Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: IDO as a drug target for cancer immunotherapy: recent developments in IDO inhibitors discovery
Shan Qian*a,
Man Zhanga,
Quanlong Chena,
Yanying Hea,
Wei Wanga and
Zhouyu Wang*b
aDepartment of Pharmaceutical Engineering, Xihua University, Chengdu 610039, P. R. China. E-mail: qians33@163.com
bDepartment of Chemistry, Xihua University, Chengdu 610039, P. R. China. E-mail: zhouyuwang77@gmail.com
First published on 27th June 2016
Abstract
Correction for ‘IDO as a drug target for cancer immunotherapy: recent developments in IDO inhibitors discovery’ by Shan Qian et al., RSC Adv., 2016, 6, 7575–7581.
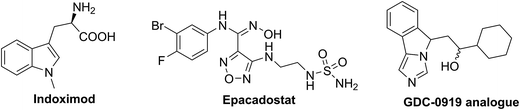
In Fig. 2 of the original manuscript, the structures given for Indoximod and Epacadostat were incorrect. In addition, the structure labelled “GDC-0919” was labelled incorrectly; the correct label is “GDC-0919 analogue”. The corrected Fig. 2 is shown below.
In Section 3 of the manuscript, “Therapeutic strategies and challenges of IDO inhibitors in cancer immunotherapy”, an incorrect IC50 value was given for Epacadostat, and a citation to ref. 17 of the original manuscript was omitted. The corrected text should read:
“Epacadostat (INCB024360) was obtained following a high throughput screening (HTS) of Incyte's corporate collection (IC50 = 0.072 μM).17,18”
In Section 4 of the manuscript, “Structure-based IDO inhibitors design”, a citation to ref. 26 of the original manuscript was omitted. The corrected text should read:
“Based on the co-crystal structure of IDO with PIM, Roerhrig et al.26 and Huang et al.30 discovered independently that 1H-1,2,3-triazole might be a new key pharmacophore of potent IDO inhibitors.”
Additionally, the following discussion should be added to the end of Section 4, and the reference cited as ref. 1 of this correction should be added:
“As Roehrig et al. were concentrating on developing 4-aryl triazoles as potent IDO inhibitors, they used computational structure-based methods to design more triazole-based IDO inhibitors with low molecular weight and high efficiency. The most potent compound has an IC50 value in the nanomolar range both in the enzymatic and in the cellular assays.1”
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
References
- U. F. Roehrig, S. R. Majjigapu, A. Grosdidier, S. Bron, V. Stroobant, L. Pilotte, D. Colau, P. Vogel, B. J. Van den Eynde, V. Zoete and O. Michielin, J. Med. Chem., 2012, 55, 5270–5290 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |