organic synthesis, its extremely high reactivity limits its use in many instances, as undesired overreactions and exotherms typically occur. Moreover, Cl2 is a highly toxic and corrosive gas and very stringent regulations are applied at laboratories and production facilities where the gas is used, especially on a large scale.1,4 The Chlorine Institute5 and EuroChlor6 in the US and Europe, respectively, provide guidelines for the safe use, handling and transportation of this substance. Production facilities in which Cl2 gas is used normally need to be isolated, and the personnel must be specifically equipped and trained for the handling of chlorine.4 Another drawback of the use of Cl2 gas for organic synthesis is its low vapor pressure (ca. 7 bar at 25 °C)1 that impedes the constant addition of the reagent to a reaction mixture. When a large amount of Cl2 needs to be added, the drop in the temperature of the gas cylinder further decreases the vapor pressure of the reagent. In such cases, or when higher flow rates/pressures of Cl2 are required, external heating of the gas cylinder or simultaneous use of several units is necessary.1,4
In the past few years, it has been demonstrated that continuous flow and microreactor technology are enabling techniques for the safe and controllable use of hazardous and highly reactive reagents in organic synthesis.7–12 Very exothermic and fast reactions can be easily handled in a continuous flow setup. In this context, the chlorination of organic compounds with Cl2 gas using microreactors has been described by the groups of Jähnisch13 and Ryu.14 In these continuous flow set-ups, Cl2 was supplied using a gas cylinder, whereby some of the safety issues associated with the handling of chlorine could not be eliminated.
Another benefit of continuous flow technology is that hazardous or unstable reagents can be generated in the reactor and consumed in situ or quenched before the reactor output, thus minimizing any risk of exposure.7 Notably, Cl2 can be easily generated from relatively safe, inexpensive and readily available chemicals such as HCl and NaOCl, MnO2, or KMnO4.15 The reaction of HCl with NaOCl is fast, spontaneous, and only generates NaCl and H2O as side products.16 We envisioned that the reaction between HCl and NaOCl in continuous flow to produce Cl2, coupled with a continuous membrane phase separator, could be used to create a laboratory-scale continuous “chlorine generator” for organic synthesis. Therefore, after a sequence composed of mixing of the reagents in the aqueous phase, addition of a suitable organic solvent and separation of the liquid phases using a continuous membrane separator, we obtained a solution of pure Cl2 that could be directly used for oxidation and chlorination reactions, thus avoiding the handling of Cl2 gas cylinders.
In this paper we report the details on the development and optimization of a continuous flow generator of Cl2 from HCl and NaOCl and, as a proof of concept, its use for a series of organic reactions, namely the chlorination of silanes, selective oxidation of secondary alcohols using a chlorine–pyridine reagent, and photochemical chlorination of benzylic compounds.
The continuous flow setup for the generation and separation of Cl2 consisted of three feeds containing NaOCl (1.5 M in water, feed A), HCl (6 M in water, feed B), and the corresponding organic solvent (feed C) (Fig. 2). The liquid streams were pumped using peristaltic pumps (Vapourtec V3, E-Series). Feeds A and B were mixed using a PEEK Y-mixer (0.5 mm id). A significant amount of gas evolution could be visually observed upon mixing of NaOCl and HCl immediately after the Y-mixer, suggesting a fast generation of Cl2. After a short residence time unit (100 μL) the organic phase was added via a second Y-mixer, and the biphasic mixture entered a second residence time unit (800 μL). Notably, all the gas immediately dissolved in all the solvents tested (CHCl3, CH2Cl2, hexane, chlorobenzene), which is in agreement with the solubility data of chlorine for these solvents.1 The aqueous and organic phases were separated using a commercial liquid–liquid membrane separator17 (Zaiput, 1.0 μm PTFE membrane).‡
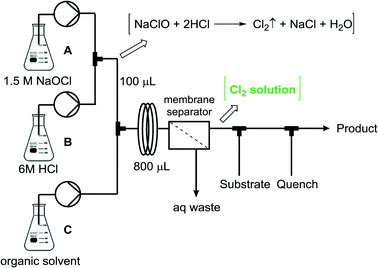 | ||
| Fig. 2 General scheme for the continuous flow setup for the generation, separation, and use of Cl2 for organic reactions (see the ESI† for further details). | ||
An initial set of experiments was carried out to optimize the generation, extraction, and separation of Cl2 (Table 1). Thus, variable amounts of the reagents and different organic solvents were tested. To determine the yield of chlorine, the organic phase coming from the phase separator outlet was collected in an acidified solution of KI in water under stirring, which was then titrated with 0.2 M Na2S2O4 using standard procedures (see the ESI† for details). All yields are calculated with respect to NaOCl. The flow rate of the NaOCl solution was kept constant at 100 μL min−1, which would correspond to a generation of 0.150 mmol min−1 of Cl2. Notably, a very good Cl2 yield was obtained even when a stoichiometric amount of HCl was used (entry 1). Using 3 equiv. of HCl, corresponding to a 50% excess, an optimal result of 91% yield was achieved (entry 2). A larger excess of HCl did not further improve this result (entry 3). Notably, moderate to very good yields were also observed using other solvents (entries 4–6), thus demonstrating that this concept can be applied for a variety of chemistries that require different solvent conditions. Immiscibility with water is the key requirement to take into account, essential for phase separation. Thus, solvents with high Cl2 solubility – but water miscible – such as AcOH or DMF could not be tested. The best results were observed for CHCl3 and CH2Cl2. Given the high solubility of Cl2 in CHCl3 (ca. 20% wt%), a variety of Cl2 concentrations could be generated by simply modifying the flow rate for the organic solvent (entries 7–10). Importantly, similar yields of ca. 90% were obtained in all cases. This yield corresponds to a generation of 0.135 mmol min−1 of Cl2 which, if required, could be tuned by modifying the initial flow rate for the 1.5 M NaOCl solution.
| Entry | Flow rate A (μL min−1) | Flow rate B (μL min−1) | Equiv. HClb | Solvent C | Flow rate C (μL min−1) | Cl2 yieldc (%) |
|---|---|---|---|---|---|---|
a Feed A: 1.5 M NaOCl; feed B: 6 M HCl.
b As the reaction stoichiometry for HCl/NaOCl is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3 equiv. represents a 50% excess.
c Determined by titration using KI/Na2S2O4 (see the ESI for details). 1, 3 equiv. represents a 50% excess.
c Determined by titration using KI/Na2S2O4 (see the ESI for details).
|
||||||
| 1 | 100 | 50 | 2 | CHCl3 | 300 | 83 |
| 2 | 100 | 75 | 3 | CHCl3 | 300 | 91 |
| 3 | 100 | 100 | 4 | CHCl3 | 300 | 86 |
| 4 | 100 | 75 | 3 | CH2Cl2 | 150 | 84 |
| 5 | 100 | 75 | 3 | Hexane | 150 | 54 |
| 6 | 100 | 75 | 3 | Chlorobenzene | 150 | 70 |
| 7 | 100 | 75 | 3 | CHCl3 | 100 | 84 |
| 8 | 100 | 75 | 3 | CHCl3 | 150 | 88 |
| 9 | 100 | 75 | 3 | CHCl3 | 200 | 90 |
| 10 | 100 | 75 | 3 | CHCl3 | 300 | 91 |
Using the optimal conditions for the generation, extraction, and separation of Cl2, a series of organic transformations using directly the solution obtained from the reactor output were carried out. The preparation of silyl chlorides of type 2 from silanes was initially attempted as a simple proof-of-concept chemical reaction (Fig. 3). Triisopropyl-, dimethylphenyl-, and triphenylsilane were chosen as model substrates. Thus, a solution of the corresponding silane in CHCl3 (0.5 M) was pumped using a syringe pump and mixed with the Cl2 solution from the membrane separator output using a Y-mixer (Fig. 3). The flow rate for the silane solution was set so that it is mixed with 1.2 equiv. of Cl2 (see the ESI† for experimental details). After a residence time of 10 min, the crude reaction mixture from the reaction output was immediately analyzed by GC-MS and GC-FID. Gratifyingly, full conversion of the silanes 1 to the desired silyl chlorides 2 was observed for the three examples tested (2a–c) (Fig. 3). The reactions were fully selective, and no traces of side products were observed. Simple evaporation of the solvent yielded quantitative amounts of the pure chlorosilanes.§ This procedure for the preparation of chlorosilanes has high potential for many examples due to its simplicity and atom-economy with respect to other procedures involving metal chloride catalysts, PCl3 or SO2Cl2.18
 | ||
| Fig. 3 Direct chlorination of silanes using the Cl2 generator. aCompound 2b hydrolyzed after being in contact with air and could not be characterized. The GC-FID yield for 2b is given. Isolated yields of 2a and 2c are shown. For a fully detailed flow scheme, see the ESI.† | ||
We next turned our attention towards a more complex transformation in which, owing to selectivity issues from the high reactivity of Cl2, the use of continuous flow technology could be beneficial. The chlorine–pyridine complex (Cl2–Py) is an oxidizing agent that is formed spontaneously and with a strong exotherm upon mixing of the two reagents.19 The chlorine–pyridine complex has been scarcely used for the oxidation of alcohols. Notably, a significant difference in the oxidation rate of secondary over primary alcohols has been reported, which permits the oxidation of the secondary group with some degree of selectivity (ca. 80%).20 We envisaged that the Cl2–Py complex could be generated in situ using the chlorine generator, and used for the selective oxidation of secondary alcohols (Fig. 4).21 A set of preliminary batch experiments, using 1,2-hexanediol (3a) as a model substrate, allowed us to establish the optimal Cl2 to pyridine ratio and the other reaction parameters (see the ESI† for details). Translation of the reaction into flow conditions was carried out using the same setup as for the chlorosilane synthesis.
 | ||
| Fig. 4 Selective oxidation of secondary alcohols using the chlorine–pyridine complex. aDetermined by GC-FID. bIsolated yields after column chromatography. For a fully detailed flow scheme, see the ESI.† | ||
The reaction mixture was quenched at the reactor output using an excess of iPrOH. Using 1.2 equiv. of Cl2 and 4 equiv. of pyridine, excellent conversion and selectivity were obtained for the selective oxidation of 1,2-hexanediol to 1-hydroxy-2-hexanone 4a after 15 min at room temperature (Fig. 4). Analogous results were achieved for an 1,3-diol (4b) and a benzylic alcohol (4c), with full conversion and excellent selectivity in both cases. Purification of the resulting hydroxyketones by extraction resulted in good yields, although the isolated products contained small amounts of pyridine. Analytically pure materials were obtained after column chromatography, which unfortunately decreased the isolated yields (Fig. 4).
Finally, we coupled the chlorine generator with a continuous flow photochemical reactor for the benzylic chlorination of a series of substituted toluenes (Fig. 5).
 | ||
| Fig. 5 Continuous photochemical chlorination of toluene derivatives. Yields are calculated with respect to Cl2, and determined by GC-FID analysis. For a fully detailed flow scheme, see the ESI.† | ||
For this purpose, a UV-150 photoreactor from Vapourtec was used. The photoreactor consisted of 10 mL FEP tubing (id 1.0 mm) and was equipped with a Hg lamp and a filter with a <300 nm cutoff.¶ A set of preliminary experiments using toluene as the model substrate was carried out to optimize the reaction conditions (see the ESI†). Thus, toluene was pumped neat and mixed with the Cl2 solution before entering the photoreactor. Using a temperature of 40 °C and ca. 15 min residence time (ca. 0.7 mL min−1 total flow rate, see experimental details in the ESI†), full conversion of chlorine and excellent selectivity for the desired benzyl chloride (6a) were obtained (the reaction was monitored by GC-MS and GC-FID using an internal standard). Very good results under the same conditions were also obtained for other toluene derivatives bearing electron-donating and electron-withdrawing groups (Fig. 5). Chlorination of 3-nitrotoluene was slower and only 86% conversion was obtained. In the case of 4-methylanisole, the high reactivity of the ring resulted in full conversion but a reduced selectivity (81%) for the monochlorination on the benzylic position (6b). In this case, chlorination of the aromatic ring and dichlorinated products were also observed. The photochlorination also proceeded well for the ortho substituted xylene with excellent conversion of chlorine and selectivity for 6d.
Notably, the high efficiency of this continuous flow photochemical chlorination (80–99% yield in 10–15 min) is in contrast with the poor results typically obtained in batch where several hours of irradiation under reflux conditions are typically required.22,23 The results are also superior to previous photochemical chlorinations using microreactor technology, where either low yields were obtained14 or high temperatures, resulting in poor selectivities, were required.13
Conclusions
We have developed a simple and convenient continuous flow setup for the generation, extraction, and separation of elemental chlorine for organic synthesis. The method is based on the reaction of HCl with NaOCl, which rapidly releases Cl2 gas that can be extracted using suitable organic solvents and separated using continuous membrane separation technology. High chlorine yields (ca. 90%) could be obtained for a variety of organic solvents. The chlorine generator has been tested for a series of organic reactions, namely the chlorination of silanes, photochlorination of benzylic compounds, and selective oxidation of secondary alcohols. Excellent results were obtained in all cases. The present protocol avoids the safety issues derived from the handling of Cl2 gas cylinders, and can be extremely useful for lab- and mesoscale experiments involving the reagent, such as preliminary experiments and reaction optimizations where a dedicated chlorine chemistry facility is not available.Notes and references
- P. Schmittinger, T. Florkiewicz, L. C. Curlin, B. Lüke, R. Scannell, T. Navin, E. Zelfel and R. Bartsch, Chlorine in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag, Weinheim, 2001 Search PubMed.
- V. Cornel, e-EROS Encyclopedia of Reagents for Organic Synthesis, 2001, DOI:10.1002/047084289X.rp071.
- K. Naumann, J. Prakt. Chem., 1999, 340, 450 Search PubMed.
- The Chlorine Manual, 6th Edition, The Chlorine Institute, Inc., Washington, D. C., 2001 Search PubMed.
- The Chlorine Institute, https://www.chlorineinstitute.org/.
- EuroChlor, http://www.eurochlor.org/.
- P. Poechlauer, S. Braune, B. Dielemans, B. Kaptein, R. Obermüller and M. Thathagar, Chim. Oggi Chem. Today, 2013, 30(4), 51 Search PubMed.
- B. Gutmann, D. Cantillo and C. O. Kappe, Angew. Chem., Int. Ed., 2015, 54, 6688 CrossRef CAS PubMed.
- D. E. Fitzpatrick, C. Battilocchio and S. V. Ley, ACS Cent. Sci., 2016, 2, 131 CrossRef CAS PubMed.
- K. F. Jensen, B. J. Reizmana and S. G. Newman, Lab Chip, 2014, 14, 3206 RSC.
- B. Gutmann and C. O. Kappe, Chim. Oggi Chem. Today, 2015, 33(3), 14–18 Search PubMed.
- S. T. R. Mueller and T. Wirth, ChemSusChem, 2015, 8, 245 CrossRef CAS PubMed.
- H. Ehrich, D. Linke, K. Morgenschweis, M. Baerns and K. Jähnisch, Chimia, 2002, 56, 647 CrossRef CAS.
- H. Matsubara, Y. Hino, M. Tokizane and I. Ryu, Chem. Eng. J., 2011, 167, 567 CrossRef CAS.
- B. S. Furniss, A. J. Hannaford, P. W. G. Smith and A. R. Tatchell, Vogel's Textbook of Practical Organic Chemistry, Wiley, New York, 5th edn, 1989 Search PubMed.
- The NaOCl/HCl system has been used for oxidation and chlorination reactions in batch using biphasic mixtures. For recent examples, see: S. W. Wright and K. N. Hallstrom, J. Org. Chem., 2006, 71, 1080 CrossRef CAS PubMed; X.-F. Zhao and C. Zhang, Synthesis, 2007, 4, 551 Search PubMed; J. Tao, T. N. Tuck and G. K. Murphy, Synthesis, 2016, 48, 772 Search PubMed.
- A. Adamo, P. L. Heider, N. Weeranoppanant and K. F. Jensen, Ind. Eng. Chem. Res., 2013, 52, 10802 CrossRef CAS.
- For recent examples using these reagents, see: SO2Cl2: L. Wang, R. K. Akhani, K. Ravish and S. L. Wiskur, Org. Lett., 2015, 17, 2408 CrossRef CAS PubMed PCl5: F. Hoffmann, J. Wagler and G. Roewer, Eur. J. Inorg. Chem., 2010, 1133 CrossRef C2Cl6/PdCl2: A. Naka and M. Ishikawa, Organometallics, 2001, 20, 1695 CrossRef.
- R. R. Goehringl, e-EROS Encyclopedia of Reagents for Organic Synthesis, 2001, DOI:10.1002/047084289X.rc062.
- J. Wicha and A. Zarecki, Tetrahedron Lett., 1974, 3059 CrossRef CAS.
- For a non-selective continuous flow method for the oxidation of alcohols using bleach, see: A. B. Leduc and T. F. Jamison, Org. Process Res. Dev., 2012, 16, 1082 CrossRef CAS.
- G. Xie, Q. Zheng, C. Huang and Y. Chen, Synth. Commun., 2003, 33, 1103 CrossRef CAS.
- M. R. Pavia, S. J. Lobbestael, C. P. Taylor, F. M. Hershenson and D. L. Miskell, J. Med. Chem., 1990, 33, 854 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data, and supplementary figures and tables. See DOI: 10.1039/c6re00135a |
| ‡ Initial experiments were performed using a Teflon AF2400 tube to directly separate Cl2 gas from the aqueous phase, in a tube-in-tube reactor. Generation and separation of Cl2 using this approach proceeded well. However, after several experiments, the Teflon AF2400 tube was significantly degraded by the chlorine and ruptured. At this point, we decided to modify our strategy and use a biphasic solvent system and a liquid–liquid membrane separator. A single PTFE membrane was used for all experiments performed for this work, without apparent degradation. |
| § Chlorodimethylphenylsilane (2b) could not be isolated in pure form due to rapid hydrolysis after being in contact with air. The yield given was determined by GC-FID. |
| ¶ The reaction could also be performed with a <365 nm cutoff filter (black light), although conversions were lower. In the absence of UV irradiation, no conversion was observed (see the ESI† for details). |
| This journal is © The Royal Society of Chemistry 2016 |