DOI:
10.1039/C6RA24627C
(Paper)
RSC Adv., 2016,
6, 102984-102996
Phosphotungstic acid supported on aminosilica functionalized perovskite-type LaFeO3 nanoparticles: a novel recyclable and excellent visible-light photocatalyst†
Received
3rd October 2016
, Accepted 24th October 2016
First published on 24th October 2016
Abstract
In this research, a novel nanohybrid compound, LaFeO3@SiO2–NH2/PTA has been prepared, in which H3PW12O40 (abbreviated as PTA) was successfully anchored onto the surface of 3-aminopropylsilica modified LaFeO3. The resultant nanohybrid compound was characterized with XRD, FT-IR, ICP-MS, FESEM, EDX, TEM, BET, and AFM techniques. The magnetic properties of all the synthesized compounds and hybrid compound were measured using a vibrating sample magnetometer (VSM) at room temperature. The photocatalytic activity of the hybrid compound was assessed by degradation of methylene blue (MB) in aqueous solution under visible light irradiation. Compared with the bare LaFeO3 and pure PTA, the newly synthesized hybrid compound exhibited enhanced photocatalytic activity under visible light irradiation. This enhancement could be attributed to the synergistic effect between LaFeO3 and PTA. After the photocatalytic reaction, the hybrid compound can be easily separated from the reaction solution and reused several times without loss of its photocatalytic activity. Moreover, a possible photocatalytic mechanism was proposed.
1. Introduction
In recent years, the problem of environmental pollution, especially water contamination is becoming increasingly serious.1 Industrially discharged water contains many harmful contaminants such as heavy metals and dyes, which are considered very carcinogenic. Among various physical, chemical and biological technologies used in pollution control, advanced oxidation processes (AOPs), including the Fenton reaction, photocatalysis, sonocatalysis, and combinations of these, are increasingly adopted in the destruction of organic contaminants, due to their high efficiency, simplicity, good reproducibility and easy handling. In general, the AOP involves in situ generation of highly reactive and nonselective chemical oxidants (i.e. H2O2, ˙OH, O2˙−, O3) to degrade persistent and non-biodegradable organic substances in industrial effluents.2,3 Photocatalysis is a procedure included in the broader field of AOPs designed to remove organic materials in wastewaters through the formation of reactive oxygen specie.4–7
Among the various photoactive materials, polyoxometalates (POMs) have been widely used and considered as suitable photocatalytic materials due to their biological and chemical inertness, low cost, strong oxidizing power, nontoxicity nature, and long term stability.8–10 POMs exhibit semiconductor-like photochemical behaviors due to analogous electronic characteristics (band gap transition for semiconductors and HOMO–LUMO transition for POMs).10–13 The ultraviolet and near-visible light and induce POM to produce oxygen-to-metal charge transfer (OMCT) with promoting electron from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO).6,7 The charge-transfer excited state (POM*) with strong oxidizing properties can direct oxidize the target pollutant, or react with water or other electron donor to generate ˙OH radical.8,9 However, the drawbacks of wide band between the well-defined HOMO–LUMO leading to low visible light photocatalytic activity, and high solubility in solution making them difficult recovering and recycling prevent their potential applications. Many researchers, have efforts in order to resolve these problems. Incorporation of homogeneous POMs with support materials, such as TiO2, ZrO2, or SiO2, and some organic polymers with UV light resistance has been extensively investigated.10–12 Those hybrids overcome some difficulties in recovering and recycling, but these methods have other shortcomings such as limited visible light activity or stability during the reaction.13 Therefore, the design and synthesis of water-insoluble POM-based photocatalyst driven by the visible light is an intriguing project, due to the ease of their structure/property tuning, recovering and recycling, as well as efficient solar light energy utilization. In this regard, the coupling of POMs with narrow band gap photoactive semiconducting materials is a novel strategy to improve their visible-light photocatalytic activity.
In recent years, perovskite-type ABO3 transition-metal oxides have received considerable attention in the photocatalytic reactions due to its narrow band-gap energy (2.0–2.5 eV) as well as its high chemical stability.6–8 Among various perovskite-type oxides, LaFeO3 (LFO) and related compounds are becoming more and more important because of their catalytic and electrical properties, which make them suitable as sensors, oxidation catalysts for air pollutants, fuel cell electrodes and oxygen permeation membranes.2 Moreover, LaFeO3 is well known photocatalyst, and has already been tested for the removal of dye pollutants due to its narrow band gap (2.1 eV), which is active under visible light.14–17 However, the photocatalytic activity of LaFeO3, is still limited, which is attributed to the weak photogenerated charge separation. Similar to other visible-responsive oxide photocatalysts, it has a low conduction band position, located below the standard hydrogen electrode reduction level, which allows fast recombination of photogenerated charge-carriers.17 To improve the photocatalytic performance of LaFeO3, coupling with wide band gap semiconducting metal-oxides such as TiO2 (ref. 18) and Ag3PO4 (ref. 19) has been reported.2,5–7 In general, it is widely accepted that the photogenerated high-energy electrons of narrow band-gap oxides could relax to the bottom of conduction band in extremely short time.20 This would lead to the fast recombination of photogenerated charges. To overcome this shortfall, the couplings of wide band gap oxides are highly desirable.
On the basis of the aforementioned considerations, among many candidates for photocatalysis,8 POMs show very efficient photoactivity under UV light irradiation (POM band gap is about 3 eV).9 On the other hand, the advantage of using LaFeO3 nanoparticles, as supported,10,11 is their activation by visible light, thanks to the smaller band gap (about 2.1 eV). Considering the above, we synthesize PTA–LFO nanohybrid compound by combining LaFeO3 and PTA, then we can use it as a photocatalyst for degradation of dye pollutant. It is worthy to mention that the prepared nanohybrid compound, exhibit higher photocatalytic activity than the bare LaFeO3 and PTA. To the best of our knowledge, there is no previous report on the enhanced visible-light activities of POMs for photocatalytic degradation of dye pollutants by coupling with perovskite nanoparticles.
2. Experimental
2.1. Materials and methods
Tetraethylorthosilicate (TEOS) (99.9%), 3-aminopropyltriethoxysilane (APTES) (98%) and phosphotungstic acid (PTA) (98%) were purchased from Merck Chemical Company. Ferrous sulphate, ferric sulphate and nickel acetate tetrahydrate were commercially acquired from Sisco Research Laboratory (SRL). All other starting materials and reagents were procured from Spectrochem pvt Ltd. and used without further purification. Organic solvents were purchased from Merck or Aldrich and used as received without further purification. Methylene blue (98%, C16H18ClN3S·3H2O, abbreviated as MB) and methyl orange (98%, C14H14N3NaO3S abbreviated as MO), were supplied from Merck and used as received. The concentration of H2O2 was 30 wt% and the studies were done by using deionized water.
2.2. Synthesis of LaFeO3@SiO2
LaFeO3 nanoparticles (LFO NPs) were prepared via the decomposition of heteronuclear hexacyano complex, La[Fe(CN)6]·5H2O, in an electric furnace at 600 °C for 2 h.21–23 LaFeO3 NPs (1.2 g) were dispersed in a mixture solvent of ethanol (45 mL) and deionized water (30 mL) by sonication for 1 h. A concentrated ammonia solution (3.6 mL) was added and the resulting mixture stirred at 40 °C for 30 min. Subsequently, tetraethylorthosilicate (TEOS, 3.6 mL) was charged to the reaction vessel and the mixture continuously stirred at 40 °C for 24 h. The precipitate was filtered off and rinsed repeatedly with deionized water until the filtrate was neutral. Then, the prepared LaFeO3@SiO2 was washed with anhydrous ethanol three times and dried at 70 °C in an oven for 24 h.24,25
2.3. Synthesis of LaFeO3@SiO2–PrNH2
LaFeO3@SiO2 (0.5 g) were suspended in dry toluene (100 mL) and concentrated ammonia solution (4 mL) was added, and the resulting mixture was stirred for 30 min. Then, a solution of 3-aminopropyltriethoxysilane (APTES, 2.0 mL) was added to the reaction vessel, and it was refluxed for 20 h.26 After completion of reaction, the LaFeO3@SiO2–PrNH2 was filtered off, washed with deionized water and ethanol, and dried at 70 °C for 5 h to give a surface bound amino group material.
2.4. Synthesis of LaFeO3@SiO2–PrNH2/PTA
For the preparation of LaFeO3@SiO2–NH2/PTA hybrid compound, a mixture of 3-aminopropylsilica coated LaFeO3@SiO2–PrNH2 (0.5 g) and phosphotungstic acid (1.0 g) in dry THF (30 mL) were sonicated for 30 min.32 The solid was filtered off, washed two times with THF and deionized water and dried at 70 °C for 5 h. The tungsten content was determined by ICP analysis, and it was found to be 14.6 wt%, confirmed a loading of 15 wt% PTA.
2.5. Characterization
Fourier-transform infrared spectra were recorded on Shimadzu FT-IR 8400S (Japan) with temperature controlled high sensitivity detector (DLATGS detector) and resolution of 4 cm−1 in the scan range of 500–4000 cm−1 using KBr pellet. PTA content was measured by inductively coupled plasma optical emission spectroscopy (ICP-OES) on a Varian VISTA-PRO model analyzer. The XRD patterns were obtained on a Rigaku D-max C III diffractometer using Ni-filtered Cu Kα radiation (λ = 1.5406 Å) for determination of the phases present in the decomposed samples. UV-Vis spectra were recorded on a on a Carry 100 Conc Varian spectrophotometer. The transmission electron microscopy (TEM) characteristics were performed with Philips CM30, a 300 kV analytical TEM Microscope (Netherlands). SEM images were obtained from MIRA3 TESCAN Field Emission Scanning Electron Microscope equipped with a link Energy-Dispersive X-ray (EDX) analyzer. Specific surface area was calculated by the BET–BJH method using N2 adsorption–desorption experiments carried out at 170 °C on a Micromeritics ASAP 2010. Magnetic measurements were carried out at room temperature using a vibrating sample magnetometer (Meghnatis Daghigh Kavir Co., Iran) with a maximum magnetic field of 10 kOe. AFM images were obtained from Ara-AFM (Iran), model Full plus, method noncontact. A multiwave ultrasonic generator (Bandeline GM 2200) equipped with a converter/transducer and titanium oscillator operating at 20 kHz with a maximum power output of 200 W was used for the ultrasonic irradiation.
2.6. Photocatalytic tests
The photocatalytic performance of the as-prepared catalyst was evaluated via the degradation of MB in an aqueous solution at 25 °C under visible light irradiation using a 400 W mercury lamp with a 420 nm cutoff filter as the light source. In each experiment, 25 mg of catalyst and 2 mL of H2O2 (0.1 mol L−1) were added into 50 mL of MB (25 mg L−1) aqueous solution in a Pyrex cell, which was cooled by recycled water to weaken the effect of thermal catalytic. Prior to illumination, the suspension was stirred gently with the speed of 300 rpm at 25 °C in dark condition to reach the adsorption–desorption equilibrium on the catalyst surface. After, that the suspension was exposed to visible light source and in several intervals, the samples were separated through centrifuge to remove the suspended particles. Then the upper clear solutions were analyzed by a UV-vis spectrophotometer. The dye concentration was determined by using Carry 100 Conc Varian spectrophotometer. The concentrations of MB and MO in the solution, were determined as a function of adsorption time from the change in absorbance at 664 and 463 nm for MB and MO solution at pH = 6 and 5 respectively. The dye degradation efficiency (R) could be calculated by R = 100(C0 − Ct)/C0, where C0 and Ct (mg L−1) are the dye concentrations at initial and t time. Control experiments in the presence of pure LaFeO3 (20 mg) and pure PTA (10 mg) were conducted under similar conditions mentioned above. In addition, a series of experiments were conducted to investigate the primary radical species during the degradation of MB over the LaFeO3@SiO2–NH2/PTA hybrid compound via introducing various scavengers into the MB solution. The experimental procedure was similar to the above photodegradation experiment. Briefly, the MB photodegradation was repeated by adding 10 mmol L−1 of tert-butyl alcohol (TBA) and ethylenediaminetetraaceticacid salt dehydrate (EDTA-2Na) as a hydroxyl radical and hole scavengers, respectively in the same condition of reaction (the initial dye concentration, catalyst dosage, and reaction time were set to 25 mg L−1, 25 mg and 15 min, respectively). Most of alcohols as TBA are capable for deactivating the hydroxyl radicals and its derivatives.
To evaluate the stability of the nanohybrid compound, recycling experiment was performed with the photodegradation of MB under visible light irradiation. After each experiment, the hybrid compound, loaded with MB was separated from the dye solutions by centrifugation and washed several times with water and ethanol at room temperature. Then the regenerated hybrid compound, was dried overnight at 70 °C and reused for the next photodegradation experiments.
3. Results and discussion
The sequence of steps in the functionalization of LaFeO3 NPs with H3PW12O40 polyoxometalate has been shown in Fig. 1. In the first step, the surface of LaFeO3 nanoparticles was coated with silica shell through hydrolysis and condensation reactions of Si(OEt)4 (TEOS) resulted in LaFeO3@SiO2. In the second step, treatment of LaFeO3@SiO2 with 3-aminopropyltriethoxysilane (APTES) afforded LaFeO3@SiO2–PrNH2. The next step involves reaction of LaFeO3@SiO2–PrNH2 NPs, with PTA to yield the nanohybrid compound. In this step, protonation of amine groups gives positively charged ammonium cations (–NH3+) which bounds electrostatically to the heteropolyanions.27–29 It has been shown that the heteropoly anion salts of organic cations are generally insoluble in water.9,30–32 So, the excess of heteropoly acids were removed by washing the resulted materials. The electrostatically attached heteropoly anions on the surface of modified LaFeO3 nanoparticles could not be leached to aqueous solution. The results of FT-IR, XRD and EDX analyses confirmed the formation of the LaFeO3@SiO2–NH2/PTA nanohybrid compound. Furthermore the results of ICP analysis revealed the tungsten content of nanohybrid compound as 14.6 wt%, which is in accordance with 15 wt% loading of PTA.
 |
| | Fig. 1 Schematic illustration for preparation of LaFeO3@SiO2–NH2/PTA nanohybrid compound. | |
3.1. Fourier transform infrared spectroscopy (FT-IR)
The FT-IR spectra of synthesized materials are shown in Fig. 2. The FT-IR spectrum of the starting complex La[Fe(CN)6]·5H2O in Fig. 2(a) shows the characteristic C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching bands at about 2150, 2060 cm−1.21 Also, the bands at 3500 and 1640 cm−1 are related to the stretching and bending vibrations of the lattice water molecules, respectively. In the spectrum of LaFeO3 nanoparticles, the strong bands at about 560 and 440 cm−1 are assigned to the Fe–O stretching and bending vibrations of the octahedral FeO6 groups in the perovskite-type structure, respectively.21,27 The presence of SiO2 in the composition of hybrid compound, was confirmed by bands at 1087 and 964 cm−1 assigned to the SiO–H and Si–O–Si groups.9,12,26,32 The two broad bands at 3429 and 1636 cm−1 can be ascribed to the stretching and bending modes of NH2 groups, respectively. The presence of the anchored propyl groups of APTES, was confirmed by C–H stretching vibrations at 2910 and 2980 cm−1 (Fig. 2(d)).9,25 It is well known that Keggin-type polyoxometalate, PTA, contains a cage of tungsten atoms linked by oxygen atoms with tetrahedral phosphate group. Oxygen atoms form four physically distinct bonds (P–Oa, W–Ot, W–Ob–W, and W–Oc–W bonds), which have distinct infrared signatures: 1080 cm−1 for asymmetric stretch vibration of P–Oa (Oa corresponds to oxygen atom of tetrahedral phosphate group), 987 cm−1 for asymmetric stretch vibration of W
N stretching bands at about 2150, 2060 cm−1.21 Also, the bands at 3500 and 1640 cm−1 are related to the stretching and bending vibrations of the lattice water molecules, respectively. In the spectrum of LaFeO3 nanoparticles, the strong bands at about 560 and 440 cm−1 are assigned to the Fe–O stretching and bending vibrations of the octahedral FeO6 groups in the perovskite-type structure, respectively.21,27 The presence of SiO2 in the composition of hybrid compound, was confirmed by bands at 1087 and 964 cm−1 assigned to the SiO–H and Si–O–Si groups.9,12,26,32 The two broad bands at 3429 and 1636 cm−1 can be ascribed to the stretching and bending modes of NH2 groups, respectively. The presence of the anchored propyl groups of APTES, was confirmed by C–H stretching vibrations at 2910 and 2980 cm−1 (Fig. 2(d)).9,25 It is well known that Keggin-type polyoxometalate, PTA, contains a cage of tungsten atoms linked by oxygen atoms with tetrahedral phosphate group. Oxygen atoms form four physically distinct bonds (P–Oa, W–Ot, W–Ob–W, and W–Oc–W bonds), which have distinct infrared signatures: 1080 cm−1 for asymmetric stretch vibration of P–Oa (Oa corresponds to oxygen atom of tetrahedral phosphate group), 987 cm−1 for asymmetric stretch vibration of W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ot (Ot corresponds to the terminal oxygen atoms), 890 cm−1 for bending vibration of W–Ob–W (Ob corresponds to oxygen atom bridging the two tungsten atoms), and 804 cm−1 for bending vibration of W–Oc–W (Oc represents oxygen atom at the corners of the Keggin structure) (Fig. 2(e)). As can see in Fig. 2(f), appearance of peaks at 1070, 985, 885 and 800 cm−1, confirm the presence of PTA in the structure of nanohybrid compound.9,10,13,27–35 The IR characteristic bands of PTA in the nanohybrid compound, have some red shifts compared with those for the pure PTA.27,30,36,37 It confirms that a strong chemical interaction, not simple physical absorption,27,30,36,37 exists between the PTA polyanions and the LaFeO3@SiO2–NH2 surface.
Ot (Ot corresponds to the terminal oxygen atoms), 890 cm−1 for bending vibration of W–Ob–W (Ob corresponds to oxygen atom bridging the two tungsten atoms), and 804 cm−1 for bending vibration of W–Oc–W (Oc represents oxygen atom at the corners of the Keggin structure) (Fig. 2(e)). As can see in Fig. 2(f), appearance of peaks at 1070, 985, 885 and 800 cm−1, confirm the presence of PTA in the structure of nanohybrid compound.9,10,13,27–35 The IR characteristic bands of PTA in the nanohybrid compound, have some red shifts compared with those for the pure PTA.27,30,36,37 It confirms that a strong chemical interaction, not simple physical absorption,27,30,36,37 exists between the PTA polyanions and the LaFeO3@SiO2–NH2 surface.
 |
| | Fig. 2 FT-IR spectra of (a) LaFe(CN)6, (b) LaFeO3 NPs, (c) LaFeO3@SiO2, (d) LaFeO3@SiO2–NH2, (e) PTA, and (f) LaFeO3@SiO2–NH2/PTA compound. | |
3.2. Phase structure study
The XRD diffraction patterns of LaFeO3 NPs and LaFeO3@SiO2–NH2/PTA nanohybrid compound, are shown in Fig. 3. Using the Debye–Scherrer equation, the average particle size of bare LaFeO3 and hybrid compound were estimated as 35 and 65 nm, respectively. The XRD pattern of LaFeO3 NPs showed only the pattern corresponding to perovskite-type LaFeO3 (JCPDS File no. 37-1493), which crystallizes in the orthorhombic system.18–21,27,38 The 2θ of strong absorption peaks of PTA are observed at 6–10°, 15–22°, 24–30°.39 By these consideration, in the XRD pattern of nanohybrid compound, all of related peaks of LaFeO3 are interestingly observed with strong intensity, also appearance of peaks at 2θ = 10.2° and 25.7° and 34.6°, confirms the presence of PTA in the structure of hybrid compound.32 The presence of the intense peaks in the XRD pattern of nanohybrid compound, indicates that there is no significant change in the structure of the PTA and it kept its structure after hybridizing with the LaFeO3@SiO2–PrNH2.32,40,41
 |
| | Fig. 3 X-ray diffraction patterns of LaFeO3 nanoparticles (a) and LaFeO3@SiO2–NH2/PTA nanohybrid compound (b). | |
3.3. BET specific surface areas analysis
Fig. 4(a) presents the N2 adsorption–desorption isotherms of nanohybrid compound. The shape of the isotherm was of type IV with a broad H3 type hysteresis loop according to the IUPAC classification,42 indicating that the hybrid compound have mesoporous structures.43,44 Furthermore the pore size distribution curve (Fig. 4(b)) shows the pores diameter are between 1 and 100 nm (maximum at 26 nm), which corresponds to the voids formed on the nanohybrid compound having a mesoporous nature. The average pore diameter of nanohybrid compound is 13 nm with a volume of 0.33 mL g−1. Also the specific surface area for the nanohybrid compound, measured as 9.44 m2 g−1. As reported in literature the specific surface areas of polyoxometalates are lower than 10 m2 g−1.36 Moreover, the nanohybrid compound has lower specific surface area, in comparison of bare LaFeO3, with specific surface area of 38.5 m2 g−1,21 It can be related to its synthesis route, performing different steps as silica and amino coating LaFeO3 and functionalization the surface of LaFeO3 for loading PTA, cause low surface area for the hybrid compound, but interestingly, due to its large average pore diameter, it can be introduce as a mesoporous compound.
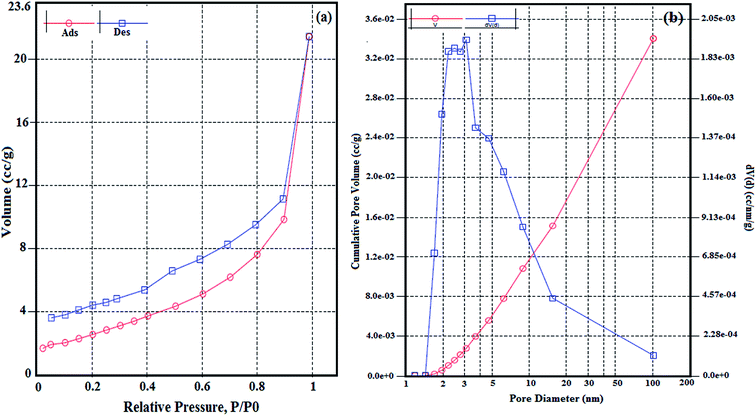 |
| | Fig. 4 Nitrogen adsorption–desorption isotherms (a), and pore size distribution of the nanohybrid compound (b). | |
3.4. Optical absorption properties
The optical absorption of the as-prepared samples was measured by a UV-vis spectrometer. Fig. 5 presents the UV-vis spectra of pure PTA, bare LaFeO3 and nanohybrid compound samples. For the LaFeO3 nanomaterials, a sharp band at 331 nm and a broad band in the range of 380–750 nm, are observed which are attributed to the electronic transition from the valence band to the conduction band of O2− → Fe3+ in the LaFeO3 lattice.21 The pure PTA, shows a sharp band at 205 nm and other weak band at 247 nm, which are attributed to charge-transfer from O2− to W6+ in Keggin-structured [PW12O40]3− at W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and W–O–W bonds, respectively.27,36,37,45 The band gap energy (Eg) of pure LaFeO3 is determined to be 2.09 eV by the equation Eg = 1240/λg.18,19,37 For hybrid compound, in comparison to pure PTA, the optical absorption is enhanced significantly in the region of 300–550 nm (in visible light region) for pure LaFeO3 and nanohybrid compound samples. By loading the PTA on the surface of LaFeO3, the ability of light absorption was enhanced for LaFeO3 and nanohybrid compound samples. More interestingly, by loading the PTA, the charge transformation bands have a redshift and shift to higher wavelength, it confirms the presence of strong chemical interactions, between the polyanion and the LaFeO3 NPs surface.36 Compared with pure PTA and LaFeO3, the absorption threshold onset of nanohybrid compound extended to visible region which corresponded to a narrow band gap energy (1.9 eV) for the nanohybrid compound sample. It reveals that the band gap energy of nanohybrid compound is to some extent lower than the bare LaFeO3 with band gap energy of 2.09 eV.19 The observed weak red shift over the nanohybrid compound, is caused by the charge-transfer transition between the PTA and the LaFeO3 conduction or valance band.36,46–48 These data confirmed that the electronic structures of LaFeO3 were slightly changed due to the introduction of Keggin-type PTA and the outstanding visible light photocatalytic activity of the nanohybrid compound, in comparison of bare PTA is attributed to the existence of the broad band in the visible region.
O and W–O–W bonds, respectively.27,36,37,45 The band gap energy (Eg) of pure LaFeO3 is determined to be 2.09 eV by the equation Eg = 1240/λg.18,19,37 For hybrid compound, in comparison to pure PTA, the optical absorption is enhanced significantly in the region of 300–550 nm (in visible light region) for pure LaFeO3 and nanohybrid compound samples. By loading the PTA on the surface of LaFeO3, the ability of light absorption was enhanced for LaFeO3 and nanohybrid compound samples. More interestingly, by loading the PTA, the charge transformation bands have a redshift and shift to higher wavelength, it confirms the presence of strong chemical interactions, between the polyanion and the LaFeO3 NPs surface.36 Compared with pure PTA and LaFeO3, the absorption threshold onset of nanohybrid compound extended to visible region which corresponded to a narrow band gap energy (1.9 eV) for the nanohybrid compound sample. It reveals that the band gap energy of nanohybrid compound is to some extent lower than the bare LaFeO3 with band gap energy of 2.09 eV.19 The observed weak red shift over the nanohybrid compound, is caused by the charge-transfer transition between the PTA and the LaFeO3 conduction or valance band.36,46–48 These data confirmed that the electronic structures of LaFeO3 were slightly changed due to the introduction of Keggin-type PTA and the outstanding visible light photocatalytic activity of the nanohybrid compound, in comparison of bare PTA is attributed to the existence of the broad band in the visible region.
 |
| | Fig. 5 UV-Vis spectra of the PTA (a), LaFeO3 (b) and nanohybrid compound (c). | |
3.5. Magnetic properties
The magnetic properties of synthesized samples were analyzed by VSM at room temperature. Fig. 6 shows the magnetization curves of the prepared samples. The LaFeO3 nanoparticle, showed paramagnetic properties with saturation magnetization about 0.33 emu g−1. After coating of LaFeO3 nanoparticles with SiO2 shell, the saturation magnetization decrease obviously. The saturation magnetizations for bare LaFeO3, LaFeO3@SiO2 and LaFeO3@SiO2–NH2 and hybrid compound were found to be 0.33, 0.13, 0.12 and 0.1 emu g−1, respectively. Decrease of the saturation magnetization, during the functional modification process, indicating the silica coating on the surface of the LaFeO3, effectively induce the magnetic property of hybrid compound.49,50 All of these four NPs showed negligible coercivity (Hc) and remanence, typical of paramagnetic materials.
 |
| | Fig. 6 Hysteresis loop of (A) LaFeO3 NPs, (B) LaFeO3@SiO2, (C) LaFeO3@SiO2–NH2, and (D) LaFeO3@SiO2–NH2/PTA nanohybrid compound. | |
3.6. SEM and TEM studies
The morphology and particle size of LaFeO3 and nanohybrid compound, were investigated by FESEM (Fig. 7). The SEM images of LaFeO3 (Fig. 7(a) and (b)) reveals uniform and spherical morphology for LaFeO3 nanoparticles. Also interestingly, the nanohybrid compound show a uniform and spherical, and to some extent aggregated morphology (Fig. 7(c)–(e)). Additionally, the weight percentage of the elements in nanohybrid compound, has been evaluated by EDX analysis, which confirms the incorporation of PTA in the nanohybrid compound (Fig. 7(f)).
 |
| | Fig. 7 SEM images (a and b) for bare LaFeO3 and (c–e) for the nanohybrid compound, EDX spectrum of nanohybrid compound (f). | |
The TEM images of the prepared hybrid compound is shown in Fig. 8 which confirm the spherical morphology of hybrid compound with the particle size in range of 60–150 nm. The TEM images are consistent with the SEM image which shows that NPs are to some extent aggregated, because of the functionalization of LaFeO3 surface, with TEOS and APTES. It is obviously shown in TEM images that the nanoparticles are in core–shell structures, black spots showing LaFeO3 and ashen part as SiO2@NH2–PTA shell.51 This indicates the successful synthesis of LaFeO3@SiO2–NH2/PTA hybrid compound.
 |
| | Fig. 8 TEM images of nanohybrid compound. | |
3.7. AFM results
The AFM images of hybrid compound are shown in Fig. 9. The results demonstrate spherical nanoparticles with coarse surface with porosity for hybrid compound. The results are in good agreement with BET results and SEM and TEM images.
 |
| | Fig. 9 AFM results of nanohybrid compound. | |
3.8. Photocatalytic activity
The photocatalytic activity of nanohybrid compound was investigated for degradation of MB and MO organic dyes in aqueous solution under visible light irradiation. The photocatalytic activity of materials, depends on the crystallinity, specific surface area, band gap energy and morphology of these materials.38 Firstly, the degradation experiments were performed for degradation of cationic dyes as MB in the presence of the nanohybrid compound. The adsorption percentage of the nanohybrid compound for MB, at 30 min of dark conditions under adsorption–desorption equilibrium, is 45% which can be related to adsorption of the cationic molecules of dye with anionic charged composite catalyst. The MB dye shows strong characteristic absorption at 664 nm which this absorption maxima gradually decreases upon increasing irradiation time. The time dependent absorption spectrum of MB solution under photo irradiation in the presence of nanohybrid compound is shown in Fig. 10(a). The absorption maxima of MB is about zero as the exposure time of photo irradiation increases to 15 min. During the photocatalytic degradation process, the intense blue colour of the initial solution, disappears and becomes almost colourless, indicating the photodegradation process of MB. Furthermore, the strong absorption bands of MB at 664 nm were not shifted, indicating that the degradation of MB is due to chromophores being destroyed.52,53 The nanohybrid compound is an effective catalyst for degradation of MB, with degradation efficiency of 100% in the period of 15 min of irradiation. The observed catalytic activity of synthesized nanohybrid compound, for degradation of MB, was found to be interestingly higher compared to some of the recently reported photocatalysts. For more comparison, some of newly synthesized nanomaterials and their photodegradation efficiency are listed in Table 1.
 |
| | Fig. 10 Time dependent absorption spectrum during photocatalytic reaction of MB (a) and MO (b) in the presence of nanohybrid compound (concentration of MB and MO = 25 mg L−1, amount of catalyst = 25 mg, 2 mL of hydrogen peroxide 0.1 M, reaction temperature = 25 °C). | |
Table 1 Application of different nanoparticles in photodegradation of organic dye pollutants
| Nanomaterial |
Irradiation |
Organic pollutant |
Degradation efficiency (%) |
Time (min) |
Ref. |
| LaFeO3@SiO2–NH2/PTA |
Visible light |
MB |
100 |
15 |
This work |
| LaFeO3/Ag3PO4 |
Visible light |
Phenol |
90 |
120 |
19 |
| LaFeO3 |
Visible light |
MB |
93 |
120 |
38 |
| TiO2/Fe3O4 |
UV light |
MB |
100 |
180 |
44 |
| HPW/BiVO4 |
Visible light |
MB |
93 |
360 |
46 |
| FeOOH–LDO |
Visible light |
MB |
95 |
180 |
53 |
| PMo12@g-C3N4 |
UV–visible |
MB |
100 |
180 |
57 |
| PW12@g-C3N4 |
UV–visible |
MB |
90 |
180 |
57 |
| MnO2-HPW |
UV light |
MB |
66 |
10 |
58 |
| HPW/TiO2 |
Visible light |
MB |
96 |
60 |
59 |
| HPW/ZrO2 |
UV light |
MB |
96 |
90 |
60 |
| Ag/AgHSiW |
Visible light |
MB |
100 |
170 |
61 |
| Ag/AgHPW/Cu2O |
Visible light |
MB |
100 |
50 |
62 |
| Fe3O4@Ag3PO4–AgCl |
Visible light |
MB |
100 |
60 |
63 |
| Ag3PO4–CoFe2O4 |
UV light |
MB |
100 |
60 |
64 |
| CoFe2O4/g-C3N4 |
Visible light |
MB |
100 |
120 |
65 |
| BiFeO3 |
Visible light |
MB |
100 |
110 |
66 |
| BiFeO3 |
Visible light |
MB |
100 |
80 |
67 |
| ZnO/RGO |
UV light |
MB |
99.5 |
180 |
68 |
| ZnO/CdS |
Visible light |
MB |
100 |
120 |
69 |
Furthermore, for more details, the photocatalytic activity of nanohybrid compound catalyst were performed for degradation of anionic dyes as MO in similar conditions. Firstly, the adsorption percentage of composite catalyst for MO, within 30 min of adsorption–desorption equilibrium, was only 9% which is related to that the anionic charges on composite catalyst repel the anionic charged MO dye molecules. The MO, shows strong characteristic absorption at 463 nm which, this absorption maxima gradually decreases upon increasing irradiation time from 0 to 100 min. The composite catalyst has degradation efficiency of 20% within 100 min of photo irradiation for MO. The curve of time dependent absorption spectra of MO solution in Fig. 10(b) reveals the hybrid compound have not good efficiency for the degradation of MO.
Further, the photocatalytic activities of bare LaFeO3 and pure PTA for degradation of MB and MO organic dyes, are evaluated and the results are shown in Fig. S1 and S2† respectively. The adsorption percentage of bare LaFeO3, within 30 min of adsorption–desorption equilibrium is 6% for MB and 7% for MO. It is observed that under photo irradiation, the LaFeO3 nanoparticles degrades 42% of MB dye within 240 min, and 27% of MO dye within 360 min. Its weak activity may be concluded from the low surface area of NPs and easily recombination of electron–hole pairs on it due to its low band gap energy.38 For the pure PTA, it can adsorb 70% of MB within 30 min of adsorption–desorption equilibrium, but it can not adsorb MO molecules during adsorption–desorption equilibrium. Its degradation efficiency within 120 min of photo light irradiation is 83% for MB and 18% for MO. Herein, the weak photodegradation of pure PTA, can be attributed to its low surface area and higher band gap. For more details, the results of photodegradation experiments for all the bare materials and hybrid compound are summarized in Fig. 11. Obviously it can be seen that the catalytic efficiency of hybrid compound catalyst, is much higher as compared to that of bare LaFeO3 NPs and pure PTA for degradation of MB under the same conditions. Basically the efficiency of photocatalysis depends on the density of photogenerated electrons and holes, and the rate of their recombination before they can reach the surface of a nanoparticle to implement the catalytic action.54,55 The recombination can be suppressed very significantly if the system has an ability to separate the electrons and holes effectively as in a p/n junction in a solar cell. Hybrid compounds with appropriate band alignment, can provide such opportunity.19,56 So the possible reason for the improved photocatalytic activity of the hybrid compound is related to increasing the additional routes of trapping holes and decreasing the recombination rate of electron–hole pairs.
 |
| | Fig. 11 The degradation efficiencies with LaFeO3 for degradation of MB (under 240 min of photo irradiation), with PTA in the period of 120 min for degradation of both MB and with nanohybrid compound for degradation of MB (in the period of 15 min of photo irradiation). | |
The control experiments were performed which consists of MB solution exposed to photo irradiation in the absence of hybrid compound, without addition of hydrogen peroxide to indicate the efficiency of photo irradiation solely on degradation of MB solution, and other experiment contains MB solution in the absence of hybrid compound catalyst but adding hydrogen peroxide (2 mL, 0.1 M). The results indicate that without hybrid compound and no hydrogen peroxide addition, only caused 10% of MB dye to be degraded and the only hydrogen peroxide addition, caused 31% of MB dye to be degraded, after 60 min of photo irradiation.
3.8.1. Effect of enhancers. To investigate the effect of hydrogen peroxide on the photodegradation reactions, several experiments were performed, using composite material for degradation of MB under the identical condition as produced before by using different enhancers. The effect of different enhancers such as hydrogen peroxide, peroxydisulfate and potassium periodate on degradation efficiency of MB was studied with an initial concentration of 0.1 M (2 mL) and the results are shown in Fig. 12. Interestingly, using the enhancers, increase the photocatalytic degradation of MB considerably.70,71 The degradation efficiency of MB dye in presence of nanohybrid compound catalyst, without any enhancer, is 68% within a process time of 15 min. The obtained degradation efficiency of MB, with different enhancers as hydrogen peroxide, peroxydisulfate and potassium periodate, are 100% within 15 min, 100% within 10 min and 100% within 5 min, respectively. In the case of the addition of hydrogen peroxide, the increase in the photocatalytic degradation of MB is mainly due to increased generation of OH radicals.71 The addition of peroxydisulfate can also enhance the photocatalytic degradation efficiency of MB because of the production of sulfate radicals and subsequently the formation of OH radicals.72 The addition of periodate ions produced the greatest favorable effect on the photocatalytic degradation efficiency of MB by capturing generated electrons.73 By considering these results, it can be concluded that the peroxydisulfate and periodate are most powerful oxidant agents and they have intensive effect on the photodegradation of MB dyes. So the optimum oxidant agent for usage in the photodegradation experiments is hydrogen peroxide, in agreement with several reports in literature.56,74–78
 |
| | Fig. 12 Effect of enhancers on the photocatalytic degradation of MB in the presence of composite catalyst (concentration of MB = 25 mg L−1, amount of catalyst = 25 mg, amount of enhancer = 2 mL with concentration of 0.1 M, reaction temperature = 25 °C). | |
3.8.2. Effect of amount of hydrogen peroxide. To determine the suitable amount of hydrogen peroxide for degradation of MB in presence of hybrid compound, three experiments were conducted with different amounts of hydrogen peroxide (0.1 M), changing from 1 to 2 and 3 mL. The results are depicted in Fig. S3.† When the amount of used hydrogen peroxide in the photodegradation experiments, increased from 1 mL to 2 and then 3 mL, degradation efficiency of MB, enhanced from 85% in 15 min, to 100% in 15 min, and 100% in 5 min, respectively (Fig. S3†). The increase in the amount of used hydrogen peroxide, significantly shortened time necessary to degradation of MB dye solution. Herein, the addition of hydrogen peroxide (2 mL) was chosen as an optimum amount for degradation of dye solution. The increase in the amount of hydrogen peroxide, generates more hydroxyl free radicals, which cause dye degradation in short time with good efficiency. However it is important that further increase in hydrogen peroxide amount, above certain limit (critical concentration), will not increase the rate of dye destruction as it acts as a radical scavenger instead of a free radical generator.74
3.8.3. Reusability of the nanohybrid compound. The stability of the catalyst materials is an important issue in the catalytic experiments. Unlike the PTA which is soluble in water, the synthesized nanohybrid compound is not soluble in water. It can easily recovered by centrifuge, washed with water and reused for a new photodegradation experiment, while other factors are kept identical. The reusability test for the nanohybrid compound was carried out for degradation of MB up to three cycles. The results showed a negligible drop in degradation efficiency after three repeated cycles, for which degradation values of 100%, 100% and 99% for MB were obtained (Fig. 13). The amount of W leached, in the clear solution of MB after three cycles of photodegradation experiments, was determined by ICP and the results showed about 1.4% of W has been leached from the nanohybrid compound. These results are confirmed with FT-IR spectra of nanohybrid compound after using for three cycles of photodegradation experiments (Fig. S4†). These results are attributed to the strong interaction between the Keggin unit PTA and the LaFeO3@SiO2–NH2 surface. More importantly, the interaction between the Keggin unit and LaFeO3 is chemical rather than physical. Therefore, the nanohybrid compound can be reused more than three cycles without finding obvious decreases in its photocatalytic activity. The synthesized nanohybrid compound, not only displays excellent photocatalytic activity under visible light irradiation, but also exhibits good reproducibility. The foregoing two aspects are of great significance for practical use of this nanohybrid compound for removal of MB dye pollutants from aqueous solution.
 |
| | Fig. 13 Effect of number of recycling runs on the photodegradation efficiency of nanohybrid compound. | |
3.8.4. Mechanism of photocatalytic degradation. It can be understand that the photocatalytic activity of bare LaFeO3 is limited due to the fast recombination rates of photogenerated electron–hole pairs. By loading of PTA on the surface of LaFeO3@SiO2–NH2 nanoparticles, the PTA can act as electron traps, making the photogenerated electrons migrate from LaFeO3 to the PTA. The better photodegradation efficiency of nanohybrid compound towards MB dye, mainly is originated from the synergistic effect caused by combination of LaFeO3 and PTA. Accordingly, the electron–hole recombination is effectively suppressed. Therefore, more holes would be preserved, reach the surface of the LaFeO3, and enhance the degradation efficiency of organic dyes.18,19,38 The position of conduction band (CB) and valence band (VB) of a semiconductor is one of the important factors that affect the photocatalytic activity. The potentials of the conduction band and valence band edges can be obtained according to the Mulliken electronegativity theory, as ECB = 0.025 eV, EVB = 2.115 eV for LaFeO3 (ref. 19) and ECB = 0.26 eV, EVB = 3.56 eV for PTA.79 The CB and VB edge potential positions of LaFeO3 are both more negative than that of PTA. The LaFeO3 nanoparticles in the composition of nanohybrid compound, would absorb photons to generate electron–hole pairs under visible light irradiation. The reaction mechanism involved during the photocatalytic activities of nanohybrid compound are schematically demonstrated in Fig. 14. During the irradiation process, the nanohybrid compound having energy greater than the threshold, so the photogenerated electrons of LaFeO3 are transferred from the VB to the CB, leaving the positive holes in the VB and result in the efficient separation of electron–hole pairs (eqn (1)). Subsequently, the photo-generated electrons on the CB bottom of LaFeO3 would easily transfer to the CB of PTA via the well connected interface as shown in eqn (2), and meanwhile holes on the VB of PTA, will diffuse into the VB of LaFeO3 as shown in eqn (3). The PTA has an ability to enhance the rate of CB electron transfer by accepting photogenerated electrons to its empty d orbitals. Such an effective electron transfer can inhibit the fast electron–hole recombination on LaFeO3. The generation of active species is related to the potential energy of the conduction band and valance band of bare materials. The CB edge of PTA is 0.26 eV (vs. NHE),19,75 which is more positive than the standard redox potential E(O2/O2˙−) (−0.33 eV vs. NHE),80 indicating that the electrons at CB of PTA, can not reduce O2 to O2˙−. Adding a suitable amount of hydrogen peroxide, facilitate the generation of ˙OH and promote the degradation efficiency. The electrons from conduction band of PTA, react with hydrogen peroxide as shown in eqn (4), to generate ˙OH and OH− anions. However, the CB edge potential of PTA, is more negative than the standard redox potential E(O2/H2O2) (0.68 eV vs. NHE),19,75 suggesting the oxygen adsorbed on the surface of nanohybrid compound can react with electrons of PTA, to form hydrogen peroxide as shown in eqn (5). Also, due to the VB edge potential of LaFeO3 (2.115 eV vs. NHE)19 is more positive than the standard redox potential of E(˙OH/OH−) (1.99 eV vs. NHE)81 suggesting that the accumulated holes on the VB of LaFeO3 can oxidize OH− to form ˙OH as shown in eqn (6). The ˙OH radicals, show strong oxidation characteristic to participate in the photodegradation reactions as shown in eqn (7). Also mainly part of holes maybe directly involved in the oxidation of MB organic dye as described in eqn (7). Based on the above discussion, the progress of photodegradation of MB with composite catalyst can be proposed as follows:| | |
LaFeO3/PTA + hν → eCB− + hVB+
| (1) |
| | |
LaFeO3(eCB−) + PTA → PTA(eCB−)
| (2) |
| | |
PTA(hVB+) + LaFeO3 → LaFeO3(hVB+)
| (3) |
| | |
PTA(eCB−) + H2O2 → ˙OH + OH−
| (4) |
| | |
PTA(2eCB−) + O2 + 2H+ → H2O2
| (5) |
| | |
LaFeO3(hVB+) + OH− → ˙OH
| (6) |
| | |
˙OH, LaFeO3(hVB+) + MB → CO2 + H2O
| (7) |
 |
| | Fig. 14 The mechanism of photocatalytic degradation of MB in presence of the nanohybrid compound. | |
3.8.5. Effect of radical and hole scavengers. In the photocatalytic process, the active species mainly involve, hole (h+) and hydroxyl radical (˙OH) and superoxide radicals (O2˙−).19 To assess active species in this photodegradation reaction, the free radical capture experiments were conducted by adding different scavengers. tert-Butyl alcohol (TBA) and disodium ethylenediaminetetraaceticacid salt dehydrate (EDTA-2Na) were used with concentration of 10 mmol L−1 as radical and hole scavengers, respectively. In the presence of TBA and EDTA, the hydroxyl radicals and holes are quenched respectively and it leads to inhibiting effect on the photocatalytic degradation. The degradation efficiency of MB, at the presence of TBA and EDTA, decreased from 100% to 46% and 62%, respectively. The results as shown in Fig. 15 gives evidence that the degradation efficiency of MB, is dominated by the oxidation reaction of hydroxyl radical and the direct hole oxidation.
 |
| | Fig. 15 Photocatalytic degradation efficiency at the presence of various scavengers. Photodegardation experiment without any scavenger (A), using TBA (B) and EDTA (C) (initial dye concentration = 25 mg L−1, amount of used catalyst = 25 mg, scavenger concentration = 10 mmol L−1). | |
4. Conclusions
In this study, the Keggin type polyoxometalate, H3PW12O40 (PTA) supported on aminosilica coated LaFeO3, for synthesis of heterogeneous nanohybrid compound LaFeO3@SiO2–NH2/PTA. The results of FT-IR, XRD, EDX and ICP analyses confirmed successfully loading of PTA species on the surface of the LaFeO3@SiO2–NH2. The bare compounds and newly synthesized hybrid compound were used for photodegradation of dye pollutants as MB and MO in aqueous solution. The results reveals that the newly synthesized nanohybrid compound has excellent efficiency for degradation of MB in compare with bare LaFeO3 or pure PTA. Supporting PTA on LaFeO3 has proven to be an efficient method to promote the photocatalytic activity of PTA, attributing to the synergistic effect between LaFeO3 and PTA. It was used as an efficient and reusable heterogeneous catalyst for degradation of MB dye molecules under visible light irradiation. The results showed that a low amount as 25 mg of nanohybrid compound was sufficient for degradation of 50 mL of MB with concentration of 25 mg L−1 with degradation efficiency of 100% within 15 min under visible light irradiation. Furthermore, the catalyst, can be recycled and reused several times without significant decrease in its photocatalytic activity.
Acknowledgements
We thank the Lorestan University and Shahid Beheshti University for support of this work.
References
- P. Benjwal, M. Kumar, P. Chamolia and K. K. Kar, RSC Adv., 2015, 5, 73249 RSC.
- Y. L. Pang, A. Z. Abdullah and S. Bhatia, Desalination, 2011, 277, 1 CrossRef CAS.
- M. Pera-Titus, V. Garcia-Molina, M. A. Banos, J. Gimenez and S. Esplugas, Appl. Catal., B, 2004, 47(4), 219 CrossRef CAS.
- A. Hiskia, A. Mylonas and E. Papaconstantinou, Chem. Soc. Rev., 2001, 30, 62 RSC.
- Y. Guo and C. Hu, J. Mol. Catal. A: Chem., 2007, 262, 136 CrossRef CAS.
- A. Dolbecq, P. Mialane, B. Keitab and L. Nadjo, J. Mater. Chem., 2012, 22, 24509 RSC.
- B. Keita and L. Nadjo, J. Mol. Catal. A: Chem., 2007, 262, 190 CrossRef CAS.
- H. Hamadi, M. Kooti, M. Afshari, Z. Ghiasifar and N. Adibpour, J. Mol. Catal. A: Chem., 2013, 373, 25 CrossRef CAS.
- R. Fazaeli, H. Aliyan, S. P. Foroushani and Z. Mohagheghian, Turk. J. Chem., 2014, 38, 372 CrossRef CAS.
- E. Rafiee and M. Khodayari, J. Mol. Catal. A: Chem., 2014, 398, 336 CrossRef.
- S. Tangestaninejad, M. Moghadam, V. Mirkhani, I. Mohammadpoor-Baltork and H. Salavati, Ultrason. Sonochem., 2008, 15, 815 CrossRef CAS PubMed.
- H. Pang, Y. Niu, J. Yu, H. Ma, Q. Song and S. Li, Inorg. Chem. Commun., 2015, 59, 5 CrossRef CAS.
- R. Sivakumara, J. Thomasa and M. Yoon, J. Photochem. Photobiol., C, 2012, 13, 277 CrossRef.
- E. Selli, Phys. Chem. Chem. Phys., 2002, 4, 6123 RSC.
- J. Wang, Y. Guo, B. Liu, X. Jin, L. Liu, R. Xu, Y. Kong and B. Wang, Ultrason. Sonochem., 2011, 18, 177 CrossRef CAS PubMed.
- C.-C. Wang, J.-R. Li, X.-L. Lv, Y.-Q. Zhang and G. Guo, Energy Environ. Sci., 2014, 7, 2831 CAS.
- A. Kubacka, M. Fernandez-Garcia and G. Colon, Chem. Rev., 2012, 112, 1555 CrossRef CAS PubMed.
- M. Humayun, Z. Li, L. Sun, X. Zhang, F. Raziq, A. Zada, Y. Qu and L. Jing, Nanomaterials, 2016, 6, 22 CrossRef.
- J. Yang, R. Hu, W. Meng and Y. Du, Chem. Commun., 2016, 52, 2621–2623 Search PubMed.
- D. H. Bremner, R. Molina, F. Martınez, J. A. Melero and Y. Segura, Appl. Catal., B, 2009, 90, 380 CrossRef CAS.
- S. Farhadi and F. Siadatnasab, J. Mol. Catal. A: Chem., 2011, 339, 108 CrossRef CAS.
- Y. Matuura, S. Matsushima, M. Sakamoto and Y. Sadaoka, J. Mater. Chem., 1993, 3(7), 768 RSC.
- N. Kondoa, H. Itoh, M. Kurihara, M. Sakamoto and Y. Sadaoka, J. Alloys Compd., 2006, 408–412, 1027 Search PubMed.
- O. P. Bajpai, J. B. Kamdi, M. Selvakumar, S. Ram, D. Khastgir and S. Chattopadhyay, eXPRESS Polym.
Lett., 2014, 8(9), 671 Search PubMed.
- J. Xu, C. Ju, J. Sheng, F. Wang, Q. Zhang, G. Sun and M. Sun, Bull. Korean Chem. Soc., 2013, 34(8), 2409 Search PubMed.
- P.-H. Li, B.-L. Li, Z.-M. An, L.-P. Mo, Z.-S. Cui and Z.-H. Zhang, Adv. Synth. Catal., 2013, 355, 2952 CrossRef CAS.
- S. Farhadi and M. Zaidi, Appl. Catal., A, 2009, 354, 119 CrossRef CAS.
- Z. Zhang, F. Zhang, Q. Zhu, W. Zhao, B. Ma and Y. Ding, J. Colloid Interface Sci., 2011, 360, 191 CrossRef PubMed.
- H. Hamadi, M. Kooti, M. Afshari, Z. Ghiasifar and N. Adibpour, J. Mol. Catal. A: Chem., 2013, 327, 27 Search PubMed.
- M. Masteri-Farahani, J. Movassagh, F. Taghavi, P. Eghbali and F. Salimi, Chem. Eng. J., 2012, 184, 342 CrossRef CAS.
- M. Riahi Farsani and B. Yadollahi, J. Mol. Catal. A: Chem., 2014, 392, 8 CrossRef.
- E. Rafiee and S. Eavani, J. Mol. Catal. A: Chem., 2013, 373, 30 CrossRef CAS.
- A. Mahrenia, A. B. Mohamad, A. A. H. Kadhum, W. R. W. Daud and S. E. Iyuke, J. Membr. Sci., 2009, 327, 38 Search PubMed.
- C. Zhana, M. Zhong, F. Chen, J. Yang, X. Cao and X. Jianga, Desalin. Water Treat., 2015, 53, 2972 Search PubMed.
- R. Tayebee, M. M. Amini, F. Nehzat, O. Sadeghi and M. Armaghan, J. Mol. Catal. A: Chem., 2013, 366, 143 Search PubMed.
- Y. H. Guo, C. W. Hu, C. J. Jiang, Y. Yang, S. C. Jiang, X. L. Li and E. B. Wang, J. Catal., 2003, 217, 141 CAS.
- H. Salavati, N. Tavakkoli and M. Hosseinpoor, Ultrason. Sonochem., 2012, 19, 546 CrossRef CAS PubMed.
- S. Thirumalairajan, K. Girija, V. Roberto Mastelaroa and N. Ponpandianb, New J. Chem., 2014, 38, 5489 RSC.
- W. Wang and S. Yang, J. Water Resour. Prot., 2010, 2, 981 Search PubMed.
- E. Rafiee, S. Eavani, S. Rashidzadeh and M. Joshaghani, Inorg. Chim. Acta, 2009, 362, 3558 CrossRef.
- E. Zhang, Y. Tang, K. Peng, C. Guo and Y. Zhang, Solid State Commun., 2008, 148, 498 Search PubMed.
- M. F. Shao, J. B. Han, M. Wei, D. G. Evans and X. Duan, Chem. Eng. J., 2011, 168, 520 CrossRef.
- K. S. W. Sung, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57(4), 612 Search PubMed.
- T. Harifi and M. Montazer, Sep. Purif. Technol., 2014, 134, 216 CrossRef.
- X. Qu, Y. Guo and C. Hu, J. Mol. Catal. A: Chem., 2007, 262, 128 CrossRef CAS.
- Z. Jin, L. Chuang, W. Bing, C. Hao, Z. J. Ping and L. Qin, Sci. China: Chem., 2013, 56(9), 1289 Search PubMed.
- Q. Zhai, L. Zhang, X. Zhao, H. Chen, D. Yin and J. Li, Appl. Surf. Sci., 2016, 377, 19 CrossRef.
- L. Fb, L. Xz, H. Mf, C. Kw and C. Wc, Appl. Catal., A, 2005, 285, 184 Search PubMed.
- J. Xu, C. Ju, J. Sheng, F. Wang, Q. Zhang, G. Sun and M. Sun, Bull. Korean Chem. Soc., 2013, 34(8), 2408–2412 CrossRef CAS.
- Z. Zhang, F. Zhang, Q. Zhu, W. Zhao, B. Ma and Y. Ding, J. Colloid Interface Sci., 2011, 360, 189–194 CrossRef CAS PubMed.
- C.-C. Wang, J.-R. Li, X.-L. Lv, Y.-Q. Zhang and G. Guo, Energy Environ. Sci., 2014, 7, 2831 CAS.
- P. P. Qian, J. L. Xue, G. X. Pan, Y. Li and Z. M. Ni, Chin. J. Inorg. Chem., 2012, 28, 1350 Search PubMed.
- S. Xia, L. Zhang, G. Pan, P. Qian and Z. Ni, Phys. Chem. Chem. Phys., 2015, 17, 5348 Search PubMed.
- R. Hu, C. Li, X. Wang, Y. Sun, H. Jia, H. Su and Y. Zhang, Catal. Commun., 2012, 29, 35 CrossRef CAS.
- H. Aono, E. Traversa, M. Sakamoto and Y. Sadaoka, Sens. Actuators, B, 2003, 94, 132 CrossRef CAS.
- W. Ramadan, P. A. Shaikh, S. Ebrahim, A. Ramadan, B. Hannoyer, S. Jouen, X. Sauvage and S. Ogale, J. Nanopart. Res., 2013, 15, 8 CrossRef.
- J. He, H. Sun, S. Indrawirawan, X. Duan, M. O. Tade and S. Wang, J. Colloid Interface Sci., 2015, 456, 15–21 CrossRef CAS PubMed.
- R. Kannan, S. G. Peera, A. Obadiah and S. Vasanthkumar, Digest Journal of Nanomaterials and Biostructures, 2011, 6(2), 829–835 Search PubMed.
- Y. Yang, Q. Wu, Y. Guo, C. Hu and E. Wang, J. Mol. Catal. A: Chem., 2005, 225, 203–212 CrossRef CAS.
- X. Qu, Y. Guo and C. Hu, J. Mol. Catal. A: Chem., 2007, 262, 128–135 CrossRef CAS.
- W. Zhou, M. Cao, S. Su, N. Li, X. Zhao, J. Wang, X. Li and C. Hu, J. Mol. Catal. A: Chem., 2013, 371, 70–76 CrossRef CAS.
- W. Zhou, N. Li, M. Cao and C. Hu, Mater. Lett., 2013, 99, 68–71 CrossRef CAS.
- X. Guo, N. Chen, C. Feng, Y. Yang, B. Zhang, G. Wang and Z. Zhang, Catal. Commun., 2013, 38, 26–30 CrossRef CAS.
- L. Gan, L. Xu and K. Qian, Mater. Des., 2016, 109, 354–360 CrossRef CAS.
- S. Vadivel, D. Maruthamani, A. Habibi-Yangjeh, B. Paul, S. Sankar Dhar and K. Selvam, J. Colloid Interface Sci., 2016, 480, 126–136 CrossRef CAS PubMed.
- W. Ramadan, P. A. Shaikh, S. Ebrahim, A. Ramadan, B. Hannoyer, S. Jouen, X. Sauvage and S. Ogale, J. Nanopart. Res., 2013, 15, 1848 CrossRef.
- T. Soltani and M. H. Entezari, J. Mol. Catal. A: Chem., 2013, 377, 197–203 CrossRef CAS.
- M. Azarang, A. Shuhaimi, R. Yousefi, A. Moradi Golsheikh and M. Sookhakian, Ceram. Int., 2014, 40, 10217–10221 CrossRef CAS.
- S. Kandula and P. Jeevanandam, J. Nanopart. Res., 2014, 16, 2452 CrossRef.
- A. Khataee, A. Karimi, S. Arefi-Oskoui, R. D. Cheshmeh Soltani, Y. Hanifehpour, B. Soltani and S. W. Joo, Ultrason. Sonochem., 2014, 22, 377 Search PubMed.
- A. Khataee, R. Darvishi Cheshmeh Soltani, A. Karimi and S. Woo Joo, Ultrason. Sonochem., 2015, 23, 226 CrossRef PubMed.
- Z. Frontistis and D. Mantzavinos, Ultrason. Sonochem., 2012, 19, 82 CrossRef PubMed.
- B. Gozmen, Environ. Prog. Sustainable Energy, 2012, 31, 302 Search PubMed.
- F. Banat, S. A. Asheh, M. Al-Rawashdeh and M. Nusair, Desalination, 2005, 181, 228 CrossRef.
- G. M. Madhu, M. A. Lourdu Antony Raj, K. V. K. pai and S. Rao, Indian J. Chem. Technol., 2007, 14, 141 Search PubMed.
- J. Jiang, J. Zou, L. Zhu, L. Huang, H. Jiang and Y. Zhang, J. Nanosci. Nanotechnol., 2011, 11, 4797 Search PubMed.
- L. G. Devi, S. Girish Kumar, K. Mohan Reddy and C. Munikrishnappa, J. Hazard. Mater., 2009, 164, 463 Search PubMed.
- S. Haji, B. Benstaali and N. Al-Bastaki, Chem. Eng. J., 2011, 168, 137 CrossRef.
- D. Zhang, X. Li, H. Tan, G. Zhang, Z. Zhao, H. Shi, L. Zhang, W. Yub and Z. Sun, RSC Adv., 2014, 4, 44322 RSC.
- J. J. Guo, S. X. Ouyang, H. Zhou, T. Kako and J. H. Ye, J. Phys. Chem. C, 2013, 117, 17716 CAS.
- T. T. Wang, J. Y. Lang, Y. J. Zhao, Y. G. Su, Y. X. Zhao and X. J. Wang, CrystEngComm, 2015, 17, 6651 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra24627c |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching bands at about 2150, 2060 cm−1.21 Also, the bands at 3500 and 1640 cm−1 are related to the stretching and bending vibrations of the lattice water molecules, respectively. In the spectrum of LaFeO3 nanoparticles, the strong bands at about 560 and 440 cm−1 are assigned to the Fe–O stretching and bending vibrations of the octahedral FeO6 groups in the perovskite-type structure, respectively.21,27 The presence of SiO2 in the composition of hybrid compound, was confirmed by bands at 1087 and 964 cm−1 assigned to the SiO–H and Si–O–Si groups.9,12,26,32 The two broad bands at 3429 and 1636 cm−1 can be ascribed to the stretching and bending modes of NH2 groups, respectively. The presence of the anchored propyl groups of APTES, was confirmed by C–H stretching vibrations at 2910 and 2980 cm−1 (Fig. 2(d)).9,25 It is well known that Keggin-type polyoxometalate, PTA, contains a cage of tungsten atoms linked by oxygen atoms with tetrahedral phosphate group. Oxygen atoms form four physically distinct bonds (P–Oa, W–Ot, W–Ob–W, and W–Oc–W bonds), which have distinct infrared signatures: 1080 cm−1 for asymmetric stretch vibration of P–Oa (Oa corresponds to oxygen atom of tetrahedral phosphate group), 987 cm−1 for asymmetric stretch vibration of W
N stretching bands at about 2150, 2060 cm−1.21 Also, the bands at 3500 and 1640 cm−1 are related to the stretching and bending vibrations of the lattice water molecules, respectively. In the spectrum of LaFeO3 nanoparticles, the strong bands at about 560 and 440 cm−1 are assigned to the Fe–O stretching and bending vibrations of the octahedral FeO6 groups in the perovskite-type structure, respectively.21,27 The presence of SiO2 in the composition of hybrid compound, was confirmed by bands at 1087 and 964 cm−1 assigned to the SiO–H and Si–O–Si groups.9,12,26,32 The two broad bands at 3429 and 1636 cm−1 can be ascribed to the stretching and bending modes of NH2 groups, respectively. The presence of the anchored propyl groups of APTES, was confirmed by C–H stretching vibrations at 2910 and 2980 cm−1 (Fig. 2(d)).9,25 It is well known that Keggin-type polyoxometalate, PTA, contains a cage of tungsten atoms linked by oxygen atoms with tetrahedral phosphate group. Oxygen atoms form four physically distinct bonds (P–Oa, W–Ot, W–Ob–W, and W–Oc–W bonds), which have distinct infrared signatures: 1080 cm−1 for asymmetric stretch vibration of P–Oa (Oa corresponds to oxygen atom of tetrahedral phosphate group), 987 cm−1 for asymmetric stretch vibration of W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ot (Ot corresponds to the terminal oxygen atoms), 890 cm−1 for bending vibration of W–Ob–W (Ob corresponds to oxygen atom bridging the two tungsten atoms), and 804 cm−1 for bending vibration of W–Oc–W (Oc represents oxygen atom at the corners of the Keggin structure) (Fig. 2(e)). As can see in Fig. 2(f), appearance of peaks at 1070, 985, 885 and 800 cm−1, confirm the presence of PTA in the structure of nanohybrid compound.9,10,13,27–35 The IR characteristic bands of PTA in the nanohybrid compound, have some red shifts compared with those for the pure PTA.27,30,36,37 It confirms that a strong chemical interaction, not simple physical absorption,27,30,36,37 exists between the PTA polyanions and the LaFeO3@SiO2–NH2 surface.
Ot (Ot corresponds to the terminal oxygen atoms), 890 cm−1 for bending vibration of W–Ob–W (Ob corresponds to oxygen atom bridging the two tungsten atoms), and 804 cm−1 for bending vibration of W–Oc–W (Oc represents oxygen atom at the corners of the Keggin structure) (Fig. 2(e)). As can see in Fig. 2(f), appearance of peaks at 1070, 985, 885 and 800 cm−1, confirm the presence of PTA in the structure of nanohybrid compound.9,10,13,27–35 The IR characteristic bands of PTA in the nanohybrid compound, have some red shifts compared with those for the pure PTA.27,30,36,37 It confirms that a strong chemical interaction, not simple physical absorption,27,30,36,37 exists between the PTA polyanions and the LaFeO3@SiO2–NH2 surface.



![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and W–O–W bonds, respectively.27,36,37,45 The band gap energy (Eg) of pure LaFeO3 is determined to be 2.09 eV by the equation Eg = 1240/λg.18,19,37 For hybrid compound, in comparison to pure PTA, the optical absorption is enhanced significantly in the region of 300–550 nm (in visible light region) for pure LaFeO3 and nanohybrid compound samples. By loading the PTA on the surface of LaFeO3, the ability of light absorption was enhanced for LaFeO3 and nanohybrid compound samples. More interestingly, by loading the PTA, the charge transformation bands have a redshift and shift to higher wavelength, it confirms the presence of strong chemical interactions, between the polyanion and the LaFeO3 NPs surface.36 Compared with pure PTA and LaFeO3, the absorption threshold onset of nanohybrid compound extended to visible region which corresponded to a narrow band gap energy (1.9 eV) for the nanohybrid compound sample. It reveals that the band gap energy of nanohybrid compound is to some extent lower than the bare LaFeO3 with band gap energy of 2.09 eV.19 The observed weak red shift over the nanohybrid compound, is caused by the charge-transfer transition between the PTA and the LaFeO3 conduction or valance band.36,46–48 These data confirmed that the electronic structures of LaFeO3 were slightly changed due to the introduction of Keggin-type PTA and the outstanding visible light photocatalytic activity of the nanohybrid compound, in comparison of bare PTA is attributed to the existence of the broad band in the visible region.
O and W–O–W bonds, respectively.27,36,37,45 The band gap energy (Eg) of pure LaFeO3 is determined to be 2.09 eV by the equation Eg = 1240/λg.18,19,37 For hybrid compound, in comparison to pure PTA, the optical absorption is enhanced significantly in the region of 300–550 nm (in visible light region) for pure LaFeO3 and nanohybrid compound samples. By loading the PTA on the surface of LaFeO3, the ability of light absorption was enhanced for LaFeO3 and nanohybrid compound samples. More interestingly, by loading the PTA, the charge transformation bands have a redshift and shift to higher wavelength, it confirms the presence of strong chemical interactions, between the polyanion and the LaFeO3 NPs surface.36 Compared with pure PTA and LaFeO3, the absorption threshold onset of nanohybrid compound extended to visible region which corresponded to a narrow band gap energy (1.9 eV) for the nanohybrid compound sample. It reveals that the band gap energy of nanohybrid compound is to some extent lower than the bare LaFeO3 with band gap energy of 2.09 eV.19 The observed weak red shift over the nanohybrid compound, is caused by the charge-transfer transition between the PTA and the LaFeO3 conduction or valance band.36,46–48 These data confirmed that the electronic structures of LaFeO3 were slightly changed due to the introduction of Keggin-type PTA and the outstanding visible light photocatalytic activity of the nanohybrid compound, in comparison of bare PTA is attributed to the existence of the broad band in the visible region.












