Ketamine-induced oxidative stress at different developmental stages of zebrafish (Danio rerio) embryos†
Luís M. Félix*abc,
Ana M. Vidald,
Cindy Serafimd,
Ana M. Valentimabc,
Luís M. Antunesabc,
Sónia Camposabc,
Manuela Matosef,
Sandra M. Monteiroa and
Ana M. Coimbraa
aCentre for the Research and Technology of Agro-Environmental and Biological Sciences (CITAB), University of Trás-os-Montes and Alto Douro (UTAD), Quinta de Prados, 5001-801, Vila Real, Portugal. E-mail: lfelix@utad.pt; Fax: +351 259 350 480; Tel: +351 259 350 000
bLaboratory Animal Science (LAS), Institute for Molecular and Cell Biology (IBMC), University of Porto (UP), Porto, Portugal
cInstitute for Research and Innovation in Health (i3S), University of Porto (UP), Porto, Portugal
dLife Sciences and Environment School (ECVA), University of Trás-os-Montes and Alto Douro (UTAD), Vila Real, Portugal
eBiosystems & Integrative Sciences Institute (BioISI), Faculty of Sciences, University of Lisboa, Lisboa, Portugal
fDepartment of Genetics and Biotechnology (DGB), University of Trás-os-Montes and Alto Douro (UTAD), Vila Real, Portugal
First published on 20th June 2016
Abstract
Ketamine, a widely used anesthetic in a variety of species, has been shown to exert a potential teratogenic effect during the early life stages of zebrafish. A number of mechanisms have been suggested for the etiology of teratogens. One of the most studied involves reactive oxygen species (ROS) formation and oxidative damage. In this study, zebrafish embryos were used to analyze oxidative stress as a potential mechanism of ketamine-induced toxicity. The changes in the accumulation and in vivo patterns of ROS, enzymatic activities (superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), lactate dehydrogenase (LDH) and acetylcholinesterase (AChE)), glutathione levels (oxidized (GSSG) and reduced (GSH)), oxidative damage (lipid peroxidation (LPO) and protein carbonyls (CO)) and gene expression (gclc, gstp1, sod1 and cat) were evaluated at 8 and 24 hours post fertilization (hpf) in zebrafish embryos exposed during 20 minutes to 0.2, 0.4 and 0.8 mg mL−1 ketamine in the course of blastula (2.5 hours post fertilization-hpf), gastrula (5.5 hpf) and segmentation (10.5 hpf). Although no changes in ROS patterns were visible after all ketamine exposures, an increase in GSH levels was observed after exposure during blastula, indicating possible alterations in cell oxidative capacity. After exposure in gastrula, an increase in SOD and CAT enzymatic activities along with an increase in GSSG levels were observed at 8 hpf. At 24 hpf, CAT activity remained higher in ketamine exposed groups. The expression of the cat gene was also augmented at this time point. The changes were related with the ability of the embryo to handle oxidative stress and to a turning point during development of the oxidative defense system. At segmentation, the exposure to ketamine induced changes in the accumulation of ROS and sod gene expression which were related to protective mechanisms against ketamine-induced oxidative stress. Changes in acetylcholinesterase were also observed which may be related to changes in ROS. The overall results show that ketamine induces phase-dependent oxidative stress misregulation that could be the key factor for ketamine toxicity and could help to elucidate and provide more information on the mechanism of embryotoxicity of ketamine.
1. Introduction
The early development of the zebrafish (Danio rerio) is characterized by a high rate of cell proliferation and differentiation as well as apoptosis,1 which depend on a balanced concentration of reactive oxygen species (ROS) and antioxidants within the cells in order to maintain homeostasis.2 During the early embryo developmental stages, the sensitivity to these reactive molecules may be particularly high leading to abnormalities that can affect the normal development, induce long-term consequences or even death.3 Despite high levels of ROS having been associated with detrimental effects,4 it is also known that at low levels, ROS acts as second messengers, regulating specific transcription factors and inducing gene expression changes in the embryos.5 Nevertheless, during the early stages of development, the oxidant–antioxidant equilibrium can be disrupted by exogenous agents leading to oxidative stress.3,5 Under this situation, and due to tolerance differences, cells may undergo cellular death6 or enter into a senescent state7 which can be associated with biochemical and morphological changes.8Anesthetics are known to affect cellular processes involved in differentiation and organogenesis9,10 and concerns have been raised regarding the effects of ketamine on development.11–14 Ketamine is a short-acting NMDA receptor blocker that is widely used for anesthetic purposes, and its effects on development have been attributed to a window of vulnerability during the period of rapid synaptogenesis.13 However, these results are still controversial15,16 and difficult to explore on the early-life stages of mammalian models due to practical and ethical restraints. In this sense, the zebrafish has emerged as a credible alternative to mammalian models and is considered a 3Rs-friendly model with a high range of experimental advantages.17 In particular, the zebrafish has already been used to study the pharmacological and toxicological effects of ketamine.18–25 These studies showed that ketamine exposure during early development induces similar effects to those observed in other vertebrate species including concentration-dependent increase in anomalies and mortality,22 modulation of behavioral responses,21 and neurogenesis alterations.23–25 However, the mechanism underlying ketamine effects during early developmental stages remains undiscovered.
Oxidative damage, caused by an imbalance of redox status, has already been associated with the mechanism of action of several teratogens.26 However, to date, and to our knowledge, no study has proposed that oxidative stress has a key process for ketamine-induced developmental toxicity. Thus, the present work aimed to study the role of oxidative biomarkers in ketamine-induced teratogenicity during the early developmental stages of the zebrafish (blastula, gastrula and segmentation), by combining genetic and biochemical methods. These experiments at such early developmental stages allow to relate results with the passage of ketamine through the placental barrier of mammals and its accumulation in embryonic and extra-embryonic tissues.27 Accordingly, the zebrafish's yolk syncytial layer, which arises during the blastula stage, has long been considered an extra-embryonic tissue with a homologous function to mammalian placenta.28,29 Ketamine is an anesthetic with a wide margin of safety and could be administered to women at very early stages of fetus development (as blastula and gastrula), when they are not aware of the pregnancy, thereby increasing the risk. Although translation from zebrafish studies to human is currently a complex task, the data from this study may help to understand the relationships between oxidative stress induction and the teratological potential of ketamine during the early developmental phases,22 which could impact the physiology, morphology and survival of individuals.
2. Experimental
All experimental procedures were conducted in compliance with licenses approved by the National Institutional Animal Care Committee (Direcção Geral de Alimentação e Veterinária, Lisboa, Portugal) and in agreement with the European Directive 2010/63/EU on the protection of animals used for scientific purposes and its transposition to the Portuguese law, Decreto-lei 113/2013, ensuring minimal animal stress and discomfort.2.1 Chemicals
Ketamine (ketamine hydrochloride, Imalgene 1000, 100 mg mL−1) was obtained from Merial Portuguesa-Saúde Animal Lda (Rio de Mouro, Portugal). All solutions were freshly made with embryo water (28 ± 0.5 °C, 200 mg L−1 Instant Ocean Salt and 100 mg L−1 sodium bicarbonate; UV sterilized) prepared from City of Vila Real filtered-tap water. Instant Ocean Salt was obtained from Aquarium Systems Inc. (Sarrebourg, France). Unless stated, all other chemicals were of analytical grade and purchased from Sigma (Sigma-Aldrich, Steinheim, Germany).2.2 Test organisms
Zebrafish maintenance and embryo collection were performed as previously described.22,30,31 Briefly, adult zebrafish from AB strain were maintained at the University of Trás-os-Montes and Alto Douro (Vila Real, Portugal) in a semi-closed water system supplied with aerated, dechlorinated, charcoal-filtered and UV-sterilized City of Vila Real tap water (pH 7.3–7.5) at 28 ± 0.5 °C in a 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 h light
10 h light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark cycle. Fish were fed twice a day with a commercial diet (Sera, Heinsberg, Germany) supplemented with Artemia sp. nauplii. In the evening before spawning induction, males and female adults (ratio of 2
dark cycle. Fish were fed twice a day with a commercial diet (Sera, Heinsberg, Germany) supplemented with Artemia sp. nauplii. In the evening before spawning induction, males and female adults (ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were randomly transferred into separate breeding tanks. Spawning was stimulated by the onset of the first light on the next morning and eggs were collected 1 hour after mating and spawning.
1) were randomly transferred into separate breeding tanks. Spawning was stimulated by the onset of the first light on the next morning and eggs were collected 1 hour after mating and spawning.
2.3 Exposure experiments
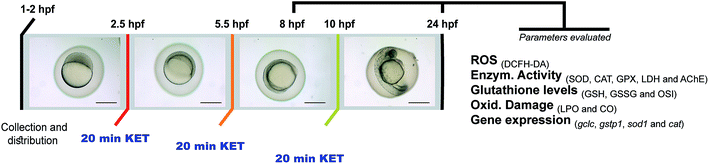
A schematic diagram of the experimental design is presented in Fig. 1. Within 1 hour after spawning, fertilized eggs were collected, bleached according to established protocols32,33 and rinsed to remove debris, and randomly distributed into 50 mL beakers. Similarly to our previous work,22 twenty minutes exposure to fresh ketamine concentrations (0.0, 0.2, 0.4 and 0.8 mg mL−1) were performed during blastula (2.5 hours post-fertilization-hpf), gastrula (5.5 hpf) and segmentation phases (10.5 hpf) according to the zebrafish described embryonic development, previously described by Kimmel et al. (1995).34 After exposure, embryos were washed three times with embryo water and allowed to develop at 28 ± 0.5 °C, with daily water renewal. For biochemical biomarkers analysis, at least four independent replicates containing 300 viable embryos were exposed to ketamine. At 8 and 24 hpf, 100 embryos were collected per replica. For gene expression studies, three independent replicates of 300 viable embryos were exposed and 50 animals/replica were collected at 8 and 24 hpf for qRT-PCR analysis. Additional experiments with five replicates of 50 eggs were carried out for dichlorofluorescein diacetate staining procedures at 24 hpf.2.4 Sample collection
For biochemical analysis, samples (100 embryos) at 8 and 24 hpf were collected in 1.5 mL microfuge tubes, washed in cold phosphate buffer saline and homogenized in cold buffer (0.32 mM of sucrose, 20 mM of HEPES, 1 mM of MgCl2, and 0.5 mM of phenylmethyl sulfonylfluoride (PMSF) that was prepared in ethanol to prevent protein degradation, pH 7.4)35 using a pellet mixer and cordless motor (VWR International, Carnaxide, Portugal). The homogenate was centrifuged at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g at 4 °C for 20 min and the supernatant was transferred to new tubes and stored at −80 °C for no more than 2 months until analysis. For gene expression analysis, at the specified time points (8 and 24 hpf), embryos were pooled in groups of 50 in RNAse free 1.5 mL microfuge tubes and stored in RNAlater at −20 °C until further analysis.
000 × g at 4 °C for 20 min and the supernatant was transferred to new tubes and stored at −80 °C for no more than 2 months until analysis. For gene expression analysis, at the specified time points (8 and 24 hpf), embryos were pooled in groups of 50 in RNAse free 1.5 mL microfuge tubes and stored in RNAlater at −20 °C until further analysis.
2.5 Reactive oxygen species accumulation and visualization
Reactive oxygen species accumulation was estimated based on a previous methodology.35 Briefly, to 20 μL of sample homogenate, 100 μL of PBS (pH 7.4) and 8.3 μL DCFH-DA (dichlorofluorescein-diacetate 10 mg mL−1 in DMSO) were added. After diffusion into cells, DCFH-DA is deacetylated by cellular esterases and oxidized by ROS into the fluorescent compound DCF (dichlorofluorescein). After incubation during 30 min at 37 °C, the fluorescence was measured in a Varian Cary Eclipse spectrofluorometer (Varian, Palo Alto, USA) equipped with microplate reader at 485 nm and 530 nm excitation and emission wavelengths, respectively, against a reagent blank. All samples were performed in duplicate and ROS accumulation was estimated based on a DCF standard curve (0–6.25 nM). Similarly, embryo intracellular reactive oxygen species (ROS) generation was assessed as previously described for blastula stage,22 at 24 hpf. Twelve embryos from each group were dechorionated using 2 mg mL−1 pronase (Roche Diagnostics, Mannheim, Germany)33 and incubated in the dark in DCFH-DA (20 μg mL−1) solution for 30 minutes at room temperature. Embryos were rinsed three times and observed under an inverted microscope (IX 51, Olympus, Antwerp, Belgium) equipped with an Olympus U-RFL-T fluorescent light source (Olympus, Antwerp, Belgium) and FITC filter, using a 4× Olympus UIS-2 objective lens (Olympus Co., Ltd., Tokyo, Japan) and data acquired using Cell R software (Olympus, Antwerp, Belgium). Fluorescent images were then processed with Adobe Photoshop CS6 (Adobe Systems, San Jose, USA).2.6 Enzymatic determinations
All enzymatic assays were carried out at 30 °C in a final volume of 200 μL using a PowerWave XS2 microplate scanning spectrophotometer (Bio-Tek Instruments, USA). All samples were performed in duplicate and measured against a reagent blank in the appropriate microplate. Superoxide dismutase (SOD) activity was assayed by measuring its ability to inhibit the photochemical reduction of nitrobluetetrazolium (NBT) at 560 nm.36 Briefly, in each well of the 96-well microplate, 10 μL of sample were mixed with 130 μL of phosphate buffer (50 mM, pH 7.4) supplemented with 1 mM EDTA, 0.5 mM hypoxanthine and 0.5 mM NBT (nitrobluetetrazolium). The reaction was started by the addition of 0.5 U mL−1 of xanthine-oxidase. The increase in absorbance due to dismutation of O2− into H2O2 was recorded for 5 minutes and SOD from bovine erythrocytes was used for construction of a standard curve (0–3.75 U mL−1). Catalase (CAT) activity was determined based on the Aebi method.37 The assay reaction consisted of 10 μL of sample with 170 μL of phosphate buffer (50 mM, pH 7.4) containing 0.1 mM EDTA. The reaction was started by the addition of fresh 3 mM H2O2 and the decrease in absorbance was monitored at 240 nm for 5 minutes. Activity was calculated as enzyme units per milligram of protein using bovine catalase as a standard (0–5 U mL−1). Glutathione peroxidase (GPx) activity was determined spectrophotometrically at 340 nm by Paglia and Valentine method.38 The reaction mixture (170 μL) contained 50 mM phosphate buffer (pH 7.4), 1 mM EDTA, 1 mM NaN3 (to inhibit CAT), 0.2 mM NADPH, 2 U mL−1 Glutathione Reductase (GR), 1 mM reduced glutathione (GSH in 0.2 mM HCl to avoid GSH autoxidation) and 10 μL of sample. After equilibration at room temperature, the reaction was started by the addition of fresh H2O2 (0.25 mM). The decrease in absorbance at 340 nm due to the oxidation of NADPH to NADP+ was observed for 5 minutes and the activity was determined using the extinction coefficient of 6.22 mM−1 cm−1. As acetylcholinesterase (AChE) and lactate dehydrogenase (LDH) are key enzymes in neurotransmission and anaerobic metabolism, respectively, their activities were also determined. For AChE, a method described for microplates39 and based on Ellman's method40 was applied. In brief, the reaction buffer consisted of 180 μL of 0.5 mM DTNB (5,5′-dithiobis-(2-nitrobenzoic acid)) in 50 mM Tris buffer (pH 7.4) and 10 μL of enzymatic sample. The reaction was started by the addition of 1 mM of acetylthiocholine iodide and the increase in the absorbance was measured at 405 nm for 5 minutes. The specific activity was determined using the TNB extinction coefficient of 13.6 mM−1 cm−1. For LDH activity determination, the method described by Domingues41 was used. Briefly, the assay system contained 10 μL of sample extract, 200 μL NADH (0.24 mM) and the reaction was started by the addition of 2 mM sodium pyruvate. The decrease in the absorbance due to the oxidation of NADH was monitored at 340 nm and the activity was calculated using the extinction coefficient of 6.22 mM−1 cm−1. Protein determination was performed using the method of Bradford42 with bovine serum albumin as a standard (0–2 mg mL−1).2.7 Glutathione levels and oxidative stress index
The glutathione levels were determined fluorometrically by measuring both the reduced (GSH) and oxidized states (GSSG) using the fluorochrome ortho-phthalaldehyde (OPA) (1 mg mL−1 methanol) at 320 nm and 420 nm, respectively excitation and emission wavelengths43 in a Varian Cary Eclipse (Varian, USA) spectrofluorometer equipped with microplate reader. Briefly, for both methods, 40 μL TCA (trichloroacetic acid 25% w/v) were added to 10 μL of sample in order to avoid errors due to oxidation of GSH. For GSH, 170 μL of reaction buffer containing Tris–HCl 0.26 M (pH 7.8), 115 μL NaOH 0.56 N (to neutralize samples) and 15 μL OPA were added to each sample. After 15 minutes of incubation at room temperature, the fluorescence was measured and GSH concentration was estimated based on a GSH standard curve (0–10 μM). For GSSG, after adding TCA, samples were mixed with 20 μL NEM (N-ethylmaleimide 40 mM) and were incubated at room temperature for 30 minutes. Then, 150 μL of a buffer containing phosphate buffer 0.23 M (pH 12), 105 μL NaOH 0.71 N and 25 μL OPA were added. After incubation for 15 minutes at room temperature, the fluorescence of samples was measured and GSSG concentration was assessed based on a GSSG standard curve (0–10 μM). The oxidative-stress index (OSI) was calculated according to the quotient between GSH and GSSG.2.8 Oxidative damage biomarkers
The lipid peroxidation was determined by a thiobarbituric (TBA) acid-based method described elsewhere.43 Briefly, 20 μL of sample were homogenized with the buffer to 150 μL and subsequently, 150 μL of TBA reagent were added. TBA reagent was made from thiobarbituric acid (TBA 0.5% w/v) prepared in 20% (w/v) TCA and 0.33 N HCl. To prevent artificial lipid peroxidation, 2 μL of BHT (butylated hydroxytoluene) 2% (w/v) were added. The mixture was incubated in boiling water for 15 minutes and left to cool at room temperature after which 300 μL of 1-butanol were added. The samples were vigorously vortexed and centrifuged at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 3 minutes and the fluorescence of the butanol extract was measured at 535 nm (excitation) and 550 nm (emission) wavelengths against butanol-treated reagent blank in a Varian Cary Eclipse (Varian, USA) spectrofluorometer equipped with a microplate reader. The major oxidative product of phospholipids, malondialdehyde (MDA), was estimated based on a standard curve (0–4 nM) of malondialdehyde bis(dimethyl acetal). The oxidative damage to proteins was also evaluated by the DNPH (2,4-dinitrophenylhydrazine) method described by Reznick and Packer.44 In a tube containing 160 μL of 10 mM DNPH in 2 M HCl, 40 μL of 2× diluted sample were added. For each sample a blank tube was made containing 160 μL of 2 M HCl and 40 μL of 2× diluted sample. The tubes were left at room temperature in the dark for 1 hour and vortexed every 15 minutes. After incubation, proteins were precipitated with 200 μL of TCA 20% (w/v) and the tubes centrifuged at 10
000 × g for 3 minutes and the fluorescence of the butanol extract was measured at 535 nm (excitation) and 550 nm (emission) wavelengths against butanol-treated reagent blank in a Varian Cary Eclipse (Varian, USA) spectrofluorometer equipped with a microplate reader. The major oxidative product of phospholipids, malondialdehyde (MDA), was estimated based on a standard curve (0–4 nM) of malondialdehyde bis(dimethyl acetal). The oxidative damage to proteins was also evaluated by the DNPH (2,4-dinitrophenylhydrazine) method described by Reznick and Packer.44 In a tube containing 160 μL of 10 mM DNPH in 2 M HCl, 40 μL of 2× diluted sample were added. For each sample a blank tube was made containing 160 μL of 2 M HCl and 40 μL of 2× diluted sample. The tubes were left at room temperature in the dark for 1 hour and vortexed every 15 minutes. After incubation, proteins were precipitated with 200 μL of TCA 20% (w/v) and the tubes centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g during 10 minutes at 4 °C. The resulting pellet was washed three times with ethanol–ethyl acetate (1
000 × g during 10 minutes at 4 °C. The resulting pellet was washed three times with ethanol–ethyl acetate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to remove free DNPH and lipid contaminants. The pellet was allowed to dry and then dissolved in 500 μL of 6 M guanidine hydrochloride solution (prepared in 20 mM phosphate buffer pH 2.3) by vortexing. Samples were then incubated for 15 min at 37 °C and 200 μL aliquots were placed in a 96-well plate and the absorbance was measured at 360 nm using a PowerWave XS2 microplate scanning spectrophotometer (Bio-Tek Instruments, USA). The results were calculated assuming the absorption coefficient of 22.0 mM−1 cm−1.
1) to remove free DNPH and lipid contaminants. The pellet was allowed to dry and then dissolved in 500 μL of 6 M guanidine hydrochloride solution (prepared in 20 mM phosphate buffer pH 2.3) by vortexing. Samples were then incubated for 15 min at 37 °C and 200 μL aliquots were placed in a 96-well plate and the absorbance was measured at 360 nm using a PowerWave XS2 microplate scanning spectrophotometer (Bio-Tek Instruments, USA). The results were calculated assuming the absorption coefficient of 22.0 mM−1 cm−1.
2.9 Gene expression analysis
Expression profiles of the genes gclc (glutamate-cysteine ligase, catalytic subunit), gstp1 (glutathione S-transferase pi 1), sod1 (Cu/Zn-superoxide dismutase) and cat (catalase) were evaluated by quantitative real-time PCR (qRT-PCR). Total RNA was extracted from independently collected pools of embryos/larvae at two time points (8 and 24 hpf) using the Illustra RNAspin kit (GE Healthcare, Munich, Germany) and treatment with RNase-free DNase I according to the manufacturer's instructions and as described before.30,31 The total RNA was recovered in 40 μL of RNAse free water and quantified by spectrophotometric readings at 260 and 280 nm in a Take3 Micro-volume plate (PowerWave XS2, BioTek Instruments, Inc. USA). RNA integrity and DNA contamination were evaluated in 1% agarose gel electrophoresis, using Green Safe Premium staining (NZYTech, Ltd., Lisbon, Portugal) and detection under UV light using BioCapt software (v99.02, Vilber Lourmat, France). RNA samples were stored at −80 °C until further use. RNA (500 ng) from each sample was reverse transcribed using the iScript cDNA synthesis (Bio-Rad Laboratories, California, USA) following the manufacturer's instructions and stored at −20 °C. The qPCR reactions were performed in a final volume of 20 μL containing 1 μL of cDNA, 200 nM of each specific primer (Table 1), using 5x HOT FIREPol EvaGreen qPCR Mix Plus (Solis Biodyne, Tartu, Estonia) in a Stratagene Mx3005P Real-Time PCR system (Stratagene, Agilent Technologies, Santa Clara, USA). The following thermal cycling conditions were used: initial denaturation at 95 °C for 10 minutes followed by 40 cycles of denaturation at 95 °C for 20 s, primer annealing for 40 s (annealing temperatures in Table 1) and extension at 72 °C for 20 s followed by a final extension at 72 °C for 5 min. This was followed by a melting curve analysis to exclude the interference of primer-dimers, DNA contaminants and other nonspecific products. Every set of qRT-PCR was carried out in three independent replicated samples, including a reaction control without cDNA. A 5-fold dilution series of cDNA, prepared from a mix of the samples, was used for standard curves construction and reaction efficiency determination. The relative expression levels were analyzed using the MxPro QPCR System software (Stratagene, Agilent Technologies, Santa Clara, USA) and were determined by normalization to the β-actin gene used as housekeeping. Values were then normalized to the control average value calculated using the ΔΔCt method with efficiency correction.45| Gene | Primer sequence (5′-3′) | Fragment size (bp) | Annealing temperature (°C) |
|---|---|---|---|
| β-Actin (NM_181601.4)30 | Fwd – ACT GTA TTG TCT GGT GGT AC | 197 | 60 |
| Rev – TAC TCC TGC TTG CTA ATC C | |||
| glcc (NM_199277.2)82 | Fwd – CTA TCT GGA GAA CAT GGA GG | 264 | 60 |
| Rev – CAT TTT CCT CTG TTG ACC GG | |||
| gstp1 (NM_131734.3)82 | Fwd – TTC AGT CCA ACG CCA TGC | 255 | 60 |
| Rev – ATG AGA TCT GAT CAC CAA CC | |||
| sod1 (Y12236.1)83 | Fwd – AAG AAG CCA GTG AAG GTG ACT | 165 | 60 |
| Rev – ACA TTA CCC AGG TCT CCG AC | |||
| cat (AF170069.1)83 | Fwd – AGA TGA AAC TGT GGA AGG AGG GTC | 269 | 60 |
| Rev – AAA CAC TTT GGC TTT GGA GTA GCG |
2.10 Statistics
For this study, a sample size calculation was performed with the G*Power 3 (University of Düsseldorf, Germany) based on standard deviations from a similar study from our group.46 In order to detect a 10% change in oxidative stress parameters, with an α error of 0.05 and a power of 90%, the sample size calculation returned the value of six animals per group. In some cases, sample size was reduced to at least four replicates per group, when outliers were identified and excluded from the analysis. A value was considered an outlier when it was 1.5 box-lengths high from the edge of the box in a boxplot graph. For gene expression analysis, sample size was based on a previous study from our group.31 Before hypothesis testing, the normal distribution and homogeneity of the data were confirmed by Kolmogorov–Smirnov and Levene's tests, respectively. Data was compared by a non-parametric independent samples Kruskal–Wallis test for non-normal distribution variables followed by Dunn's pairwise comparison tests and data expressed as median and interquartile range (25th; 75th percentiles) or by one-way analysis of variance (ANOVA) followed by Tukey's pairwise comparison tests for variables with normal distribution and data expressed as mean ± standard deviation. For data following both parametric and non-parametric distributions, non-parametric tests were used as they are more conservative and best suited for small datasets. In all cases, statistical analyses were carried out using SPSS for Windows (Version 22.0; Chicago, IL, USA) and differences were considered significant at p < 0.05.3. Results
3.1 ROS levels and in vivo accumulation
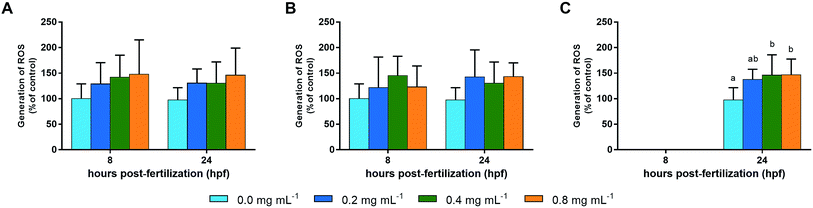
The generation of ROS was detected using DCFH-DA and expressed as a percentage of control (Fig. 2). The levels observed for control samples were, respectively, 373.8 ± 108.8 and 316.3 ± 77.7 nmol DCF per mg−1 of protein, for 8 and 24 hpf. Despite a dose-dependent increase (up to 50%), no significant differences were observed for the groups exposed to ketamine during the blastula stage. Similar results were observed when embryos were exposed during gastrula, resulting in an increase in the ROS levels detected that also did not reach the significance level. The exposure to ketamine during segmentation showed significant increases of about 38% (p = 0.113), 46% (p = 0.027) and 47% (p = 0.020) of the control mean level for 0.2, 0.4 and 0.8 mg mL−1, respectively. In relation to the ROS distribution pattern (Fig. 3), no significant alterations in this pattern were observed independently of the ketamine dose and exposure period. | ||
| Fig. 3 Representative DCF fluorescence images of 144 hpf larvae exposed to ketamine during gastrula (A) and segmentation (B). Data from blastula was previously published.22 No significant changes were observed in exposed embryos despite variations observed in the yolk extension (ys), yolk sac (ys) and pericardium zone (pc). Scale bar represents 500 μm. | ||
3.2 Enzymatic activities
The effects of ketamine on the activities of SOD, CAT, GPx, LDH and AChE at 8 and 24 hpf are presented in Tables 2 and 3. After exposure during blastula, the SOD, CAT, GPX, LDH and AChE activities were similar in the experimental groups despite a tendency to vary with ketamine doses at both time points. When exposure was performed during gastrula stage, significant differences were observed for SOD, CAT and AChE activities. An increase was observed in SOD activity at 8 hpf, showing statistical differences between 0.2 mg mL−1 (p = 0.045), 0.4 mg mL−1 (p = 0.002) and 0.8 mg mL−1 (p = 0.001) exposed embryos comparatively to control group. However, at 24 hpf the values returned to average values. An increase was also observed in CAT activity at 8 and 24 hpf, with significant differences between 0.4 mg mL−1 (p8 hpf = 0.014; p24 hpf = 0.023), 0.8 mg mL−1 (p8 hpf = 0.006; p24 hpf = 0.002) and control group. The AChE activity was shown to be irregular with a significant increase observed in 0.4 mg mL−1 comparatively to control group (p = 0.020), 0.2 mg mL−1 (p = 0.010) and 0.8 mg mL−1 (p = 0.008), at 8 hpf. On the other hand, at 24 hpf, 0.2 mg mL−1 ketamine exposure increased AChE activity compared to control (p = 0.044). No differences were perceived for the glutathione peroxidase and LDH activities at both time-points. When exposure was performed during segmentation, no significant variations where observed for SOD, CAT, GPx and LDH despite the slight variations detected among experimental groups. Considering the AChE activity, results varied with ketamine concentrations significantly increasing in the highest ketamine dose when compared to control (p = 0.024).| Developmental phase | Dose (mg mL−1) | 8 hpf | 24 hpf | ||||
|---|---|---|---|---|---|---|---|
| SOD (U mg−1 of protein) | CAT (U mg−1 of protein) | GPx (nmol NADPH per min−1 mg−1 of protein) | SOD (U mg−1 of protein) | CAT (U mg−1 of protein) | GPx (nmol NADPH per min−1 mg−1 of protein) | ||
| a Data from at least four independent replicates of 100 animals each, is expressed as mean ± SD for parametric data distribution or median and interquartile range for non-parametric data. Statistical analysis was performed using one-way ANOVA followed by Tukey's multiple-comparison test or Kruskal–Wallis followed by Dunn's test. Different lowercase letters indicate significant differences between groups (p < 0.05). | |||||||
| Blastula | 0.0 | 93.54 (77.05–126.2) | 47.64 (46.45–60.10) | 17.83 (12.05–22.76) | 97.54 ± 23.96 | 82.60 (67.05–88.18) | 6.370 (5.643–15.26) |
| 0.2 | 118.6 (90.43–172.6) | 61.76 (60.22–77.26) | 22.62 (11.95–27.32) | 130.6 ± 27.57 | 71.18 (69.86–84.39) | 17.65 (7.089–31.71) | |
| 0.4 | 128.5 (108.9–181.9) | 65.98 (47.88–73.13) | 5.365 (2.260–16.21) | 130.9 ± 42.59 | 86.84 (80.70–95.11) | 27.29 (17.40–37.32) | |
| 0.8 | 131.4 (102.3–203.0) | 82.07 (67.17–94.25) | 6.550 (6.245–15.14) | 146.7 ± 53.17 | 73.29 (61.44–77.74) | 10.27 (5.223–17.59) | |
| Statistical test | X2(3) = 5.553 | X2(3) = 6.237 | X2(3) = 7.037 | F(3,24) = 0.359 | X2(3) = 7.650 | X2(3) = 6.455 | |
| p | 0.135 | 0.101 | 0.071 | 0.783 | 0.054 | 0.091 | |
| Gastrula | 0.0 | 93.54 (77.05–126.2)a | 47.64 (46.45–60.10)a | 17.55 ± 2.802 | 97.54 ± 23.96 | 82.60 (67.05–88.18)a | 6.370 (5.643–15.26) |
| 0.2 | 120.4 (65.07–178.8)b | 69.98 (60.11–82.46)ab | 15.39 ± 4.132 | 142.4 ± 53.04 | 113.5 (85.93–117.4)ab | 13.63 (10.04–22.42) | |
| 0.4 | 126.6 (114.8–186.3)b | 78.21 (64.39–85.56)b | 17.17 ± 3.503 | 130.3 ± 41.37 | 101.9 (99.32–107.0)b | 15.48 (9.205–18.60) | |
| 0.8 | 120.1 (87.80–160.8)b | 74.64 (63.80–84.30)b | 12.31 ± 4.759 | 143.4 ± 27.55 | 110.9 (96.64–115.2)b | 14.35 (10.04–17.53) | |
| Statistical test | X2(3) = 13.39 | X2(3) = 8.872 | F(3,17) = 0.219 | F(3,24) = 2.192 | X2(3) = 10.51 | X2(3) = 0.767 | |
| p | 0.004 | 0.031 | 0.882 | 0.115 | 0.015 | 0.857 | |
| Segmentation | 97.54 ± 23.96 | 79.28 ± 5.866 | 6.370 (5.643–15.26) | ||||
| 137.6 ± 20.02 | 87.54 ± 7.215 | 13.63 (4.732–21.85) | |||||
| 146.7 ± 40.14 | 84.61 ± 1.439 | 12.16 (6.464–23.73) | |||||
| 146.6 ± 30.82 | 81.55 ± 4.096 | 10.72 (4.923–19.01) | |||||
| Statistical test | F(3,24) = 0.514 | F(3,19) = 0.254 | X2(3) = 0.707 | ||||
| p | 0.677 | 0.857 | 0.872 | ||||
| Developmental phase | Dose (mg mL−1) | 8 hpf | 24 hpf | ||
|---|---|---|---|---|---|
| LDH (nmol NADH per min−1 mg−1 of protein) | AChE (nmol TNB per min−1 mg−1 of protein) | LDH (nmol NADH per min−1 mg−1 of protein) | AChE (nmol TNB per min−1 mg−1 of protein) | ||
| a Data from at least four independent replicates of 100 animals each, is expressed as mean ± SD for parametric data distribution or median and interquartile range for non-parametric data. Statistical analysis was performed using one-way ANOVA followed by Tukey's multiple-comparison test or Kruskal–Wallis followed by Dunn's test. Different lowercase letters indicate significant differences between groups (p < 0.05). | |||||
| Blastula | 0.0 | 108.6 ± 13.89 | 1.073 ± 0.100 | 141.4 (86.92–150.5) | 0.810 (0.320–1.255) |
| 0.2 | 109.7 ± 11.57 | 0.380 ± 0.059 | 137.8 (124.2–138.5) | 1.400 (0.960–1.605) | |
| 0.4 | 113.0 ± 43.37 | 1.463 ± 1.143 | 139.4 (129.4–145.0) | 0.160 (0.100–0.225) | |
| 0.8 | 150.9 ± 10.85 | 0.313 ± 0.253 | 150.8 (140.5–159.7) | 1.240 (0.460–1.530) | |
| Statistical test | F(3,12) = 2.836 | F(3,12) = 3.572 | X2(3) = 4.823 | X2(3) = 6.898 | |
| p | 0.083 | 0.050 | 0.185 | 0.075 | |
| Gastrula | 0.0 | 108.6 ± 13.89 | 1.073 ± 0.100a | 129.3 ± 21.83 | 0.943 ± 0.404a |
| 0.2 | 109.7 ± 35.49 | 0.913 ± 0.758a | 143.1 ± 23.06 | 2.643 ± 0.582b | |
| 0.4 | 109.4 ± 19.27 | 2.455 ± 0.663b | 114.04 ± 11.51 | 2.197 ± 0.363ab | |
| 0.8 | 132.1 ± 16.28 | 0.873 ± 0.482a | 117.9 ± 14.73 | 0.507 ± 0.147ab | |
| Statistical test | F(3,12) = 1.001 | F(3,12) = 7.286 | F(3,14) = 0.783 | F(3,14) = 4.998 | |
| p | 0.426 | 0.005 | 0.523 | 0.015 | |
| Segmentation | 0.0 | 141.4 (86.92–150.5) | 0.943 ± 0.404a | ||
| 0.2 | 72.06 (86.92–80.10) | 2.050 ± 0.272ab | |||
| 0.4 | 127.8 (66.89–123.9) | 1.637 ± 0.680ab | |||
| 0.8 | 118.5 (117.8–127.1) | 3.493 ± 0.032b | |||
| Statistical test | X2(3) = 4.596 | F(3,14) = 3.836 | |||
| p | 0.204 | 0.034 | |||
3.3 Glutathione levels
The embryonic glutathione levels measured at 8 and 24 hpf are shown in Table 4. After exposure to ketamine during blastula, an increase of GSH levels was observed at 8 hpf, with the highest ketamine dose presenting significantly higher levels of GSH, compared to control group (p = 0.024) and to the lowest ketamine dose (p = 0.014). Regarding the levels of GSSG, in blastula exposure, an increase with ketamine was also observed at 8 hpf with statistical differences between 0.2 mg mL−1 (p = 0.021) and 0.8 mg mL−1 (p = 0.003) relatively to control group. No differences were observed neither at 24 hpf nor in the oxidative stress index at both time-points. When exposure was performed during gastrula, the levels of GSH remained similar among treatments, both at 8 hpf and at 24 hpf. At 8 hpf, the GSSG levels were increased in all ketamine treatments with significant differences between 0.2 mg mL−1 (p = 0.0004), 0.4 mg mL−1 (p = 0.006) and 0.8 mg mL−1 (p = 0.0002) relatively to control group. No differences were observed neither among ketamine treatments, at 24 hpf, nor for the oxidative stress index at 8 and 24 hpf. Relatively to segmentation exposure, no differences were observed in GSH and GSSG levels or in the oxidative stress index, despite slight variations occurred.| Developmental phase | Dose (mg mL−1) | 8 hpf | 24 hpf | ||||
|---|---|---|---|---|---|---|---|
| GSH (nmol mg−1 of protein) | GSSG (nmol mg−1 of protein) | OSI | GSH (nmol mg−1 of protein) | GSSG (nmol mg−1 of protein) | OSI | ||
| a Data from at least four independent replicates of 100 animals each, is expressed as mean ± SD for parametric data distribution or median and interquartile range for non-parametric data. Statistical analysis was performed using one-way ANOVA followed by Tukey's multiple-comparison test or Kruskal–Wallis followed by Dunn's test. Different lowercase letters indicate significant differences between groups (p < 0.05). | |||||||
| Blastula | 0.0 | 18.07 (12.14–28.18)a | 94.47 (77.05–124.1)a | 0.220 (0.105–0.288) | 51.57 ± 11.96 | 345.8 (235.1–607.7) | 0.150 (0.133–0.210) |
| 0.2 | 13.27 (12.48–20.81)a | 140.9 (107.2–213.8)b | 0.160 (0.060–0.230) | 24.34 ± 7.017 | 221.0 (184.9–312.0) | 0.105 (0.088–0.125) | |
| 0.4 | 50.80 (19.42–52.61)ab | 114.7 (106.5–128.2)ab | 0.200 (0.150–0.395) | 76.18 ± 20.02 | 249.6 (108.9–617.9) | 0.130 (0.120–0.220) | |
| 0.8 | 30.00 (22.09–35.80)b | 167.5 (138.8–193.3)b | 0.195 (0.135–0.428) | 48.81 ± 15.62 | 181.9 (160.1–345.8) | 0.230 (0.043–0.298) | |
| Statistical test | X2(3) = 8.286 | X2(3) = 10.33 | X2(3) = 1.423 | F(3,17) = 1.627 | X2(3) = 3.446 | X2(3) = 7.525 | |
| p | 0.040 | 0.016 | 0.700 | 0.220 | 0.328 | 0.057 | |
| Gastrula | 0.0 | 18.07 (12.14–28.18) | 99.07 ± 9.117a | 0.220 (0.105–0.288) | 51.14 (22.88–80.69) | 345.8 (235.1–607.7) | 0.150 (0.133–0.210) |
| 0.2 | 19.34 (8.103–34.65) | 165.9 ± 10.39b | 0.200 (0.160–0.220) | 172.2 (69.58–264.1) | 676.8 (238.9–1043) | 0.176 (0.040–0.233) | |
| 0.4 | 21.28 (9.700–22.57) | 149.8 ± 7.303b | 0.420 (0.320–0.550) | 22.65 (9.730–24.67) | 296.9 (211.2–842.9) | 0.185 (0.132–0.185) | |
| 0.8 | 31.15 (24.47–37.03) | 161.0 ± 9.840b | 0.175 (0.065–0.180) | 30.64 (11.00–79.21) | 237.2 (198.9–792.7) | 0.159 (0.056–0.185) | |
| Statistical test | X2(3) = 4.723 | F(3,20) = 11.72 | X2(3) = 2.287 | X2(3) = 4.729 | X2(3) = 1.557 | X2(3) = 2.228 | |
| p | 0.193 | 0.0001 | 0.515 | 0.193 | 0.669 | 0.526 | |
| Segmentation | 0.0 | 51.14 (22.88–80.69) | 345.8 (235.1–607.7) | 0.165 ± 0.019 | |||
| 0.2 | 12.04 (8.300–102.0) | 470.9 (165.3–855.0) | 0.137 ± 0.036 | ||||
| 0.4 | 9.330 (5.290–55.86) | 199.1 (157.0–784.3) | 0.190 ± 0.040 | ||||
| 0.8 | 20.80 (15.55–148.8) | 180.3 (130.4–601.4) | 0.220 ± 0.065 | ||||
| Statistical test | X2(3) = 0.613 | X2(3) = 0.866 | F(3,24) = 0.596 | ||||
| p | 0.894 | 0.834 | 0.614 | ||||
3.4 Oxidative damage biomarkers
Data on lipid and protein oxidative damage is presented in ESI 1.† The results show that after ketamine exposures during the blastula, gastrula and segmentation stages neither lipid peroxidation (LPO) nor protein carbonylation (CO) were increased in zebrafish embryos.3.5 Gene expression analysis
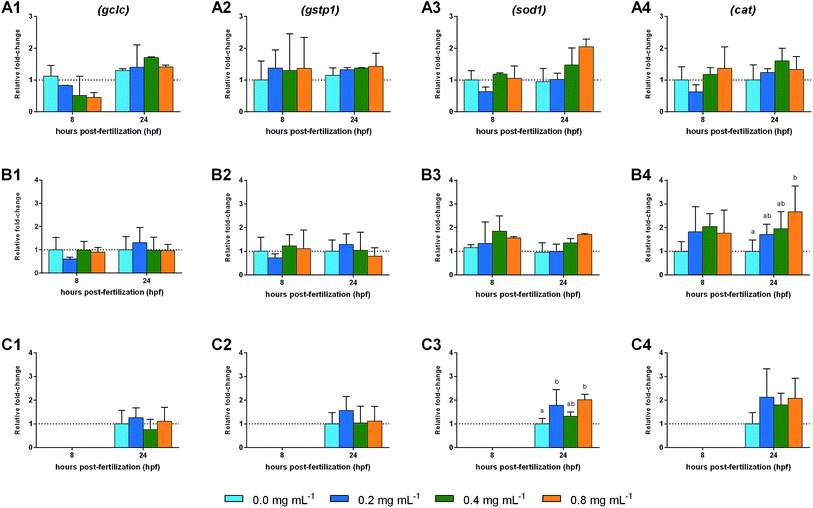
The results of the oxidative stress related genes expression analysis for blastula, gastrula and segmentation exposures are presented in Fig. 4. No significant changes were observed in oxidative stress related genes after exposure of blastula embryos to ketamine, despite the slight variations observed. After exposure of embryos during gastrula stage, a 1.66-fold increase in cat expression levels was observed at 24 hpf with the highest ketamine dose showing statistical differences relatively to control group (p = 0.022). At 24 hpf, the sod1 expression was observed to be up-regulated in the groups exposed during segmentation, with a 0.78-fold and 1.02-fold increases towards control in the lowest (p = 0.035) and highest (p = 0.007) doses, respectively.4. Discussion
In a previous study, it was found that ketamine causes toxicity to zebrafish embryos.22 To assess whether this toxicity can result from oxidative stress induction, two time-points (8 and 24 hpf) were evaluated after exposure to ketamine during blastula, gastrula and segmentation. The selection of these time points was based on the bioenergetic profiling of zebrafish embryonic development. Mitochondrial respiration increases between 7 and 24 hpf and reach a plateau at 30 and 48 hpf.47 It is known that disruption of mitochondrial respiration increases ROS levels and ketamine has been shown to impair mitochondrial functions in rodents.48,49 Although the results were not particularly conclusive, ketamine induced variations in enzymatic and non-enzymatic defenses, such as the increase of reduced glutathione at 8 hpf after blastula exposure, superoxide dismutase at 8 hpf and catalase activities at both 8 and 24 hpf, as well as the increase of the oxidized glutathione at 8 hpf, especially after exposure at gastrula stage. Ketamine exposure during this phase up-regulated catalase gene expression in 24 hpf zebrafish embryos, whereas only slight variations were observed in the remaining antioxidant-related genes. Furthermore, an increase in ROS production, acetylcholinesterase activity and sod1 were observed after exposure during the segmentation stage.Embryos are extremely susceptible to oxidative stress which may induce developmental disruptions, resulting in abnormalities.5,50,51 Indeed, it was previously reported that ROS can affect cellular functions essential for the normal development of the embryo.52 For normal development, the embryo requires a constant energy supply to control major physiological events. Adenosine triphosphate (ATP) is the primary chemical energy source for the majority of cellular functions, being mainly produced by oxidative phosphorylation. In addition, ATP may also be generated through anaerobic glycolysis,53 which is known to be active in embryos at different developmental stages.54 Early in embryogenesis, and due to rapid cell divisions, large amounts of energy are required which are obtained by glycolysis. This process produces energy at a much faster rate than oxidative phosphorylation.47,54,55 Compounds affecting anaerobic metabolism through a spurt of ROS could be very harmful to the zebrafish embryo as its cells are still not equipped with defensive mechanisms,55 although the embryo may be protected by maternally transferred substances.56
It was previously reported that ketamine exposure during early developmental stages induces embryo malformations, namely when exposure is performed during the blastula stage.22 However, no significant changes in enzymatic activities, oxidative damage biomarkers and gene expression were observed after exposure during the blastula stage, supporting the lack of differences observed in DCFH-DA.22 Notwithstanding, the GSH levels were significantly increased after ketamine exposure, at 8 hpf, but not at 24 hpf, compared with control. No changes were observed in the oxidative stress index. GSH plays a crucial role in ROS regulation in cytoplasm by reducing protein disulfides and other cellular molecules53,57 maintaining redox homeostasis.58 GSH concentrations are strongly correlated with developmental cellular processes (proliferation, differentiation and apoptosis) through complex mechanisms, such as DNA synthesis, transcription factors and epigenetic modifications.5,58 Based on these results, ketamine exposure during blastula induces a protective response to slight oxidative stress that affected GSH homeostasis possibly by increasing GSH synthesis without affecting the overall oxidative stress of the embryo. However, little is known about the redox-based mechanisms that mediate teratogenesis phenotypes58 but similar results were observed when embryos were exposed to other chemicals.59 Moreover, at the same time-point (8 hpf), there was a tendency to decrease gclc expression, which is involved in GSH synthesis.58 Therefore, ketamine effects can be resultant of the mRNA translation process and GSH protein synthesis. GSH synthesis is controlled by several signaling pathways including Nrf2, NFkB and mitogen-activated protein kinases (MAPK) pathways5,60,61 that are regulated by intracellular calcium.62 It is worth noting that the range of concentrations used in this study (0.84, 1.68 and 3.37 mM) are within the range of concentrations that inhibit calcium oscillation frequency (from 0.3 mM up).63 Furthermore, exposure to inhibitors of c-Jun N-terminal kinase (JNK) signaling, a member of the MAPK family, results in morphological defects during embryogenesis64 which are similar to those previously observed.22 Thus, future work on the effects of ketamine in calcium homeostasis would be central to understand how GSH production could be affected by ketamine.
The shift to aerobic metabolism is initiated during gastrula and early organogenesis.47,54,55 At this point, and due to higher oxygen concentrations, the embryo must develop its oxidative defense mechanism by slowly increasing the activity of the major antioxidant enzymes (SOD, CAT and GPx),54 coinciding with an increase in ROS production and availability65 that is accompanied by an increase in the embryo mRNA levels.54 This has been described as the most susceptible phase to the action of teratogens.54,55 In fact, when embryos were exposed during the gastrula stage, an increase in SOD at 8 hpf and in CAT activity, at both 8 and 24 hpf, was observed in the ketamine groups. This increase was also noted in cat expression, at 24 hpf, without ROS production increment and biological damage being observed. Previous studies have shown that the main enzymatic activities detected at embryonic ages are from SOD and CAT66 and that an increase in their activities is responsible for diminishing teratogen-induced oxidative stress by maintaining a steady-state concentration of the produced ROS.67 In fact, an increased catalase activity was already correlated with improved developmental parameters.68 These results indicate that ketamine induced oxidative stress at this stage that was balanced by the increase of these enzymes, protecting the embryo against DNA damage, oxidative damage and embryopathies. In fact, it is known that anesthetics induce an elevated production of ROS69 and in particular, that ketamine increases ROS production in embryonic stem cells.70–72 Moreover, supporting our hypothesis of ketamine-induced oxidative stress in zebrafish embryos, it has been shown that ketamine promotes a H2O2 production through mitochondrial impairment in rodents.49 Furthermore, it has been shown that an increase in GSSG levels, as seen at 8 hpf after gastrula exposure, may be indicative of some controlled processes of oxidative stress during embryogenesis.73 Additionally, an adaptive response to oxidative stress can be reflected in gene expression changes. After gastrula exposure to ketamine, cat levels were significantly increased which, besides controlling redox homeostasis, may also prevent abnormalities seen in zebrafish embryos exposed at early stages,22 thus playing an important role in protecting the embryo against ketamine-induced teratogenesis.
Later on development, during segmentation, an increase in mitochondrial respiration is observed comparatively to earlier stages corresponding to a maximum proton leak.47 Among other changes, this proton leak induces a decrease in the rate of ROS generation.74 When embryos were exposed to ketamine during segmentation, a significant increase in ROS production was detected. However, the increase of antioxidant activity, necessary to re-establish the normal redox potentials and decrease potential ketamine damage potential was not observed. As previously stated, ketamine is known to induce ROS production through mitochondrial dysfunction,75 which helps in the justification of the present results as mitochondria are fully functional at this stage.48 Additionally, Cu/Zn-SOD genes are up-regulated in response to oxidative stress76 which supports our results as a stimulation of sod1 expression levels were observed after ketamine exposure, indicating a higher production of ROS. Still, an overexpression of sod1 is known to elicit a protective effect to the embryo against malformations,77,78 as it constitutes the first line of defense against ROS.79 As no differences were observed for oxidative damage biomarkers neither for cell membrane integrity (LDH activity), the observed response could result from cellular metabolic activity changes induced by ketamine. However, an in vitro study showed contradictory results after ketamine treatment (3 mM) in embryonic stem cells,70 indicating potential differences in results when using a whole organism model. Additionally, a significant increase in acetylcholinesterase activity was observed when embryos in the segmentation stage were exposed to the high dose of ketamine. Although an interference by phenylmethylsulfonyl fluoride in the storage buffer may occur, a previous report showed that AChE activity is only significantly affected from 2 mM up,80 thus supporting the current results. Although the relevance of acetylcholinesterase changes during development is not clear, it has been reported that disturbances in calcium homeostasis result in an increase of AChE activity, which are strictly related with increased ROS levels.81 Hence, the identification of biological alterations related to the calcium signaling during ketamine exposure may render some important insights about the cellular and molecular targets involved in the teratogenicity of ketamine.
5. Conclusions
The data supports that embryos are responsive to ketamine at early life stages and that ketamine is capable of interfering with oxidative stress processes, which was detected by the changes in some antioxidant parameters, without causing severe damage and not affecting the overall redox status in zebrafish embryos. These changes induced by ketamine exposure were developmental stage-dependent, and related with the gradual development of the antioxidant defense system of the embryo, which is dependent on changes in energy-sensing pathways. This misregulation could have important implications in the general embryonic and fetal development, including in humans, following exposure to ketamine. In addition, as calcium signaling mediates a number of developmental processes through energy balance, more attention should be focused on the relationship between ketamine and calcium homeostasis during early developmental stages.Acknowledgements
This work was supported by European Investment Funds by FEDER/COMPETE/POCI – Operational Competitiveness and Internationalization Programme, under Project POCI-01-0145-FEDER-006958 and FCOMP-01-0124-FEDER-028683 and National Funds by FCT – Portuguese Foundation for Science and Technology, under the projects PTDC/CVT-WEL/4672/2012 and UID/AGR/04033.Bibliographic references
- L. K. Cole and L. S. Ross, Dev. Biol., 2001, 240, 123–142 CrossRef CAS PubMed.
- Y. L. Guo, S. Chakraborty, S. S. Rajan, R. Wang and F. Huang, Stem Cells Dev., 2010, 19, 1321–1331 CrossRef CAS PubMed.
- P. A. Dennery, Free Radicals Biol., 2010, 49, 1147–1151 CrossRef CAS PubMed.
- R. T. Di Giulio, P. C. Washburn, R. J. Wenning, G. W. Winston and C. S. Jewell, Environ. Toxicol. Chem., 1989, 8, 1103–1123 CrossRef CAS.
- P. A. Dennery, Birth Defects Res., Part C, 2007, 81, 155–162 CrossRef CAS PubMed.
- S. Tan, Y. Sagara, Y. Liu, P. Maher and D. Schubert, J. Cell Biol., 1998, 141, 1423–1432 CrossRef CAS PubMed.
- T. Lu and T. Finkel, Exp. Cell Res., 2008, 314, 1918–1922 CrossRef CAS PubMed.
- S. Fulda, A. M. Gorman, O. Hori and A. Samali, Int. J. Cell Biol., 2010, 2010, 214074 Search PubMed.
- H. G. Kress, Eur. J. Anaesthesiol., 1995, 12, 83–97 CAS.
- J. E. Sturrock and J. F. Nunn, Anesthesiology, 1975, 43, 21–33 CrossRef CAS PubMed.
- C. Ikonomidou, F. Bosch, M. Miksa, P. Bittigau, J. Vockler, K. Dikranian, T. I. Tenkova, V. Stefovska, L. Turski and J. W. Olney, Science, 1999, 283, 70–74 CrossRef CAS PubMed.
- A. C. Scallet, L. C. Schmued, W. Slikker Jr, N. Grunberg, P. J. Faustino, H. Davis, D. Lester, P. S. Pine, F. Sistare and J. P. Hanig, Toxicol. Sci., 2004, 81, 364–370 CrossRef CAS PubMed.
- W. Slikker, X. Zou, C. E. Hotchkiss, R. L. Divine, N. Sadovova, N. C. Twaddle, D. R. Doerge, A. C. Scallet, T. A. Patterson, J. P. Hanig, M. G. Paule and C. Wang, Toxicol. Sci., 2007, 98, 145–158 CrossRef CAS PubMed.
- X. Zou, T. A. Patterson, N. Sadovova, N. C. Twaddle, D. R. Doerge, X. Zhang, X. Fu, J. P. Hanig, M. G. Paule, W. Slikker and C. Wang, Toxicol. Sci., 2009, 108, 149–158 CrossRef CAS PubMed.
- A. T. Bhutta, Semin. Perinatol., 2007, 31, 303–308 CrossRef PubMed.
- J. Yan and H. Jiang, J. Neurosurg. Anesthesiol., 2014, 26, 155–160 CrossRef PubMed.
- S. Ali, D. L. Champagne, H. P. Spaink and M. K. Richardson, Birth Defects Res., Part C, 2011, 93, 115–133 CrossRef CAS PubMed.
- R. Riehl, E. Kyzar, A. Allain, J. Green, M. Hook, L. Monnig, K. Rhymes, A. Roth, M. Pham, R. Razavi, J. Dileo, S. Gaikwad, P. Hart and A. V. Kalueff, Neurotoxicol. Teratol., 2011, 33, 658–667 CrossRef CAS PubMed.
- S. M. Zakhary, D. Ayubcha, F. Ansari, K. Kamran, M. Karim, J. R. Leheste, J. M. Horowitz and G. Torres, Synapse, 2011, 65, 160–167 CrossRef CAS PubMed.
- E. G. De Campos, A. T. Bruni and B. S. De Martinis, Behav. Brain Res., 2015, 292, 537–546 CrossRef PubMed.
- H. A. Burgess and M. Granato, J. Neurosci., 2007, 27, 4984–4994 CrossRef CAS PubMed.
- L. M. Felix, L. M. Antunes and A. M. Coimbra, Neurotoxicol. Teratol., 2014, 41, 27–34 CrossRef CAS PubMed.
- J. Kanungo, E. Cuevas, S. F. Ali and M. G. Paule, J. Appl. Toxicol., 2013, 33, 410–417 CrossRef CAS PubMed.
- J. Kanungo, E. Cuevas, S. F. Ali and M. G. Paule, Reprod. Toxicol., 2012, 33, 205–212 CrossRef CAS PubMed.
- S. Lantz-McPeak, X. Guo, E. Cuevas, M. Dumas, G. D. Newport, S. F. Ali, M. G. Paule and J. Kanungo, J. Appl. Toxicol., 2015, 35, 261–272 CrossRef CAS PubMed.
- M. E. Hahn, A. G. McArthur, S. I. Karchner, D. G. Franks, M. J. Jenny, A. R. Timme-Laragy, J. J. Stegeman, B. R. Woodin, M. J. Cipriano and E. Linney, PLoS One, 2014, 9, e113158 CrossRef PubMed.
- A. Ellingson, K. Haram, N. Sagen and E. Solheim, Acta Anaesthesiol. Scand., 1977, 21, 41–44 CrossRef CAS PubMed.
- S. K. Hong, C. S. Levin, J. L. Brown, H. Wan, B. T. Sherman, W. Huang da, R. A. Lempicki and B. Feldman, BMC Dev. Biol., 2010, 10, 42 CrossRef PubMed.
- L. Carvalho and C. P. Heisenberg, Trends Cell Biol., 2010, 20, 586–592 CrossRef CAS PubMed.
- A. Luzio, S. M. Monteiro, A. A. Fontainhas-Fernandes, O. Pinto-Carnide, M. Matos and A. M. Coimbra, Aquat. Toxicol., 2013, 128–129, 183–189 CrossRef CAS PubMed.
- D. Santos, M. Matos and A. M. Coimbra, Neurotoxicol. Teratol., 2014, 46, 18–25 CrossRef CAS PubMed.
- Z. M. Varga, Methods Cell Biol., 2011, 104, 453–478 Search PubMed.
- M. Westerfield, The zebrafish book: a guide for the laboratory use of zebrafish (Danio rerio), University of Oregon press, 5th edn, 2007 Search PubMed.
- C. B. Kimmel, W. W. Ballard, S. R. Kimmel, B. Ullmann and T. F. Schilling, Dev. Dyn., 1995, 203, 253–310 CrossRef CAS PubMed.
- J. Deng, L. Yu, C. Liu, K. Yu, X. Shi, L. W. Yeung, P. K. Lam, R. S. Wu and B. Zhou, Aquat. Toxicol., 2009, 93, 29–36 CrossRef CAS PubMed.
- I. Durak, Z. Yurtarslanl, O. Canbolat and O. Akyol, Clin. Chim. Acta, 1993, 214, 103–104 CrossRef CAS.
- H. Aebi, Methods Enzymol., 1984, 105, 121–126 CAS.
- D. E. Paglia and W. N. Valentine, J. Lab. Clin. Med., 1967, 70, 158–169 CAS.
- G. Rodriguez-Fuentes, F. J. Rubio-Escalante, E. Norena-Barroso, K. S. Escalante-Herrera and D. Schlenk, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2015, 172–173, 19–25 CAS.
- G. L. Ellman, K. D. Courtney, V. Andres Jr and R. M. Feather-Stone, Biochem. Pharmacol., 1961, 7, 88–95 CrossRef CAS PubMed.
- I. Domingues, R. Oliveira, J. Lourenco, C. K. Grisolia, S. Mendo and A. M. Soares, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2010, 152, 338–345 Search PubMed.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed.
- S. P. Gartaganis, N. E. Patsoukis, D. K. Nikolopoulos and C. D. Georgiou, Eye, 2007, 21, 1406–1411 CrossRef CAS PubMed.
- A. Z. Reznick and L. Packer, Methods Enzymol., 1994, 233, 357–363 CAS.
- M. W. Pfaffl, Nucleic Acids Res., 2001, 29, e45 CrossRef CAS PubMed.
- M. M. Oliveira, J. C. Teixeira, C. Vasconcelos-Nobrega, L. M. Felix, V. A. Sardao, A. A. Colaco, P. A. Oliveira and F. P. Peixoto, J. Appl. Toxicol., 2013, 33, 434–443 CrossRef CAS PubMed.
- K. D. Stackley, C. C. Beeson, J. J. Rahn and S. S. Chan, PLoS One, 2011, 6, e25652 CrossRef CAS PubMed.
- C. Venancio, L. Antunes, L. Felix, P. Rodrigues, T. Summavielle and F. Peixoto, Life Sci., 2013, 93, 464–470 CrossRef CAS PubMed.
- C. Venancio, L. Felix, V. Almeida, J. Coutinho, L. Antunes, F. Peixoto and T. Summavielle, Anesth. Analg., 2015, 120, 320–328 Search PubMed.
- P. G. Wells, Y. Bhuller, C. S. Chen, W. Jeng, S. Kasapinovic, J. C. Kennedy, P. M. Kim, R. R. Laposa, G. P. McCallum, C. J. Nicol, T. Parman, M. J. Wiley and A. W. Wong, Toxicol. Appl. Pharmacol., 2005, 207, 354–366 CrossRef PubMed.
- P. C. Jenkinson, D. Anderson and S. D. Gangolli, Teratog., Carcinog., Mutagen., 1986, 6, 547–554 CrossRef CAS.
- R. C. Harbeitner, M. E. Hahn and A. R. Timme-Laragy, Ecotoxicology, 2013, 22, 387–401 CrossRef CAS PubMed.
- A. J. Harvey, K. L. Kind and J. G. Thompson, Reproduction, 2002, 123, 479–486 CrossRef CAS PubMed.
- A. Ornoy, Reprod. Toxicol., 2007, 24, 31–41 CrossRef CAS PubMed.
- J. M. Hansen, Birth Defects Res., Part C, 2006, 78, 293–307 CrossRef CAS PubMed.
- C. T. Lin, W. C. Tseng, N. W. Hsiao, H. H. Chang and C. F. Ken, Fish Shellfish Immunol., 2009, 27, 318–324 CrossRef CAS PubMed.
- D. N. Laub, N. O. Elmagbari, N. M. Elmagbari, M. A. Hausburg and C. S. Gardiner, Toxicol. Sci., 2000, 56, 150–155 CrossRef CAS PubMed.
- A. R. Timme-Laragy, J. V. Goldstone, B. R. Imhoff, J. J. Stegeman, M. E. Hahn and J. M. Hansen, Free Radical Biol. Med., 2013, 65, 89–101 CrossRef CAS PubMed.
- M. Wu, H. Xu, Y. Shen, W. Qiu and M. Yang, Environ. Toxicol. Chem., 2011, 30, 2335–2341 CrossRef CAS PubMed.
- N. E. Huseby, C. Ravuri and U. Moens, Free Radical Res., 2016, 50, 1–13 CrossRef CAS PubMed.
- J. M. Hansen and C. Harris, Reprod. Toxicol., 2013, 35, 165–179 CrossRef CAS PubMed.
- C. D. White and D. B. Sacks, Methods Mol. Biol., 2010, 661, 151–165 CrossRef CAS PubMed.
- L. Huang, Y. Liu, P. Zhang, R. Kang, Y. Liu, X. Li, L. Bo and Z. Dong, PLoS One, 2013, 8, e59804 CrossRef CAS PubMed.
- E. G. Valesio, H. Zhang and C. Zhang, J. Appl. Toxicol., 2013, 33, 32–40 CrossRef PubMed.
- T. H. Shepard, L. A. Muffley and L. T. Smith, Anat. Rec., 1998, 252, 383–392 CrossRef CAS PubMed.
- V. Zaken, R. Kohen and A. Ornoy, Early Pregnancy: Biol. Med., 2000, 4, 110–123 CAS.
- J. Si, H. Zhang, Z. Wang, Z. Wu, J. Lu, C. Di, X. Zhou and X. Wang, Mutat. Res., 2013, 745–746, 26–33 CrossRef CAS PubMed.
- J. P. Abramov and P. G. Wells, FASEB J., 2011, 25, 2188–2200 CrossRef CAS PubMed.
- C. Wang, X. Zhang, F. Liu, M. G. Paule and W. Slikker Jr, Sci. World J., 2010, 10, 1473–1482 CrossRef CAS PubMed.
- Z. J. Bosnjak, Y. Yan, S. Canfield, M. Y. Muravyeva, C. Kikuchi, C. W. Wells, J. A. Corbett and X. Bai, Curr. Drug Saf., 2012, 7, 106–119 CrossRef CAS PubMed.
- X. Bai, Y. Yan, S. Canfield, M. Y. Muravyeva, C. Kikuchi, I. Zaja, J. A. Corbett and Z. J. Bosnjak, Anesth. Analg., 2013, 116, 869–880 Search PubMed.
- H. Ito, T. Uchida and K. Makita, PLoS One, 2015, 10, e0128445 CrossRef PubMed.
- C. Harris, in Drug Toxicity in Embryonic Development I, ed. R. Kavlock and G. Daston, Springer, Berlin Heidelberg, 1997, vol. 124/1, ch. 18, pp. 519–548 Search PubMed.
- P. S. Brookes, Free Radical Biol. Med., 2005, 38, 12–23 CrossRef CAS PubMed.
- M. P. Murphy, Biochem. J., 2009, 417, 1–13 CrossRef CAS PubMed.
- I. N. Zelko, T. J. Mariani and R. J. Folz, Free Radical Biol. Med., 2002, 33, 337–349 CrossRef CAS PubMed.
- Z. J. Hagay, Y. Weiss, I. Zusman, M. Peled-Kamar, E. A. Reece, U. J. Eriksson and Y. Groner, Am. J. Obstet. Gynecol., 1995, 173, 1036–1041 CrossRef CAS PubMed.
- F. Wang, E. A. Reece and P. Yang, Am. J. Obstet. Gynecol., 2013, 209(345), e341–347 Search PubMed.
- K. Kumari, A. Khare and S. Dange, BioMed Res. Int., 2014, 2014, 782493 Search PubMed.
- P. Z. Leite, T. C. Margarido, D. de Lima, C. Rossa-Feres Dde and E. A. de Almeida, Environ. Sci. Pollut. Res. Int., 2010, 17, 1411–1421 CrossRef CAS PubMed.
- G. Sberna, J. Saez-Valero, K. Beyreuther, C. L. Masters and D. H. Small, J. Neurochem., 1997, 69, 1177–1184 CrossRef CAS PubMed.
- C. Y. Usenko, D. C. Hopkins, S. J. Trumble and E. D. Bruce, Toxicol. Appl. Pharmacol., 2012, 262, 43–51 CrossRef CAS PubMed.
- C. Liu, H. Xu, S. H. Lam and Z. Gong, PLoS One, 2013, 8, e83954 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra08298j |
| This journal is © The Royal Society of Chemistry 2016 |