Continuous photochemistry: the flow synthesis of ibuprofen via a photo-Favorskii rearrangement†
M.
Baumann
and
Ian R.
Baxendale
*
Department of Chemistry, University of Durham, South Road, Durham, DH1 3LE, UK. E-mail: i.r.baxendale@durham.ac.uk
First published on 4th November 2015
Abstract
A new enabling technology for performing photochemical reactions in a continuous fashion is presented. This photo-reactor is compatible with existing flow systems and can be furthermore linked to a photo-spectrometer in order to allow for real time analysis of photochemical reactions. In this communication we wish to report the profiling of this system and its application to the continuous synthesis of ibuprofen based on a photo-Favorskii rearrangement reaction of a readily available α-chloropropiophenone precursor.
Due to its many benefits over conventional batch methods, flow chemistry has seen widespread applications in modern synthetic chemistry in areas such as synthesis methodology,1 material science2 or the efficient preparation of bio-active target compounds.3 Specifically the latter area has witnessed great attention from academia as well as pharmaceutical and agrochemical industries as it provided a fertile ground for validating claims of safer and more efficient processing. This area has been reviewed recently summarizing the current standing of the community regarding the continuous manufacture of active pharmaceutical ingredients (API's) and highlighting numerous accomplishments in this fast developing field.4 Despite these milestones further efforts are needed in order to improve the overall processes by increasing the material throughput of such continuous approaches and at the same time utilising more atom economical and environmentally benign strategies. Consequently, recent focus has shifted towards the application of alternative continuous methods, for instance those based on electro-5 and photochemical protocols.6 In this article we wish to report on our results leading to a continuous synthesis of the high volume drug ibuprofen utilising a photo-Favorskii rearrangement as the key step.
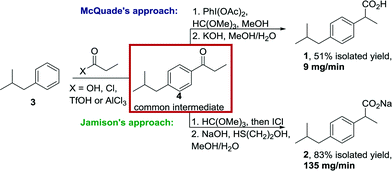
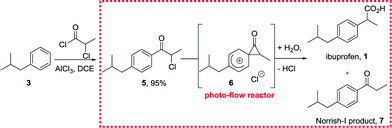
Ibuprofen (1, isobutylphenyl propionic acid) is a nonsteroidal anti-inflammatory drug (NSAID) widely used for alleviating pain, fever and inflammation. It is sold over the counter and annual production is estimated to surpass 9.8 kilotonnes.7 Since its discovery in the early 1960's several routes have been reported8 also including two recent flow approaches by McQuade (2009)9 and Jamison (2015).10 Although the latter were not intended to rival the known manufacturing routes regarding modern process metrics (productivity, reagent cost, waste removal etc.), these examples represent important proof of concept studies towards generating a simple, yet high demand API in a continuous fashion. Both flow approaches share the use of a Friedel–Crafts acylation between isobutylbenzene (3) and propionic acid activated in situ using excess triflic acid or propionyl chloride and AlCl3 (Scheme 1). A subsequent 1,2-aryl migration step is achieved through treatment of the intermediate propiophenone species with either PhI(OAc)2/trimethyl orthoformate (HC(OMe)3) or ICl/trimethyl orthoformate yielding after alkaline ester hydrolysis crude ibuprofen or its sodium salt. Whereas McQuade's earlier approach delivers 9 mg ibuprofen per minute (51% isolated yield), the improved strategy reported by the Jamison lab generates up to 135 mg ibuprofen Na-salt per minute (83% isolated yield).
We initiated our own studies by devising a synthetic strategy based on a photo-Favorskii rearrangement of α-halopropio-phenones and related compounds11 to directly furnish the desired arylpropionic acid scaffold present in ibuprofen. As such we prepared key building block 5 by a Friedel–Crafts acylation between isobutylbenzene and chloropropionyl chloride in the presence of AlCl3. Performing this reaction in batch yielded the desired product with high regioselectivity (>9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 para
1 para![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ortho) and yield in a straightforward fashion. Although the chloropropionyl chloride used in this step is on small scale more expensive than propionic acid itself, we deemed this rapid access into the desired building block more favourable as (1) no further functionalisation or functional group interconversion is needed and (2) chloropionyl chloride can be cheaply prepared by chlorination of readily available and biorenewable lactic acid through literature-known procedures.12
ortho) and yield in a straightforward fashion. Although the chloropropionyl chloride used in this step is on small scale more expensive than propionic acid itself, we deemed this rapid access into the desired building block more favourable as (1) no further functionalisation or functional group interconversion is needed and (2) chloropionyl chloride can be cheaply prepared by chlorination of readily available and biorenewable lactic acid through literature-known procedures.12
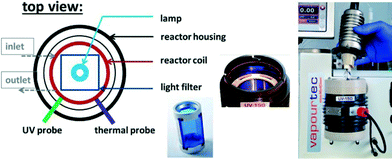
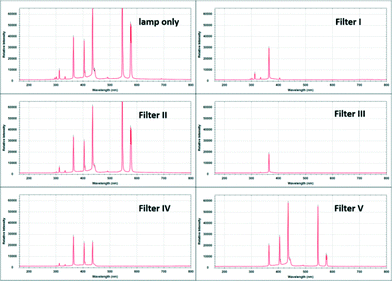
In order to study the interconversion of α-halopropiophenone 5 into ibuprofen we decided to use a commercially available Vapourtec E-series flow system in conjunction with its UV-150 photo-reactor. This system is ideally suited for exploratory studies as it accommodates the bespoke photo-reactor that can be operated at various temperatures via active cooling or heating scenarios (−5–80 °C). Furthermore, the power setting of its high intensity medium pressure metal halide lamp can be selected and easily adjusted by dimming (50–100% equivalent to 75–150 W) whilst several filters can be used in order to select a specific wavelength range. In order to evaluate these filters towards our intended application we used a portable ExemplarLS spectrometer that was readily connected to the photo-flow reactor set-up allowing us to record transmission spectra of the lamp as well as the selected filters (I–V) provided with the system (Fig. 1).
Screening the different filters by passing water as solvent through the flow set-up at 50% power setting (75 W) indicated that filter I and III (Fig. 2) would specifically remove wavelengths above 400 nm which we deemed desirable as blocking the infrared part of the light emitted by the lamp would also reduce the necessity to actively adjust the temperature of the reactor coil.
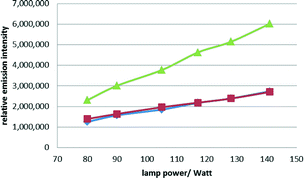
In a further set of experiments we profiled filter III regarding its response towards different lamp power settings for either a) the reaction solvent alone (10 vol% water in acetone; green graph Fig. 3), b) solvent containing α-chloropropiophenone substrate 5 (0.1 M; red graph Fig. 3) or c) solvent with substrate 5 (0.1 M) and scavenger (propylene oxide, 2 vol%; blue graph Fig. 3). Plotting the integral of the emission signal at 365 nm against the power setting (in 50–100% or 75–150 W) confirmed that the solvent as well as the scavenger barely absorb at this wavelength, whereas the substrate absorbs about 50% of the emitted light. Furthermore, the reaction products do not seem to significantly absorb light of this wavelength either.
After this initial profiling of the photo-reactor set-up we commenced our synthetic studies by subjecting substrate 5 (0.1 M solutions) to a set of reaction conditions in an attempt to prepare the desired ibuprofen product (Scheme 2). As such we elected to use acetone/water (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 vol/vol) as a green solvent system that would convert the putative spirocyclic intermediate 6 directly into the acid form of ibuprofen. Furthermore, we decided to add a small quantity of propylene oxide (2 vol%) to this solvent system to act as a scavenger for the hydrochloric acid formed in the process. Having defined these parameters we next studied a selection of reaction conditions as summarised in Table 1.
10 vol/vol) as a green solvent system that would convert the putative spirocyclic intermediate 6 directly into the acid form of ibuprofen. Furthermore, we decided to add a small quantity of propylene oxide (2 vol%) to this solvent system to act as a scavenger for the hydrochloric acid formed in the process. Having defined these parameters we next studied a selection of reaction conditions as summarised in Table 1.
| Entry | Filter | Concentration/M [scavenger/vol%] | Reactor temperature/°C | Lamp power setting | tRes/min |
1H-NMR ratio 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (reactions in duplicate) 7 (reactions in duplicate) |
Productivity of 1/mmol h−1 |
|---|---|---|---|---|---|---|---|
| 1 | None, II, IV, V | 0.1 (vol%) | 30 to >80 or 30 (cooled) | 80% | 20 | >90% conv., complex mixture, 70–80% conv., cleaner reaction | n.a. |
| 2 | III | 0.1 (vol%) | 55 | 80% | 40 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
1.10 |
| 3 | I | 0.1 (vol%) | 65 | 80% | 40 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 86 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
1.29 |
| 4 | I | 0.08 (vol%) | 65 | 80% | 40 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
1.28 |
| 5 | I | 0.12 (vol%) | 65 | 80% | 40 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
1.17 |
| 6 | I | 0.1 (vol%) | 65 | 80% | 30 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 84 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
1.68 |
| 7 | I | 0.1 (vol%) | 65 | 80% | 20 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 84 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
2.52 |
| 8 | I | 0.1 (vol%) | 65 | 80% | 15 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 81 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
3.65 |
| 9 | I | 0.1 (vol%) | 65 | 80% | 10 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
4.20 |
| 10 | I | 0.1 (vol%) | 65 | 80% | 7.5 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
4.77 |
| 11 | I | 0.1 (vol%) | 70 | 90% | 10 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 68 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
4.08 |
| 12 | I | 0.1 (vol%) | 75 | 100% | 10 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
4.38 |
| 13 | I | 0.1 (vol%) | 70 | 90% | 7.5 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
5.13 |
| 14 | I | 0.1 (vol%) | 75 | 100% | 7.5 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 63 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
5.67 |
| 15 | I | 0.1 (ref. 1) | 65 | 80% | 15 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
3.38 |
| 16 | I | 0.1 (ref. 3) | 65 | 80% | 15 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 81 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
3.65 |
| 17 | I | 0.1 (vol%) | 20 (cooled) | 80% | 15 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
2.79 |
We quickly established that employing either no filter or filters II, IV or V was unfavourable to the reaction outcome as the heat generated by the lamp led to a significant temperature increase greater than 80 °C and consequently resulted in substantial by-product formation. On the other hand regulating the reactor temperature to between 30–35 °C, by active cooling with a stream of cold nitrogen, under the same conditions gave a cleaner reaction albeit at the cost of residual starting material (>20%). We also encountered defunctionalised propiophenone 7, which arises from an undesired Norrish type I fragmentation pathway (~15%). Next we utilised filters I and III exposing the reactor coil to almost monochromatic light of ~365 nm wavelength. Pleasingly, both filters resulted in a much cleaner conversion of 5 into ibuprofen 1 (with very small amounts of Norrish product 7) allowing the further optimisation of these conditions. For the proceeding optimisation study filter I was selected over filter III as it allowed for a higher power input at the same major wavelength (see Fig. 2). Subsequently, it was established that a concentration of 0.1 M with 200 μL scavenger/10 mL solution was best, whilst a lower concentration (0.08 M) did not improve the reaction and a higher concentration resulted in more Norrish-I product formation (Table 1, entries 3, 4, 5).
Next the residence time was optimised indicating that just over 15 minutes was sufficient to achieve almost complete conversion of substrate 5 producing ibuprofen in >80% (Table 1, entries 3, 6, 7, 8). Although it was established that lower residence times of 10 or 7.5 minutes would give diminished conversions, the overall productivity of ibuprofen would under these conditions be significantly higher (Table 1, entries 9 and 10). This effect can be further enhanced by increasing the lamp power setting from 80% to 90 or 100% resulting in an increased productivity of up to 5.67 mmol ibuprofen per hour but at the cost of incomplete conversion of starting material 5 (Table 1, entries 9–14). In addition, a higher amount of scavenger was not found to be beneficial, whilst less scavenger led to increased amounts of the Norrish-I product (Table 1, entries 8, 15, 16). Finally, decreasing the reaction temperature from 65 °C to 20 °C was found to lead to lower conversion with more Norrish-I product (Table 1, entries 8 and 17) indicating that the conditions of entry 8 would seem to balance best the substrate conversion and ibuprofen productivity.
In order to further profile this transformation selected cases were evaluated at larger scales (10 mmol scale, 20 min residence time, 4 h total processing time). As such using the conditions of entry 7 delivered ibuprofen in 76% isolated yield, whereas scale-up of entry 9 and 14 generated the target compound in 63% and 54% respectively thus showing good agreement with the findings of the initial optimisation study. Besides the ease of performing this photo-Favorskii reaction in a continuous fashion, it was found that product isolation through solvent evaporation and acid–base extraction rapidly and reliably yielded ibuprofen upon trituration from hexanes as a white solid.
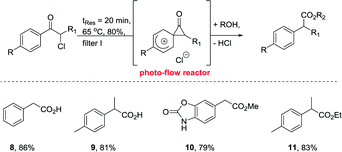
Finally, we decided to validate our continuous flow protocol by subjecting a small selection of different substrates to the optimised reaction conditions (Table 1, entry 7) in order to prepare the corresponding photo-Favorskii products. As such we were pleased to see that different α-chloroaceto- and propiophenones yielded the desired products (8–11) in high yield (Scheme 3). In addition, this methodology was successfully extended to also prepare selected ester products (10, 11) by substituting water with methanol or ethanol as the minor component in the solvent system (i.e. 10% alcohol in acetone).
Conclusions
In conclusion, we have developed a fast and efficient continuous process yielding ibuprofen via a photo-Favorskii rearrangement of a readily available α-chloropropiophenone precursor. Importantly, this has been achieved through a newly available enabling technology in the form of a photochemical reactor unit that can be used in combination with existing and commercially available flow reactor systems. Aided by real-time analysis through a small footprint photo-spectrometer a rapid profiling of various reaction conditions (including different filters, residence times, concentrations etc.) was conducted, establishing conditions for high productivity of ibuprofen. The best conditions were subsequently verified at larger scale allowing photochemical production of multi-gram quantities of ibuprofen within a single working day. This demonstrates that this new photochemical reactor can be used efficiently for the continuous lab scale synthesis of valuable compounds.Acknowledgements
We gratefully acknowledge financial support from the Royal Society (to MB and IRB). Furthermore we are grateful to Duncan Guthrie and Stacey Crane (Vapourtec) for lending the photo-spectrometer used in this project as well as helpful discussions.Notes and references
- For selected recent reviews on flow chemistry, see:
(a) S. V. Ley, D. E. Fitzpatrick, R. M. Myers, C. Battilocchio and R. J. Ingham, Angew. Chem., 2015, 54, 10122 CrossRef CAS PubMed
; (b) J. Yoshida, H. Kim and A. Nagaki, ChemSusChem, 2011, 4, 331 CrossRef CAS PubMed
; (c) K. F. Jensen, B. J. Reizman and S. G. Newman, Lab Chip, 2014, 14, 3206 RSC
; (d) J. Wegner, S. Ceylan and A. Kirschning, Adv. Synth. Catal., 2012, 354, 17 CrossRef CAS
; (e) V. Hessel, D. Kralisch, N. Kockmann, T. Noël and Q. Wang, ChemSusChem, 2013, 6, 746 CrossRef CAS PubMed
; (f) I. R. Baxendale, J. Chem. Technol. Biotechnol., 2013, 88, 519 CrossRef CAS
; (g) I. R. Baxendale, C. J. Mallia and L. Brocken, Green Process. Synth., 2013, 2, 211 CAS
.
- For the use of continuous methods in materials chemistry, see:
(a) R. M. Myers, D. E. Fitzpatrick, R. M. Turner and S. V. Ley, Chem. – Eur. J., 2014, 30, 12348 CrossRef PubMed
; (b) D. T. McQuade and P. H. Seeberger, J. Org. Chem., 2013, 78, 6384 CrossRef CAS PubMed
.
- For recent reviews on flow synthesis of bioactive molecules, see:
(a) J. C. Pastre, D. L. Browne and S. V. Ley, Chem. Soc. Rev., 2013, 42, 8849 RSC
; (b) M. Baumann, I. R. Baxendale and S. V. Ley, Mol. Diversity, 2011, 15, 613 CrossRef CAS PubMed
, Also see ref. 4.
-
(a) M. Baumann and I. R. Baxendale, Beilstein J. Org. Chem., 2015, 11, 1194 CrossRef CAS PubMed
; (b) B. Gutmann, D. Cantillo and C. O. Kappe, Angew. Chem., 2015, 54, 6688 CrossRef CAS PubMed
.
- For recent examples of flow electrochemistry, see:
(a) M. A. Kabeshov, B. Musio, P. R. D. Murray, D. L. Browne and S. V. Ley, Org. Lett., 2014, 16, 4618 CrossRef CAS PubMed
; (b) K. Watts, A. R. Baker and T. Wirth, J. Flow Chem., 2014, 4, 2 CrossRef
; (c) K. Arai and T. Wirth, Org. Process Res. Dev., 2014, 18, 1377 CrossRef CAS
; (d) K. Watts, W. Gattrell and T. Wirth, Beilstein J. Org. Chem., 2011, 7, 1108 CrossRef CAS PubMed
.
- For recent examples of flow photochemistry, see:
(a) J. W. Beatty and C. R. J. Stephenson, J. Am. Chem. Soc., 2014, 136, 10270 CrossRef CAS PubMed
; (b) L. D. Elliott, J. P. Knowles, P. J. Koovits, K. G. Maskill, M. J. Ralph, G. Lejeune, L. J. Edwards, R. I. Robinson, I. R. Clemens, B. Cox, D. D. Pascoe, G. Koch, M. Eberle, M. B. Berry and K. I. Booker-Milburn, Chem. – Eur. J., 2014, 20, 15226 CrossRef CAS PubMed
; (c) F. Lévesque and P. H. Seeberger, Angew. Chem., Int. Ed., 2012, 51, 1706 CrossRef PubMed
; (d) D. Kopetzki, F. Lévesque and P. H. Seeberger, Chem. – Eur. J., 2013, 19, 5450 CrossRef CAS PubMed
; (e) J. P. Knowles, L. D. Elliott and K. I. Booker-Milburn, Beilstein J. Org. Chem., 2012, 8, 2025 CrossRef CAS PubMed
; (f) J. P. Knowles, L. D. Elliott and K. I. Booker-Milburn, Beilstein J. Org. Chem., 2012, 8, 2025 CrossRef CAS PubMed
; (g) S. Cludius-Brandt, L. Kupracz and A. Kirschning, Beilstein J. Org. Chem., 2013, 9, 1745 CrossRef PubMed
; (h) K. Gilmore and P. H. Seeberger, Chem. Rec., 2014, 14, 410 CrossRef CAS PubMed
; (i) D. B. Ushakov, K. Gilmore, D. Kopetzki, D. T. McQuade and P. H. Seeberger, Angew. Chem., Int. Ed., 2014, 53, 557 CrossRef CAS PubMed
.
- Global Ibuprofen Industry Research Report 2015 from LifeScienceIndustryResearch.com for details see website: http://www.lifescienceindustryresearch.com/purchase?rn ame=38384, accessed 18/08/2015.
- For selected patent references towards ibuprofen, see:
(a)
D. D. Lindley, T. A. Curtis, T. R. Ryan, E. M. de la Garza, C. B. Hilton and T. M. Kenesson, Process for the production of 4'-isobutylacetophenone, US Pat. US 5,068,448, Nov 26, 1991 Search PubMed
; (b) D. A. Ryan, Method for producing 1-(4'-isobutylphenyl)ethanol, US Pat. US 4,929,773, May 29, 1990 Search PubMed
; (c) V. Elango, M. A. Murphy, B. L. Smith, K. G. Davenport, G. Mott, E. G. Zey and G. L. Moss, Method for producing ibuprofen, US Pat. US 4,981,995, Jan 1, 1991 Search PubMed
.
- A. R. Bogdan, S. L. Poe, D. C. Kubis, S. J. Broadwater and D. T. McQuade, Angew. Chem., Int. Ed., 2009, 48, 8547 CrossRef CAS PubMed
.
- D. R. Snead and T. F. Jamison, Angew. Chem., 2015, 54, 983 CrossRef CAS PubMed
.
- H. Sonawane, N. S. Bellur, D. G. Kulkarni and N. R. Ayyangar, Tetrahedron, 1994, 50, 1243 CrossRef CAS
.
- For example:
(a)
J. S. Chirtel, A. M. Mark and S. Brelant, (Hydroxyacyl)amino acids and their polymers, US Pat. US 2,786,045, Jan 21, 1953 Search PubMed
; (b) J. Xing, X. Zhang, J. Liu and F. Gu, A process for preparing chiral 2-chloro-propanoyl chloride, Chinese Pat. CN 102,190,574, Mar 11, 2010 Search PubMed
.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c5re00037h |
| This journal is © The Royal Society of Chemistry 2016 |