Synthesis and characterization of novel solid–solid phase change materials with a polyurethaneurea copolymer structure for thermal energy storage
Abstract
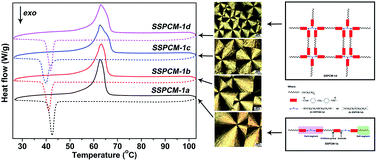
A series of novel polymeric solid–solid phase change materials (SSPCMs) with polyurethaneurea (PUU) copolymer structure were synthesized by the crosslinking copolymerization of polyethylene glycol (PEG) and multiamino compounds (diethylenetriamine, triethylenetetramine, tetraethylenepentamine) with different molar ratios. The results from the polarization optical microscopy (POM) and WAXD patterns indicate that the SSPCMs have typical spherocrystal morphology and characteristic diffraction peaks like neat PEG, but their spherulite size and diffraction peak intensity are smaller than those of PEG. According to the thermal analyses, the SSPCMs possess high phase transition enthalpies, good reusability and high thermal stability for their potential applications in thermal energy storage. With the decrease of crosslinking density of SSPCMs, the crystalline properties and thermal properties of SSPCMs have improved apparently, which is closely related to the free movement of PEG segments in the SSPCMs.


 Please wait while we load your content...
Please wait while we load your content...