DOI:
10.1039/C6RA24614A
(Paper)
RSC Adv., 2016,
6, 114244-114255
Gas phase dehydration of glycerol catalyzed by gamma Al2O3 supported V2O5: a statistical approach for simultaneous optimization
Received
2nd October 2016
, Accepted 2nd December 2016
First published on 2nd December 2016
Abstract
Selective gas-phase dehydration of glycerol to acrolein over V2O5/γ-Al2O3 catalysts was investigated. The catalysts with various V2O5 loading were facilely synthesized by an impregnation method. The prepared catalysts were characterized by several techniques such as scanning electron microscopy (SEM), temperature-programmed desorption of NH3 (TPD-NH3), temperature programmed reduction (H2-TPR), Fourier transform infrared spectroscopy (FT-IR), Brunauer–Emmett–Teller (BET) and X-ray diffraction (XRD). The effects of V2O5 loading (5–25 wt%), gas hourly space velocity (GHSV) of O2/N2 (120–540 h−1) and reaction temperature (250–320 °C) on yield of acrolein were studied by Box–Behnken design. The influence of independent factors and their quadratic interactions were examined by means of the analysis of variance (ANOVA). The present results indicate that temperature has a different effect on the conversion and selectivity of acrolein. The catalyst with 14.80 wt% of V2O5 loading, GHSV of 268.60 h−1 and reaction temperature of 286.35 °C produced the maximum yield of acrolein (73.05%). The analysis revealed that the predicted results agree well with experimental data (12.5 wt% loading of V2O5 on γ-Al2O3, GHSV of the carrier gas = 330 h−1 and reaction temperature of 285 °C with 68.31% yield).
1. Introduction
Nowadays, the investigation of biomass-derivable feedstocks as starting materials for the production of chemical materials is an attractive research topic.1,2 Because of its continuous availability in large amounts from the biodiesel production process, glycerol has gained too much attention.3 Depletion of fossil fuels and the impacts of global warming have also been identified as critical problems, and the utilization of biomass-derivable feedstocks such as catalytic dehydration of glycerol to acrolein can be an efficient way to overcome this challenge. Therefore, it is essential to obtain cost-effective ways of utilizing glycerol that will be beneficial for both biodiesel manufacturing and the environment. Acrolein is currently produced by oxidation of propylene over Bi–Mo catalysts.4 It is usually converted to acrylic acid, which is widely used in adhesives, plastics, fibers, polymer dispersions and other chemical intermediates. The most significant direct application of acrolein is as a herbicide to control the growth of aquatic plants.3 Many studies have been reported on the dehydration of glycerol and confirmed that the selectivity to acrolein is related to the presence of Brønsted acid sites.1,5 Therefore it is crucial to understand and find the best combination of parameters such as: reaction temperature, gas hourly space velocity and catalyst textural for improving the selectivity of acrolein in this reaction. Researchers have applied various experimental and statistical techniques to optimize and improve the process parameters. Using a one factor at a time optimization method is an intricate approach to evaluate the effects of different variables on an experimental outcome. The application of modeling tools such as response surface methodology (RSM) can extremely reduce the need for a large number of laboratory tests and associated costs. The catalytic developments in the reaction dehydration of glycerol to acrolein has widely been investigated in the gas phase over solid acid catalysts such as heteropoly acids6,7 metal oxides,8,9 supported zeolites.10,11 In general, dehydration of glycerol to acrolein in the gas phase has higher yield than in the liquid phase even when using the same type of solid catalyst.12 Talebian et al. reported gas-phase glycerol dehydration to acrolein over supported silicotungstic acid catalyst and used central composite design (CCD) to evaluate the interactions on reaction conversion.2 Dubois et al. used WO3–ZrO2 catalysts and obtained high selectivity of acrolein (60%) for dehydration of glycerol.13 Regardless of the reaction phase, the strength and nature of the acid sites is a critical factor in selective production of acrolein from glycerol, as well as the influence of the morphology and textural properties of the solids.1,14 Wang et al. reported dehydration of glycerol over VPO oxides catalysts because of their mild acid–basic properties and added molecular oxygen into reaction in order to reduce side reactions and avoid coke formation.15 Finally, they concluded that vanadyl hydrogen phosphate hemihydrate VOHPO4·0.5H2O has the best performance with 66% selectivity for acrolein at 100% conversion of glycerol.16 Kamiya et al. reported that the total amount of Brønsted and Lewis acid sites of VPO catalyst with different preparation methods is an important parameter in the yield of acrolein.17 Cecilia et al. prepared a series of catalysts, based on vanadium and vanadium-phosphorous containing Zr-SBA-15 and concluded that dehydration reaction of glycerol to acrolein could take place inside the meso channels for vanadium based catalysts.18 Wang et al. reported a new route for selective and efficient glycerol dehydration to acrolein and found that Brønsted and Lewis acid sites significantly improved the yield of acrolein.5 Alhanash et al. suggested two reaction pathways based on the nature of acid sites and reported that dehydration of primary hydroxyl groups of glycerol at Lewis acid sites enhance the acetol production but dehydration of the internal secondary and terminal primary hydroxyl groups both occurring at Brønsted acid sites and greatly enhanced the production of acrolein.19
Chieregato et al. synthesized bicomponent W–Mo, tricomponent W–Mo–V oxides, VO–WOx and V–Co–AlPO4 as a new class of catalysts for the one-pot conversion of glycerol to acrylic acid and obtained high yields of acrylic acid (up to 51%).20,21 Souza et al. synthesized NbV, NbMo and pure Nb catalysts and used Box–Behnken design (BBD) to evaluate the metal doping, solvent and effect of temperature in the oxidation of glycerol to cyclic ethers.22 Arkema company patented a way to reduce side products through addition of oxygen during the dehydration reaction and obstruct catalyst deactivation base on acidic materials such as ZrO2, SO4, WO3 and Al2O3.23 Omata et al. synthesized W–Nb–O catalysts by hydrothermal method and achieved high yield of acrolein in gas phase glycerol dehydration in the presence of these catalysts, water and oxygen.24 But there have been a few reports on the favorable outcome optimization of RSM in glycerol conversion processes and dehydration of glycerol to acrolein. These researches had different problems such as large amounts of by-products, severe coke formation, low thermal stability and harsh reaction conditions. These problems must be solved in order to gain economical and continuous industrial production.
In this work, for the first time, a novel V2O5/Al2O3 catalyst with different loading of V2O5 was prepared by impregnation method. Since V2O5 have low surface areas so γ-Al2O3 nanoparticle was added as the second component to the catalyst in order to increase the surface area. This causes good dispersion of vanadium particles on the support surface without aggregation and also can affect the relative strength of acid sites. The influence of the preparation condition, such as loading of V2O5 supported on γ-Al2O3 (B) and operation conditions such as reaction temperature (A) and gas hourly space velocity (GHSV) (C) on the activity of the catalyst for acrolein yield were investigated. The optimization of the dehydration reaction was carried out using response surface methodology (RSM). The relationships between the glycerol conversion and reaction parameters (factors) were evaluated to determine the optimum conditions for acrolein production. Scanning electron microscopy (SEM), Fourier transform infrared (FTIR) spectra of adsorbed pyridine, Brunauer–Emmett–Teller (BET), powder X-ray diffraction (XRD), temperature-programmed desorption of NH3 (TPD-NH3) and H2-TPR (temperature programmed reduction) were employed to characterize the texture and structure of the catalysts.
2. Experimental
2.1. Materials
All materials were of commercial reagent grade. V2O5 (99.6% Alfa Aesar), oxalic acid (99% Aldrich), glycerol (purity > 99%), aluminum nitrate (Al(NO3)3·9H2O, 99% Merck), sodium carbonate (Na2CO3, 99.5% Merck) and deionized water were used as received without further purification. Gases employed were N2 (99.9999%, Air Liquid) and dry air (99.99%, 20 wt% O2/N2).
2.2. Catalyst preparation
2.2.1. Preparation of nano γ-Al2O3 powder. Nano-sized porous gamma alumina was synthesized according to a literature procedure.25 Initially, 200 mL water was heated to 65 °C in a 2 liter round bottomed double capped flask. Aluminum nitrate (0.05 M) and sodium carbonate (0.075 M) solutions were prepared by dissolving 18.75 g and 7.96 g in 250 mL of deionized water respectively and then added (drop by drop from two separate burettes) to 200 mL deionized water. The gel was kept under vigorous stirring at 65 °C for 8 h to precipitate Al cations in the form of hydroxides and then aged for an additional 1 h. After vacuum filtration, the solid was washed with distilled water and dried at room temperature for 1 day then was calcined in a programmable muffle furnace at 600 °C for 5 h in air with heating rate of 2 °C min−1 to produce γ-Al2O3 powders. The surface area of prepared γ-Al2O3 was 230 m2 g−1.
2.2.2. Preparation of the nano V2O5 and nano V2O5/γ-Al2O3. In the first, V2O5 (9.09 g, 0.5 mol) and H2C2O4 (13.50 g, 1.5 mol) in a stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 were dissolved to 100 mL distilled water under active stirring (800 RPM) at room temperature until the color of the solution changed from yellow to blue. The resulting solution was introduced into a 200 cm3 Teflon-lined stainless steel autoclave and submitted to a hydrothermal treatment at 250 °C for 24 h. The solid was separated by filtration, dried at 80 °C and then calcined in the furnace at 400 °C in air for 2 h (ref. 26) and finally labeled as nano V2O5. The V2O5/γ-Al2O3 catalysts containing V2O5 with 5, 10, 15, and 20 wt% were prepared by impregnation method as described in (ref. 27). In a typical synthesis, 1.0 g of calcined and out gassed γ-Al2O3 was impregnated with certain concentration of vanadyl oxalate solution. Above solution was prepared by mixing vanadium pentoxide with desire amounts of oxalic acid. The wet solid was then dried at 120 °C over night under vacuum and calcined in air under static conditions at 600 °C for 5 h. Finally, the obtained samples were crushed and sieved to obtain catalyst particle size of 0.077–0.300 mm.
3 were dissolved to 100 mL distilled water under active stirring (800 RPM) at room temperature until the color of the solution changed from yellow to blue. The resulting solution was introduced into a 200 cm3 Teflon-lined stainless steel autoclave and submitted to a hydrothermal treatment at 250 °C for 24 h. The solid was separated by filtration, dried at 80 °C and then calcined in the furnace at 400 °C in air for 2 h (ref. 26) and finally labeled as nano V2O5. The V2O5/γ-Al2O3 catalysts containing V2O5 with 5, 10, 15, and 20 wt% were prepared by impregnation method as described in (ref. 27). In a typical synthesis, 1.0 g of calcined and out gassed γ-Al2O3 was impregnated with certain concentration of vanadyl oxalate solution. Above solution was prepared by mixing vanadium pentoxide with desire amounts of oxalic acid. The wet solid was then dried at 120 °C over night under vacuum and calcined in air under static conditions at 600 °C for 5 h. Finally, the obtained samples were crushed and sieved to obtain catalyst particle size of 0.077–0.300 mm.
2.3. Catalyst characterization
The structure of these samples was studied by X-ray diffraction (XRD) experiments. A Philips model PW 1800 diffractometer with Cu Kα (λ = 0.15406 nm) radiation and Ni filter operating at 40 kV and 10 mA in the 2θ range of 10–80° at a scanning rate of 4° min−1 was used to collect the X-ray data. Scanning electron microscopy (SEM) images were obtained with a Philips XL30 instrument. The specific surface areas were measured at −196 °C using the N2 adsorption/desorption by the Brunauer–Emmett–Teller (BET) method on a Micromeritics ASAP 2010 instrument. The pore size and average pore volume were calculated using the Barrett–Joyner–Halenda (BJH) method. The samples were degassed at 200 °C for 8 h to remove impurities and physisorbed water prior to surface area measurements. Fourier transform infrared (FTIR) spectra of adsorbed pyridine were recorded on a Shimadzu 8300 spectrometer. The samples (10 mg) were activated at 400 °C under a vacuum of 1.33 × 10−3 Pa for 3 h, followed by adsorption of purified pyridine vapor at room temperature for 30 min. After this step, the samples were evacuated at 100 °C and the pyridine – IR spectra were recorded. NH3-TPD (ammonia temperature-programmed desorption) and H2-TPR (temperature programmed reduction) spectra were recorded on a Micromeritics Autochem 2920 chemisorption analyzer. The samples were pretreated by passage of high purity helium (50 mL min−1) at 220 °C for 1 h, then saturated with high purity anhydrous ammonia at 100 °C for 30–40 min and subsequently flushed at the same temperature for 30 min to remove physisorbed NH3. TPD analysis was carried out from 50 to 600 °C at a heating rate of 10 °C min−1. The amount of NH3 consumed was monitored by using a thermal conductivity detector (TCD). TPR analysis was carried out from 50 to 850 °C with gaseous mixture of 10% H2/Ar at a heating rate of 10 °C min−1 and total gases flow rate of 50 mL min−1. The H2 consumption was monitored by a calibrated thermal conductivity detector (TCD). Thermogravimetric-differential thermal analysis (TG-DTA) of the used catalyst (after 3 and 9 h) were performed with a Shimadzu TGA-51 thermogravimetric analyzer. The samples were heated from room temperature to 800 °C, at a heating rate of 10 °C min−1, under a 100 mL min−1 flow of dry air.
2.4. Experimental design and catalytic reaction
In this study, the Box–Behnken design of experiment was employed to find the optimal conditions for gas phase dehydration of glycerol (GLY) and acrolein (ACR) production. The effects of temperature (A), V2O5 loading on the support (B) and GHSV (C) were investigated. The conversion of glycerol aqueous solution (GLY Conv%), acrolein selectivity (ACR Sele%) and yield (%) were calculated in the final reaction mixture according to the following equations:| | |
Glycerol conversion (%) = (moles of glycerol reacted/moles of glycerol in the feed) × 100
| (1) |
| | |
Product selectivity (%) = (moles of carbon in a product defined/mole of carbon in glycerol reacted) × 100
| (2) |
| | |
Product yield (%) = (conversion of glycerol × selectivity of acrolein)/100
| (3) |
Glycerol dehydration reactions were conducted at atmospheric pressure in a vertical stainless steel reactor of 50 cm length and 0.9 cm internal diameter. The experimental set-up is shown schematically in Fig. 1. A mixture of 5.0 g catalyst and 3.0 g corundum (50–70 mesh) was loaded in the middle section of the reactor, with quartz wool packed in both ends and was pretreated at 300 °C in GHSV of 120 mL h−1 (dry air 20% O2/N2 was used as carrier gas) for 1 h before the reaction. The dry air was controlled by a Brooks mass flow controller. The reaction temperature was monitored by three thermocouples (TC 1–3) inserted into the middle of the catalyst. Aqueous glycerol (30%, w/w) was fed at a speed of 1 mL h−1 by a HPLC pump. The composition of fed gas was N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2
O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol, equal to 13.5
glycerol, equal to 13.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in molar ratio. After 3 h, reaction products were collected in an ice-water cold trap and n-butanol was added as internal standard. Products were quickly analyzed by Varian Star 3400 gas chromatograph equipped with a 10 wt% FFAP/chromosorb W-AW column (2 mm i.d., 2.5 m long) and FID detector. The main products identified by GC analysis were acrolein, acetol, allyl alcohol and acetaldehyde. The mass balance was determined and an acceptable carbon balance (>85%) was achieved for each trap. Besides, some chromatographic signals in lower quantities have not been identified. Glycerol conversion, selectivity and yield were calculated by eqn (1)–(3) respectively.
1 in molar ratio. After 3 h, reaction products were collected in an ice-water cold trap and n-butanol was added as internal standard. Products were quickly analyzed by Varian Star 3400 gas chromatograph equipped with a 10 wt% FFAP/chromosorb W-AW column (2 mm i.d., 2.5 m long) and FID detector. The main products identified by GC analysis were acrolein, acetol, allyl alcohol and acetaldehyde. The mass balance was determined and an acceptable carbon balance (>85%) was achieved for each trap. Besides, some chromatographic signals in lower quantities have not been identified. Glycerol conversion, selectivity and yield were calculated by eqn (1)–(3) respectively.
 |
| | Fig. 1 Continuous fixed-bed reactor applied for dehydration. | |
2.5. Response surface exploration
Several preliminary tests have been performed to evaluate the effect of parameters on glycerol conversion and acrolein selectivity over V2O5/γ-Al2O3 catalysts. Reaction temperature (A), V2O5 loading on support (B) and gas hourly speed velocity (GHSV) (C) were regarded as most significant factors. In order to investigate the optimum levels of those variables and to study their relation, Box–Behnken experimental design was found to be more suitable one. One of the advantages of Box–Behnken design (BBD) is that they are all spherical design, the sample combinations are processed such that they are located at mid points of edges constituted by any two factors and require factors to be run at only of three levels that is represented by [−1,0,1].28 The three examined levels and experimental ranges of each independent variable are given in Table 1. The relationship between the variables and the response was calculated by the second order polynomial equation. The form of the full quadratic model is shown in eqn (4) as follows:| | |
Y = β0 + ∑βixi + ∑βiixi2 + ∑βijxixj
| (4) |
where Y is the predicted response or output (dependent variable), xi and xij are the coded independent variables, β0 is the intercept term, βi the linear effect (first order), βii the squared effect, and βij is the interaction effect, i and j are the index numbers for variables.
Table 1 Experimental range and levels for Box–Behnken design
| Independent variables |
Range and level |
| −1 |
0 |
1 |
| Temperature (°C), A |
250 |
285 |
320 |
| Loading V2O5 (wt%), B |
5 |
12.5 |
20 |
| GHSV (h−1), C |
120 |
330 |
540 |
Experimental design and data analysis were performed by using the Design of Experiment software (trial version 10).
3. Results and discussion
3.1. Catalysts characterization
The XRD patterns of the support (γ-Al2O3), V2O5 and V2O5/γ-Al2O3 with different loading of 5 wt%, 10 wt%, 15 wt% and 20 wt% after calcinations are shown in Fig. 2. The γ-Al2O3 characteristic peaks appeared at 45.8, 67 and 37.6° (JCPDS 10-425) (Fig. 2a). For reference, the diffractogram of pure V2O5 is also showed. The peaks located at 2θ = 20.52°, 21.96°, 26.39° and 31.30° can be assigned to the (010), (110), (101) and (400) planes of V2O5, respectively (Fig. 2b). All of the diffraction peaks of V2O5 are not distinct when its loading is lower than 15 wt% but for higher than 15 wt%, the diffraction peaks appear (Fig. 2e) and their intensity increases with the V2O5 loading, which is attributing to formation of a crystalline aluminum vanadium oxide (V2O5/γ-Al2O3) phase (Fig. 2f). The average crystallite size of (20 wt%) V2O5/γ-Al2O3 sample determined from the diffraction peak broadening by using the Scherer's formula was 22.4 nm (Fig. 2f). For the 15 wt% catalyst obtained some low intense broad peaks which confirmed the lowest crystallinity of V2O5/γ-Al2O3 catalysts and high dispersion of V2O5 particles on the support surface.
 |
| | Fig. 2 XRD patterns of (a) γ-Al2O3, (b) V2O5, (c) (5 wt%) V2O5/γ-Al2O3, (d) (10 wt%) V2O5/γ-Al2O3, (e) (15 wt%) V2O5/γ-Al2O3 and (f) (20 wt%) V2O5/γ-Al2O3 calcined at 600 °C for 5 h. | |
In addition, the SEM images (Fig. 3) of the pure γ-Al2O3, V2O5 and V2O5 (15 wt%)/γ-Al2O3 sample indicated that agglomerated spherical vanadium oxides particles were well deposited on the surface of γ-Al2O3. According to Fig. 3f the particle sizes of V2O5 (15 wt%)/Al2O3 sample was within the range of 17–35 nm.
 |
| | Fig. 3 SEM images of (a, b) V2O5, (c, d) V2O5 (15 wt%)/Al2O3, (e) pure γ-Al2O3 and (f) V2O5 (15 wt%)/Al2O3 along with particle sizes of V2O5 calcined at 600 °C for 5 h. | |
NH3-TPD profiles of V2O5/γ-Al2O3 catalysts with different V2O5 loadings are shown in Fig. 4. For γ-Al2O3 support, three desorption steps can be observed, which represent weak acidic sites (77–300 °C), medium acidic sites (300–450 °C) and strong acidic sites (450–600 °C), respectively. These profiles were deconvoluted with the Gaussian curve fitting method, with results reported in Table 2. As observed in Fig. 4, by increasing the amount of V2O5 on the γ-Al2O3 from 0 wt% to 20 wt%, the number of weak-medium acid sites continuously increased which implies that the addition of vanadium could cover strong acidic sites. Therefore, it seems that by increasing the V2O5 loading, the peaks of strong-strength acid sites shifted to low temperatures. The results are summarized in Table 2, (area of the peaks at the low temperature was larger than the peaks at the high temperature).
 |
| | Fig. 4 NH3 desorption curves from NH3-TPD over: (a) γ-Al2O3, (b) (5 wt%) V2O5/γ-Al2O3, (c) (10 wt%) V2O5/γ-Al2O3, (d) (15 wt%) V2O5/γ-Al2O3 and (e) (20 wt%) V2O5/γ-Al2O3 calcined at 600 °C for 5 h. | |
Table 2 Acid distribution of V2O5/γ-Al2O3 catalysts
| Sample |
Weak acid (mmol per NH3 per g) |
Middle acid (mmol per NH3 per g) |
Strong acid (mmol per NH3 per g) |
Total (mmol per NH3 per g) |
Brønsted sites/Lewis sitesa |
| Ratio of the concentration of Brønsted and Lewis acid sites calculated from the adsorption of pyridine evacuated at 100 °C. |
| γ-Al2O3 |
0.715 |
0.310 |
0.263 |
1.288 |
0.0 |
| 5V2O5/γ-Al2O3 |
0.810 |
0.322 |
0.213 |
1.345 |
0.31 |
| 10V2O5/γ-Al2O3 |
0.908 |
0.330 |
0.182 |
1.420 |
0.55 |
| 15V2O5/γ-Al2O3 |
0.985 |
0.395 |
0.099 |
1.479 |
0.89 |
| 20V2O5/γ-Al2O3 |
1.023 |
0.464 |
0.059 |
1.546 |
0.63 |
In many studies have been reported that deactivation is related to the acid sites strength. Therefore, high strength sites may be leading reactions to produce heavy products which deposited on the catalyst surface. Their catalytic results showed a straight forward relationship between amount of V2O5 and weak moderate acidic properties. This behavior could be inferred from the shift of the acid sites distribution toward lower desorption temperatures, as observed in the corresponding NH3-TPD curves.29
Several catalytic systems reported that Brønsted acid sites with combination of weak-medium acid strength have important influences on reaction performance and improve acrolein selectivity in the glycerol transformation.30–32 Therefore pyridine adsorption coupled to FTIR analysis were taken to evaluate the nature of acidic sites. The spectra of pyridine adsorbed γ-Al2O3 and V2O5/γ-Al2O3 catalysts with different amount of V2O5 after out gassing at 100 °C are shown in Fig. 5a–e. Lewis and Brønsted acid sites were identified by two bands at around 1450, 1610 cm−1 (attributing to the absorptions of pyridine on the Lewis acid centers) and 1545, 1637 cm−1 (attributing to pyridine coordinated to Brønsted acid sites). Furthermore, the band at about 1495 cm−1 attributed to combination of both Brønsted and Lewis (B + L) acid sites.12,29,33 These results demonstrate that the γ-Al2O3 support had only Lewis acid sites and the total acidity of bare γ-Al2O3 was 1.288 mmol NH3 per g cat. (Table 2). However spectra for the V2O5 on the γ-Al2O3 catalysts showed the presence of both Brønsted and Lewis acid sites. As shown in Table 2, the 15 wt% V2O5/γ-Al2O3 catalyst had the highest Brønsted sites concentration and then dramatically decreased at higher loading of vanadium (20 wt% V2O5/γ-Al2O3), which is maybe due to decrease in surface area and low dispersion of the active sites because of the agglomeration of vanadium species and also their interval diffusion limitations which enhanced the coke formation.14,27 The specific surface area, pore structure property and vanadium oxide surface density for V2O5/γ-Al2O3 catalysts of this study are tabulated in Table 3. It is apparent that by increasing of V2O5 loading, BET surface areas are gradually decreased from 230 m2 g−1 with average pore volume of 0.28 cm3 g−1 to 168 m2 g−1 with average pore volume of 0.20 cm3 g−1, indicating that penetration of surface vanadium species into the pores of gamma alumina. This feature was confirmed by the pore size distribution, which shows the existence of pores ranging between 7 to 6.2 nm and consistent with previous report that vanadium species tend to have a strong interaction with the γ-Al2O3 support.
 |
| | Fig. 5 FTIR spectra of pyridine adsorbed on: (a) pure γ-Al2O3, (b) (5 wt%) V2O5/γ-Al2O3, (c) (10 wt%) V2O5/γ-Al2O3, (d) (15 wt%) V2O5/γ-Al2O3 and (e) (20 wt%) V2O5/γ-Al2O3. | |
Table 3 Texture properties of V2O5/γ-Al2O3 and vanadium surface density (V/nm2) of catalysts after calcination at 600 °C
| Sample |
Surface area (m2 g−1) |
Average pore volume (cm3 g−1) |
Average pore size (nm) |
Surface density (V/nm2) |
| γ-Al2O3 |
230 |
0.28 |
7.0 |
0 |
| 5V2O5/γ-Al2O3 |
222.5 |
0.26 |
6.8 |
1.7 |
| 10V2O5/γ-Al2O3 |
198 |
0.26 |
6.4 |
3.6 |
| 15V2O5/γ-Al2O3 |
190.5 |
0.24 |
6.4 |
5.9 |
| 20V2O5/γ-Al2O3 |
168 |
0.20 |
6.2 |
8 |
The temperature programmed reduction (TPR) of supported V2O5 (with different amount of vanadium oxides from 5 to 20 wt%) were performed to evaluate the reducibility of catalysts, (Fig. 6a–d). According to the literature34–36 three peaks at approximately 350–420 °C, 480–550 °C and 650–720 °C, are attributed to isolated vanadium species (with highly dispersion), polymeric vanadium species and bulk-like V2O5, respectively. As can be seen, by increasing of V2O5 loadings from 5.0 to 15.0 wt%, the reduction temperature of the supported V2O5 catalysts shifted to higher temperature, this is mainly because of polymeric vanadium species deposited on the support that might be more difficult to reduce than isolated vanadium species under the TPR conditions or significant interaction between V2O5 and γ-Al2O3 support along with the formation of crystalline phases of V2O5. The existence of a shoulder for 15 wt% V2O5/γ-Al2O3 is attributed to the reduction of the highly dispersed vanadium species gradually from V5+ to V4+ and V4+ to V3+.37 However, by increasing of V2O5 content, the reduction peak area at 700 °C was markedly increased due to high concentration of crystalline phases of V2O5. This clearly suggests that the high V2O5 content sample (20 wt% V2O5/γ-Al2O3) has more amounts of reducible V5+ species, which is also confirmed by X-ray diffraction pattern, Fig. 2f.
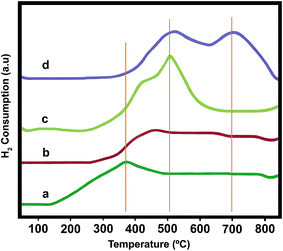 |
| | Fig. 6 H2-TPR profiles of (a) (5 wt%) V2O5/γ-Al2O3, (b) (10 wt%) V2O5/γ-Al2O3, (c) (15 wt%) V2O5/γ-Al2O3 and (d) (20 wt%) V2O5/γ-Al2O3. | |
3.2. Analysis of data and development of the response surface model
In the Box–Behnken design, 17-experimental observations were undertaken at random orders for the optimization of glycerol conversion (%) and acrolein selectivity (%) in the dehydration reaction and then the yield of acrolein was determined. Table 4 shows the data resulting from the effect of three variables: temperature (A), V2O5 (wt%) loading on the support (B) and gas hourly speed velocity (GHSV) (C), while the other factors are constant.
Table 4 Box–Behnken design matrix and results of the glycerol conversion, acrolein selectivity and yield
| Run |
Temperature (°C) |
Loading of V2O5 (wt%) |
GHSV (h−1) |
Glycerol conversion (%) |
Acrolein selectivity (%), (yield)a (%) |
| A |
B |
C |
Experimental |
Predicted |
Experimental |
Predicted |
| Numbers in bracket related to yield of acrolein. |
| 1 |
320.0 |
20.00 |
330 |
90.15 |
91.11 |
22.18(20.01) |
22.82(20.79) |
| 2 |
285.0 |
20.00 |
540 |
55.14 |
54.05 |
63.88(35.29) |
62.39(33.72) |
| 3 |
285.0 |
12.50 |
330 |
92.45 |
94.30 |
71.15(65.77) |
71.67(67.58) |
| 4 |
285.0 |
12.50 |
330 |
94.03 |
94.30 |
72.65(68.31) |
71.67 (67.58) |
| 5 |
285.0 |
20.00 |
120 |
83.26 |
83.33 |
54.30(45.21) |
55.43(46.18) |
| 6 |
250.0 |
20.00 |
330 |
54.37 |
54.43 |
51.11(27.78) |
50.84(27.67) |
| 7 |
285.0 |
12.50 |
330 |
95.78 |
94.30 |
71.13(66.12) |
71.67(67.58) |
| 8 |
250.0 |
5.00 |
330 |
57.23 |
56.27 |
26.44(15.13) |
25.80(14.51) |
| 9 |
320.0 |
12.50 |
540 |
47.63 |
47.75 |
42.28(20.14) |
43.13(20.59) |
| 10 |
320.0 |
5.00 |
330 |
58.98 |
58.91 |
16.24(9.57) |
16.50(9.72) |
| 11 |
285.0 |
12.50 |
330 |
93.22 |
94.30 |
69.31(64.61) |
71.67 (67.58) |
| 12 |
250.0 |
12.50 |
540 |
43.44 |
44.47 |
59.14(25.69) |
60.89(27.07) |
| 13 |
285.0 |
5.00 |
120 |
65.89 |
66.97 |
39.42(25.98) |
40.91(27.39) |
| 14 |
250.0 |
12.50 |
120 |
56.31 |
56.19 |
56.85(32.05) |
55.99(31.46) |
| 15 |
285.0 |
5.00 |
540 |
40.11 |
40.05 |
46.69(18.72) |
45.55(18.24) |
| 16 |
320.0 |
12.50 |
120 |
93.25 |
92.23 |
38.19(35.61) |
36.43(33.60) |
| 17 |
285.0 |
12.50 |
330 |
95.03 |
94.30 |
71.50(67.94) |
71.67 (67.58) |
Analysis of variances (ANOVA) was used to evaluate the effects of main factors and their interactions. The results of the second order response surface model fitting in the form ANOVA for glycerol conversion and acrolein selectivity are shown in Tables 5 and 6 respectively. The ‘Pred R-Squared’ of 0.9835 is in reasonable agreement with the ‘Adj R-Squared’ of 0.9948. ‘Adeq Precision’ measures the signal to noise ratio. The ratio of 46.515 indicates an adequate signal and this model can be used to navigate the design space. The model F-value of 340.06 implies that the model is significant. There is only a 0.01% chance that a ‘Model F-value’ this large could occur due to noise. The large value of F indicates that the most of the variation in the response can be explained by the regression equation. Values of ‘Prob > F’ less than 0.0500 indicate that the model terms are significant. In this case A, B, C, AB, AC, BC, A2, B2, C2 are significant model terms. The lack of fit F-value of 2.28 implies that the lack of fit is not significant relative to the pure error. There is a 22.12% chance that a lack of fit F-value this large could occur due to noise (non-significant lack of fit is good). A quadratic model with statistical significance from a combination of estimates for the variables and the ANOVA results can be produced. The quadratic model was used to explain the mathematical relationship between the independent variables and dependent responses which are represented by eqn (5) and (6).
| | |
GLY Conv. (%) = 94.30 + 9.83A + 7.59B − 14.05C + 8.51AB − 8.19AC − 0.59BC − 15.03A2 − 14.09B2 − 9.11C2
| (5) |
| | |
ACR Sele. (%) = 71.67–9.33A + 7.84B + 2.90C − 4.68AB + 0.45AC + 0.58BC − 22.32A2 − 20.36B2 − 0.24C2
| (6) |
Table 5 ANOVA of the model for the conversion of glycerol aqueous solution from Box–Behnkena
| Source |
Sum of squares |
df |
Mean square |
F-Value |
p-Value Prob > F |
|
| R-Squared = 0.9977, Adj R-squared = 0.9948, Pred R-squared = 0.9835, Adeq precision = 46.515. |
| Model |
7078.46 |
9 |
786.50 |
340.06 |
<0.0001 |
Significant |
| A |
773.42 |
1 |
773.42 |
334.41 |
<0.0001 |
| B |
460.71 |
1 |
460.71 |
199.20 |
<0.0001 |
| C |
1578.94 |
1 |
1578.94 |
682.70 |
<0.0001 |
| AB |
289.51 |
1 |
289.51 |
125.18 |
<0.0001 |
| AC |
268.14 |
1 |
268.14 |
115.94 |
<0.0001 |
| BC |
1.37 |
1 |
1.37 |
0.59 |
0.4669 |
| A2 |
951.29 |
1 |
951.29 |
411.31 |
<0.0001 |
| B2 |
835.73 |
1 |
835.73 |
361.35 |
<0.0001 |
| C2 |
1538.21 |
1 |
1538.21 |
665.09 |
<0.0001 |
| Residual |
16.19 |
7 |
2.31 |
|
|
| Lack of fit |
6.34 |
3 |
2.11 |
0.86 |
0.5309 |
Not significant |
| Pure error |
9.85 |
4 |
2.46 |
|
|
| Cor total |
7094.65 |
16 |
|
|
|
Table 6 ANOVA of the model for the selectivity of acrolein from Box–Behnkena
| Source |
Sum of squares |
df |
Mean square |
F-Value |
p-Value Prob > F |
|
| R-Squared = 0.9958, Adj R-squared = 0.9903, Pred R-squared = 0.9568, Adeq precision = 38.275. |
| Model |
5428.54 |
9 |
603.17 |
170.84 |
<0.0001 |
Significant |
| A |
696.58 |
1 |
696.58 |
197.29 |
<0.0001 |
| B |
491.10 |
1 |
491.10 |
139.09 |
<0.0001 |
| C |
67.45 |
1 |
67.45 |
19.10 |
0.0033 |
| AB |
87.70 |
1 |
87.70 |
24.84 |
0.0016 |
| AC |
0.81 |
1 |
0.81 |
0.23 |
0.6466 |
| BC |
1.33 |
1 |
1.33 |
0.38 |
0.5582 |
| A2 |
2096.95 |
1 |
2096.95 |
593.92 |
<0.0001 |
| B2 |
1745.22 |
1 |
1745.22 |
494.30 |
<0.0001 |
| C2 |
0.24 |
1 |
0.24 |
0.067 |
0.8036 |
| Residual |
24.71 |
7 |
3.53 |
|
|
| Lack of fit |
15.60 |
3 |
5.20 |
2.28 |
0.2212 |
Not significant |
| Pure error |
9.12 |
4 |
2.28 |
|
|
| Cor total |
5453.26 |
16 |
|
|
|
A positive sign before a term indicates a synergistic effect, while a negative sign indicates an antagonistic effect.2,28 The presence of the significant XX cross terms in the model confirms that response depends on both single and mixture variables. According to the eqn (5) and (6) the binary terms indicates that there is a synergistic effect between temperature (A) and loading of V2O5 (B) for conversion of glycerol and there are two synergistic effects between other variables (BC and AC) for selectivity of acrolein, whereas an antagonistic effect resulted between V2O5 loading and reaction temperatures.
The comparison between experimental and predicted values of response variables GLY Conv. (%) and ACR Sele. (%) are shown in Fig. 7. A line of unit slope, corresponding to zero error between actual and predicted values is also presented. The determination coefficients of the R2 (0.9977 and 0.9955) were also high, indicating that the predicted results are matching satisfactorily with the experimental values.
 |
| | Fig. 7 Comparison between experimental and predicted values. Small: for GLY conversion. Large: for ACR selectivity. | |
3.3. Effect of process variables on the conversion of glycerol and selectivity of acrolein
The main effects of factors (A, B, C) on conversion of glycerol (GLY) and selectivity of acrolein (ACR) have been evaluated. Each response surface was created by keeping one of three variables constant at their center points. Although the conversion of GLY and selectivity of ACR are significantly increased by simultaneous increasing amount of V2O5 on support, for higher than 15 wt% of V2O5 loading, the selectivity of ACR decreased dramatically. Other than the individual effect contributed by each main variable, the responses were also influenced by the interaction variables. In order to gain a better understanding of the interaction effects of variables on conversion and selectivity, 3D surface plot for the measured responses were formed based on the model equations (eqn (5) and (6)). The resulted surface response 3-D plots of GLY conversion and ACR selectivity as a function of two independent variables, (a, d) loading of V2O5 on the γ-Al2O3 and temperature; (b, e) GHSV and temperature; (c, f) loading of V2O5 on the γ-Al2O3 and GHSV are shown in Fig. 8a–f, respectively.
 |
| | Fig. 8 3D surface plots describing the response surface for: (Left: GLY conversion, Right: ACR selectivity) as function of: (a, d) loading of V2O5/γ-Al2O3 vs. temperature at GHSV = 330 h−1. (b, e) GHSV vs. temperature at loading of V2O5/γ-Al2O3 = 12.5 wt%. (c, f) GHSV vs. Loading of V2O5/γ-Al2O3 at temperature = 285 °C. | |
As can be seen in Fig. 8, temperature had a different effect on the conversion of GLY and selectivity of ACR. The conversion of glycerol is significantly increased by simultaneous increasing of temperature (A), while it seems that optimum temperature for ACR selectivity is lower than 300 °C, Fig. 8(a, b, d and e). When the loading of V2O5 on γ-Al2O3 increases from 5 wt% to 15 wt%, selectivity of ACR will increase because there are more Brønsted acid sites available for the reaction mechanism leading to the production of ACR. Fig. 8c shows that conversion of GLY decreases with increasing GHSV from 120 to 540 h−1 and increases by increasing V2O5 loaded on support. At high level of GHSV (540 h−1) and middle level of V2O5 loading and low temperature maximum selectivity of ACR was obtained, while high level of catalyst and temperature and low level of GHSV maximized GLY conversion. This simply means that the GHSV and temperature are two factors which could be adjusted within the range to obtain a high yield of ACR. Thus, we can conclude that the yield of ACR is significantly increased by increasing of gas hourly space velocity, at constant V2O5 loading. In high temperatures the fast conversion leads to a high concentration of products and this might interfere by blocking the acidic sites, indicating that the selectivity of ACR is reduced considerably. Therefore, desorption rate of unwanted products is facilitated by increasing in GHSV. On the other hand, it seems to eliminate or slow down coke deposition on catalyst surface.38
3.4. Validation of the experimental model
Response optimizer helps to identify the combination of input variable settings that jointly optimize a single response or a set of responses.28 The software was used to predict the best conditions for the yield of acrolein (ACR) by pre-setting certain criteria, as shown in Table 7. The optimum values of the independent variables are obtained considering the lower and higher values of temperature, V2O5 loading and gas hourly space velocity (GHSV). It was found that yield of higher than 68.31% for ACR could be achieved when using the V2O5 loading higher than 12.5 (wt%), the temperature of higher than 250 °C and the GHSV lower than 540 h−1. It was predicted that for amount of V2O5 on the γ-Al2O3 = 14.80 wt%, GHSV of 268.60 h−1 and temperature = 286.35 °C the yield of the ACR would be 73.05%, while the actual result for the maximum yield was 68.31% for 12.5 wt% loading of V2O5 on the γ-Al2O3, GHSV = 330 h−1 and reaction temperature of 285 °C. These results demonstrated that the predicted values are matching satisfactorily with the experimental ones. Therefore, obtained data from RSM technique, as mentioned above, let us to suggest the better operation conditions for dehydration reaction of glycerol (GLY) to acrolein (ACR).
Table 7 The preset criteria for optimization of the maximum conversion, selectivity and yielda
| Factor/response |
Goal |
Lower limit |
Upper limit |
| Composite desirability = 1.00000. |
| Temperature (°C) A |
In range |
250 |
320 |
| Loading of V2O5 (wt%) B |
In range |
5 |
20 |
| GHSV (h−1) C |
In range |
120 |
540 |
| GLY conversion (%) |
Maximize |
40.11 |
95.03 |
| ACR selectivity (%) |
Maximize |
16.24 |
72.65 |
| ACR yield (%) |
Maximize |
9.57 |
69.23 |
3.5. Effect of reaction time
The effect of reaction time on catalytic activity of 12.5 wt% V2O5/γ-Al2O3 at 285 °C is shown in Fig. 9. To evaluate the stability of catalyst, dehydration of glycerol was carried out under atmospheric pressure for 1, 3, 6 and 9 h under GHSV of 330 h−1. The reactor was fed with aqueous solution contained 30% (in mass) of glycerol at a feed flow rate of 2 mL h−1 by means of HPLC pump. According to Table 8, maximum glycerol conversion (99.28%) can be achieved in the early hour (TOS 1 h) and then gradually decreased by further increasing of reaction progress, which it is probably due to the high reactivity of acrolein, the side reactions and formation of larger molecules and coke formation, as previously mentioned.18 For better understanding, the TG and DTA curves of spent catalyst after 3 and 9 h are shown in Fig. 10. It can be seen from the TG curve that total weight loss for spent catalyst after 3 h is smaller than after 9 h. Furthermore, two exothermic peaks are appeared in the DTA curves. First peak is attributed to reoxidation of V4+ to V5+ species which take place at lower temperature (around 350 °C) and second peak is due to formation of poly-glycols or large molecules (around 500 °C), respectively.15,16 Also, a small peak around 220 °C is due to decomposition of carbonaceous species after 9 h on the catalyst surface while, no carbon content was observed at 3 h of reaction progress, Fig. 10a. As expected, coke formation over the active sites was mainly responsible for decreasing acid site density and will result in catalyst deactivation during the reaction. Therefore, the highest acrolein yield (68.31%) was achieved after 3 h. The low selectivity is expected due to limited availability of active vanadium sites and consecutive reactions such as acrolein degradation or polymerization, which confirmed by above findings. Hence, the acrolein yield after 9 h (52.30%) is much lower than after 3 h. Similarly to acrolein, the yield of other products such as acetol, acetaldehyde, allyl alcohol and acrylic acid was decreased dramatically after 9 h.
 |
| | Fig. 9 Glycerol conversion and acrolein selectivity and yield over 12.5 wt% V2O5/γ-Al2O3 with time. | |
Table 8 Product distribution results of glycerol dehydration over 12.5 wt% V2O5/γ-Al2O3 catalyst
| Time (h) |
Conversion (%) |
Yield (%) |
| Acrolein |
Acetol |
Allyl alcohol |
Acetaldehyde |
Acrylic acid |
Others |
| 1 |
99.28 |
57.96 |
19.63 |
10.65 |
5.23 |
2.04 |
4.49 |
| 3 |
94.03 |
68.31 |
16.17 |
7.02 |
5.01 |
1.95 |
1.55 |
| 6 |
92.85 |
59.07 |
15.69 |
6.95 |
4.89 |
1.14 |
12.26 |
| 9 |
85.47 |
52.30 |
12.45 |
5.21 |
4.85 |
0.93 |
24.26 |
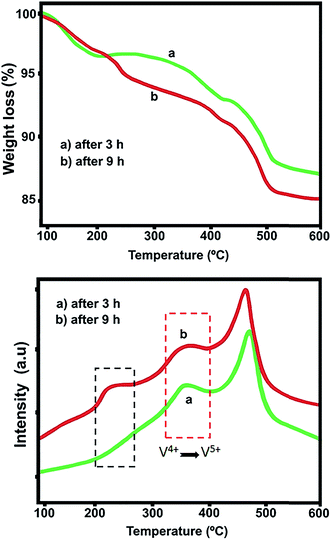 |
| | Fig. 10 TG and DTA curves of the spent 12.5 wt% V2O5/γ-Al2O3 catalyst after 3 and 9 h of reaction. | |
4. Conclusions
A response surface model, based on the Box–Benkhen technique, was developed to describe the conversion of glycerol, acrolein selectivity and yield in gas phase dehydration reaction of glycerol. Temperature programmed desorption of ammonia (NH3-TPD) showed that the amount of strong acid sites decreased by increasing of V2O5 loading while number of total acid sites significantly increased. Temperature programmed reduction (H2-TPR) showed that the high V2O5 content sample (20 wt% V2O5/γ-Al2O3) has more amounts of reducible V5+ species, which is mainly because of crystalline phase of V2O5. The obtained results from ANOVA showed that the most significant factor affecting the conversion of GLY and ACR selectivity were gas hourly space velocity of carrier gas and reaction temperature, respectively. In addition, the interactions between temperature and V2O5 loading (A × B), V2O5 loading had significant effects on the GLY conversion and ACR selectivity. Coefficient of determination (R2) value of 0.9977 and 0.9958 obtained from eqn (5) and (6) shown that quadratic polynomial regression model could properly interpret the experimental data.
Also, we found that the catalytic activity and amount of Brønsted acid site depend on GHSV and amount of V2O5 on the support and both are required for the high yield of ACR. The spent catalyst was also investigated by TG/DTA analysis and results showed that there is a significant decrease in the weight loss of the spent samples which leading to a decrease in the conversion and yield of acrolein after 9 h reaction. Thus, weak-medium strength acid sites efficiently promoted the dehydration reaction. With 14.80 wt% of V2O5/γ-Al2O3, reaction temperature of 286.35 °C and low level of GHSV (268.60 h−1), we could achieve the highest yield of acrolein (73.05%). Also, to show the validity of this prediction, we run the experiment at this condition and gained 68.31% for the yield of acrolein. These studies confirm that the predicted values are fully compatible with the experimental values.
References
- B. Katryniok, S. B. Paul and F. Dumeignil, ACS Catal., 2013, 3, 1819 CrossRef CAS.
- A. Talebian-Kiakalaieh and N. A. S. Amin, Ind. Eng. Chem. Res., 2015, 54, 8113 CrossRef CAS.
- X. Feng, Y. Yao, Q. Sua, L. Zhaoa, W. Jianga and W. Ji, Appl. Catal., B, 2015, 164, 31 CrossRef CAS.
- M. M. Bettahar, G. Costentin, L. Savary and J. C. Lavalley, Appl. Catal., A, 1996, 145, 1 CrossRef CAS.
- Z. Wang, L. Wang, Y. Jiang, M. Hunger and J. Huang, ACS Catal., 2014, 4, 1144 CrossRef CAS.
- D. Stosic, S. Bennici, S. Sirotin, P. Stelmachowski, J. Couturier, J. Dubois, A. Travert and A. Auroux, Catal. Today, 2014, 226, 167 CrossRef CAS.
- L. Shen, H. Yin, A. Wang, Y. Feng, Y. Shen, Z. Wu and T. Jiang, Chem. Eng. J., 2012, 180, 277 CrossRef CAS.
- W. Yan and G. J. Suppes, Ind. Eng. Chem. Res., 2009, 48, 3279 CrossRef CAS.
- L. Shen, H. Yin, A. Wang, X. Lu and C. Zhang, Chem. Eng. J., 2014, 244, 168 CrossRef CAS.
- A. S. de Oliveira, S. J. S. Vasconcelos, J. R. de Sousa, F. F. de Sousa, J. M. Filho and A. C. Oliveira, Chem. Eng. J., 2011, 168, 765 CrossRef CAS.
- L. G. Possato, R. N. Diniz, T. Garetto, S. H. Pulcinelli, C. V. Santilli and L. A. Martins, J. Catal., 2013, 300, 102 CrossRef CAS.
- R. Estevez, S. Lopez-Pedrajas, F. Blanco-Bonilla, D. Luna and F. M. Bautista, Chem. Eng. J., 2015, 282, 179 CrossRef CAS.
- J. L. Dubois, C. Duquenne and W. Holderich, Arkema FR 2884818 or WO2006087083 A2, 2006.
- A. Talebian-Kiakalaieh, N. A. S. Amin and H. Hezaveh, Renewable Sustainable Energy Rev., 2014, 40, 28 CrossRef CAS.
- F. Wang, J. L. Dubois and W. Ueda, Appl. Catal., A, 2010, 376, 25 CrossRef CAS.
- F. Wang, J. Ldubois and W. Ueda, J. Catal., 2009, 268, 260 CrossRef CAS.
- Y. Kamiya, H. Nishiyama, M. Yashiro, A. Satsuma and T. Hattori, J. Jpn. Pet. Inst., 2003, 46, 62 CrossRef.
- J. A. Cecilia, C. García-Sancho, J. M. Mérida-Robles, J. Santamaría-González, R. Moreno-Tost and P. Maireles-Torres, Catal. Today, 2015, 254, 43 CrossRef CAS.
- A. Alhanash, E. F. Kozhevnikova and I. V. Kozhevnikov, Appl. Catal., A, 2010, 378, 11 CrossRef CAS.
- A. Chieregato, M. Dolores Soriano, E. Garcia-Gonzalez, G. Puglia, F. Basile, P. Concepcion, C. Bandinelli, J. M. Lopez Nieto and F. Cavani, ChemSusChem, 2015, 8, 398 CrossRef CAS PubMed.
- A. Chieregato, C. Bandinelli, P. Concepcion, M. D. Soriano, F. Puzzo, F. Basile, F. Cavaniand and J. M. Lopez Nieto, ChemSusChem, 2016, 9, 1 CrossRef.
- P. J. Souza, Th. Melo, M. A. L. de Oliveira, R. M. Paniago, P. P. de Souza and L. C. A. Oliveira, Appl. Catal., A, 2012, 443, 153 CrossRef.
- J. L. Dubois, C. Duquenne, W. Holderich and J. Kervennal, Arkema FR 2882053 A1 or US2008214880 A1, 2006.
- K. Omata, S. Izumi, T. Murayama and W. Ueda, Catal. Today, 2013, 201, 7 CrossRef CAS.
- K. M. Parida, A. C. Pradhan and J. D. NruparajSahu, Mater. Chem. Phys., 2009, 113, 244 CrossRef CAS.
- A. Pan, J. G. Zhang, Z. Nie, G. Cao, B. W. Arey, G. Li, S. Liang and J. Liu, J. Mater. Chem., 2010, 20, 9193 RSC.
- X. Hu, C. Li and C. Yang, Catal. Today, 2010, 158, 504 CrossRef CAS.
- V. Mahdavi and A. Monajemi, J. Taiwan Inst. Chem. Eng., 2014, 45, 2286 CrossRef CAS.
- C. García-Sancho, J. A. Cecilia, A. Moreno-Ruiz, J. M. Mérida-Robles, J. Santamaría-González, R. Moreno-Tost and P. Maireles-Torres, Appl. Catal., B, 2015, 179, 139 CrossRef.
- B. Katryniok, S. Paul, V. Bellière-Baca, P. Reye and F. Dumeignil, Green Chem., 2010, 12, 2079 RSC.
- A. Talebian-Kiakalaieh and N. A. S. Amin, J. Taiwan Inst. Chem. Eng., 2016, 59, 11 CrossRef CAS.
- N. PethanRajan, G. Srinivasa Rao, B. Putrakumar and K. V. R. Chary, RSC Adv., 2014, 4, 53419 RSC.
- N. P. Rajan, G. Srinivasa Rao, V. Pavankumar and K. V. R. Chary, Catal. Sci. Technol., 2014, 4, 81 Search PubMed.
- M. Banares, J. Cardoso, F. Agulló Rueda, J. Correa Bueno and J. Fierro, Catal. Lett., 2000, 64, 191–196 CrossRef CAS.
- A. Held, J. Kowalska-Kuś and K. Nowińska, Catal. Commun., 2012, 17, 108–113 CrossRef CAS.
- B. Solsona, T. Blasco, J. L. Nieto, M. Pena, F. Rey and A. Vidal-Moya, J. Catal., 2001, 203, 443–452 CrossRef CAS.
- S. Besselmann, C. Freitag, O. Hinrichsen and M. Muhler, Phys. Chem. Chem. Phys., 2001, 3, 4633 RSC.
- A. Talebian-Kiakalaieh, N. A. S. Amin and Z. Y. Zakaria, J. Ind. Eng. Chem., 2016, 34, 300 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 were dissolved to 100 mL distilled water under active stirring (800 RPM) at room temperature until the color of the solution changed from yellow to blue. The resulting solution was introduced into a 200 cm3 Teflon-lined stainless steel autoclave and submitted to a hydrothermal treatment at 250 °C for 24 h. The solid was separated by filtration, dried at 80 °C and then calcined in the furnace at 400 °C in air for 2 h (ref. 26) and finally labeled as nano V2O5. The V2O5/γ-Al2O3 catalysts containing V2O5 with 5, 10, 15, and 20 wt% were prepared by impregnation method as described in (ref. 27). In a typical synthesis, 1.0 g of calcined and out gassed γ-Al2O3 was impregnated with certain concentration of vanadyl oxalate solution. Above solution was prepared by mixing vanadium pentoxide with desire amounts of oxalic acid. The wet solid was then dried at 120 °C over night under vacuum and calcined in air under static conditions at 600 °C for 5 h. Finally, the obtained samples were crushed and sieved to obtain catalyst particle size of 0.077–0.300 mm.
3 were dissolved to 100 mL distilled water under active stirring (800 RPM) at room temperature until the color of the solution changed from yellow to blue. The resulting solution was introduced into a 200 cm3 Teflon-lined stainless steel autoclave and submitted to a hydrothermal treatment at 250 °C for 24 h. The solid was separated by filtration, dried at 80 °C and then calcined in the furnace at 400 °C in air for 2 h (ref. 26) and finally labeled as nano V2O5. The V2O5/γ-Al2O3 catalysts containing V2O5 with 5, 10, 15, and 20 wt% were prepared by impregnation method as described in (ref. 27). In a typical synthesis, 1.0 g of calcined and out gassed γ-Al2O3 was impregnated with certain concentration of vanadyl oxalate solution. Above solution was prepared by mixing vanadium pentoxide with desire amounts of oxalic acid. The wet solid was then dried at 120 °C over night under vacuum and calcined in air under static conditions at 600 °C for 5 h. Finally, the obtained samples were crushed and sieved to obtain catalyst particle size of 0.077–0.300 mm.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2
O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol, equal to 13.5
glycerol, equal to 13.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in molar ratio. After 3 h, reaction products were collected in an ice-water cold trap and n-butanol was added as internal standard. Products were quickly analyzed by Varian Star 3400 gas chromatograph equipped with a 10 wt% FFAP/chromosorb W-AW column (2 mm i.d., 2.5 m long) and FID detector. The main products identified by GC analysis were acrolein, acetol, allyl alcohol and acetaldehyde. The mass balance was determined and an acceptable carbon balance (>85%) was achieved for each trap. Besides, some chromatographic signals in lower quantities have not been identified. Glycerol conversion, selectivity and yield were calculated by eqn (1)–(3) respectively.
1 in molar ratio. After 3 h, reaction products were collected in an ice-water cold trap and n-butanol was added as internal standard. Products were quickly analyzed by Varian Star 3400 gas chromatograph equipped with a 10 wt% FFAP/chromosorb W-AW column (2 mm i.d., 2.5 m long) and FID detector. The main products identified by GC analysis were acrolein, acetol, allyl alcohol and acetaldehyde. The mass balance was determined and an acceptable carbon balance (>85%) was achieved for each trap. Besides, some chromatographic signals in lower quantities have not been identified. Glycerol conversion, selectivity and yield were calculated by eqn (1)–(3) respectively.









