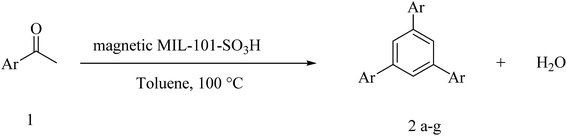
Magnetic MIL-101-SO3H: a highly efficient bifunctional nanocatalyst for the synthesis of 1,3,5-triarylbenzenes and 2,4,6-triaryl pyridines†
Mahmoud Borjian Boroujeni,
Alireza Hashemzadeh,
Mohammad-Tayeb Faroughi,
Ahmad Shaabani* and
Mostafa Mohammadpour Amini*
Faculty of Chemistry, Shahid Beheshti University, G. C., P. O. Box 19396-4716, Tehran, Iran. E-mail: a-shaabani@sbu.ac.ir; m-pouramini@sbu.ac.ir
First published on 14th October 2016
Abstract
A magnetic MIL-101-SO3H was synthesized and successfully used as a highly active nanocatalyst for the synthesis of 1,3,5-triarylbenzenes and 2,4,6-triaryl pyridines. The prepared nanocatalyst was characterized by Fourier transform infrared spectroscopy, scanning electron microscopy, energy-dispersive X-ray and X-ray powder diffraction. It is found that the catalyst can be easily separated from the reaction mixture by an external magnetic field and recycled several times without a significant loss of activity.
1. Introduction
Metal–organic frameworks (MOFs) are an important new class of porous material widely used in separation, sensors, gas storage and catalysis due to their unique combination properties, such as adjustable pore size, crystalline open structures, high density of active sites and functionality.1–3 MOFs, with their high surface area, can be used as heterogeneous catalysts or supports for organic transformations such as C–H bond activation, C–C bond formation, aerobic oxidation, and multi-component reactions (MCRs).4–9Among the various metal organic frameworks that have been investigated, MIL-101 has been widely used due to its excellent stability under catalytic conditions, high metal content, and high surface area.10,11 MIL-101 by its 3D pore structure is used as a reusable solid catalyst for autoxidation of benzylic hydrocarbons and aerobic oxidative desulfurization of dibenzothiophene.12,13 As well as, MIL-101 is widely used as a support for metal phthalocyanine complexes,14 polyoxometalates15 and nanoparticles.16–18 It is important to note that the most important strategy to improve properties of MOFs for catalytic applications is functionalizing of MOFs in monofunctional and multifunctional active sites.19,20 For instance, sulfonation of MIL-101 has been developed and has been used for one-pot deacetalization–nitroaldol reaction and the ring opening of epoxides with alcohols.21–23 Bifunctional Au@MIL-53(NH2) and MIL-101-Cr-SO3H-Al(III) are used for aerobic oxidation/Knoevenagel condensation and benzylation reaction of aromatic hydrocarbons, respectively.24,25 Recently, the synthesis of magnetic framework composites (MFCs) has attracted a considerable attention in catalyst because of their low toxicity, high catalytic activity, and easy separation of the catalyst after the end of reactions. Immobilization of magnetic nanoparticles on MOFs such as ZIf-8 and MIL-101 has produced highly efficient adsorbent for anionic dyes, removing textile dyes, and efficient catalyst for oxidation of alcohols.26–29 To the best of our knowledge, there is no report on utilization of magnetic MIL-101-SO3H for an organic transformation.
1,3,5-Triarylbenzenes are an important polycyclic aromatic compounds with potential applications in fields of conducting polymers, organic light emitting diodes, synthetic dendrimers, special ligands and resistance materials.30 Consequently, numerous methods for synthesis of these compounds have been developed in recent years. para-Toluene sulfonic acid,30 Bi(OTf)3,31 TiCl4,32 and H6P2W18O62/nanoclinoptilolite33 are used as catalysts for synthesis of triarylbenzenes. Despite the efficiency of these methods, there are some limitations such as tedious workup, none-recyclability of the catalyst and long reaction times.
2,4,6-Trisubstituted pyridines are an important materials in supramolecular chemistry due to their p-stacking ability and excellent thermal stability that were used in high-performance Flrpic based organic light-emitting device (OLED).34 Consequently, the synthesis of these materials remains interesting topic in modern synthetic chemistry.35 In recent years, various methods have been developed for their synthesis, including classical heating condition, microwave irradiation, and sonication in the presence of bismuth triflate, copper triflate, and mesoporous nanocrystalline MgAl2O4 catalysts.36–39 Nevertheless, the development of new heterogeneous catalyst for the synthesis of 2,4,6-trisubstituted pyridines is in high demand.
In continuation of our ongoing research in the sustainable benign pathway for organic transformations and nanocatalysis,40–43 we have introduced a novel bifunctional nanocatalyst and investigated its catalytic activity for the synthesis of 1,3,5-triarylbenzenes and 2,4,6-trisubstituted pyridines.
2. Experimental
2.1. Materials and methods
The FT-IR spectra were recorded on a Bomem MB-Series FT-IR spectrometer. The X-ray powder diffraction (XRD) pattern was recorded on a STOE diffractometer with Cu-Kα radiation (λ = 1.5418 Å). The scanning electron microscopy (SEM) and the energy dispersive X-ray spectroscopy (EDS) analyses were performed using a Philips XL-30 instrument. All samples for morphological studies were sputtered with gold before observation. The 1H NMR spectra were recorded on a BRUKER DRX-300 AVANCE spectrometer using CDCl3 and DMSO-d6 as solvents and tetramethylsilane (TMS) as internal standard. The melting points of the products were measured by an electrothermal 9100 apparatus. The concentrations of iron were estimated using an inductively coupled plasma optical emission spectrometer (ICP-OES) Varian Vista PRO Radial. The CHN analysis was performed using a Perkin-Elmer 2400 CHN analyzer.2.2. General procedure for the synthesis of the MIL-101
The MIL-101 was prepared by hydrothermal reaction according to the previous report.44 Briefly, Cr(NO3)3·9H2O (2.00 g, 5.00 mmol), terephthalic acid (0.82 g, 5.00 mmol) and deionized water (24 mL) were loaded in a hydrothermal chamber and heated at 220 °C for 18 h. The resulted pale green solid was collected and washed several times with deionized water, DMF and hot ethanol and then soaked in ethanol 95% for 24 h. Finally, the powder was dried overnight at 150 °C.2.3. General procedure for the synthesis of magnetic MIL-101
The synthesis of magnetic MIL-101 was carried out according to the literature procedure.28 Briefly, 2.00 mmol FeCl3·6H2O and 1.00 mmol FeCl2·4H2O were added to 100 mL an aqueous suspension containing 0.50 g of MIL-101. The suspension was sonicated for 10 min and then vigorously was stirred and was degassed with nitrogen for 2 h, and this followed by addition of 15 mL NH4OH solution to form black suspension. The suspension was separated by the magnet decantation, washed with distilled water, ethanol and, finally was dried under vacuum at the room temperature. ICP-AES analysis showed 17 wt% Fe is loaded in magnetic MIL-101.2.4. General procedure for the synthesis of magnetic MIL-101-SO3H
The activated MIL-101 (2.00 g) was stirred in 30 mL CHCl3 at 25 °C for 15 min, ClSO3H (0.60 g, 5.10 mmol) in 15 mL CHCl3 was added dropwise, and then the mixture was stirred for 2 h at room temperature. The catalyst was separated by an external magnet, was washed three times with CHCl3, and was dried to give magnetic MIL-101-SO3H.2.5. General procedure for the synthesis of 1,3,5-triarylbenzenes
The magnetic MIL-101-SO3H (30 mg), acetophenone (0.36 g, 3.00 mmol), and toluene (5 mL) were added to a 25 mL round bottom flask and the reaction mixture was stirred for 3 h at 100 °C. Upon completion of the reaction, the reaction mixture was cooled to the room temperature and the catalyst was separated by an external magnet and crude product was obtained by evaporation of solvent. The solid product was purified through recrystallization in mixture of EtOH and H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).
1, v/v).
2.6. General procedure for the synthesis of 2,4,6-triaryl pyridines
A mixture of acetophenones (2.00 mmol), aromatic aldehydes (1.00 mmol), ammonium acetate (2.00 mmol) and the magnetic MIL-101-SO3H (30 mg) were added to a 25 mL round bottom flask and the reaction mixture was stirred for 2 h at 110 °C. Upon completion the reaction, the reaction mixture was cooled to the room temperature, eluted with hot ethanol and the catalyst was separated by an external magnet. The product was obtained by recrystallization of ethanol solution.3. Results and discussion
The magnetic MIL-101-SO3H nanocatalyst was prepared according to the following two-step procedure: (i) magnetic MIL-101 was synthesized according to the reference procedure28 and (ii) the magnetic MIL-101 was sulfonated by simple treatment with chlorosulfonic acid to give the bifunctional catalyst. Powder XRD patterns of the MIL-101, magnetic MIL-101 and magnetic MIL-101-SO3H are shown in Fig. 1. The diffraction peaks of the MIL-101 and Fe3O4 nanoparticles are observed in the XRD pattern of the magnetic MIL-101 which confirms the formation of Fe3O4 nanoparticles in the nanocomposite. The relative intensities of the diffraction peaks are decreased in the magnetic MIL-101 that confirms encapsulation of the Fe3O4 nanoparticles within the pores of MIL-101 was occurred.28 The intensity of the Fe3O4 nanoparticles in the XRD pattern of the magnetic MIL-101 was relatively low due to the low content of the Fe3O4 nanoparticles in the nanocomposite. | ||
| Fig. 1 The XRD patterns of the MIL-101 (A), magnetic MIL-101 (B) and the magnetic MIL-101-SO3H nanocatalyst (C). | ||
The FT-IR spectra of MIL-101, magnetic MIL-101, and magnetic MIL-101-SO3H nanocatalyst are shown in Fig. 2. Characteristic vibration bands are observed around 1390 and 1510 cm−1 for the (O–C–O) groups which confirmed the presence of the dicarboxylate. The broad band around 3340 cm−1 is attributed to the water molecules within the pores of the MIL-101. The FT-IR spectrum of the magnetic MIL-101 shows an absorption band at 570 cm−1 ascribes the Fe–O group vibration. The FT-IR spectrum of the magnetic MIL-101-SO3H nanocatalyst exhibits broad bands around 1000–1250 cm−1 which can be attributed to the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O symmetric and asymmetric starching vibration modes that confirmed the MIL-101 was functionalized. According to the elemental analysis data, sulfur content of catalyst was 1.3 mmol g−1. The number of H+ site of the catalyst determined by acid–base titration was 1.2 meq. g−1.
O symmetric and asymmetric starching vibration modes that confirmed the MIL-101 was functionalized. According to the elemental analysis data, sulfur content of catalyst was 1.3 mmol g−1. The number of H+ site of the catalyst determined by acid–base titration was 1.2 meq. g−1.
 | ||
| Fig. 2 FT-IR spectra of the MIL-101 (A), the magnetic MIL-101 (B), and the magnetic MIL-101-SO3H (C). | ||
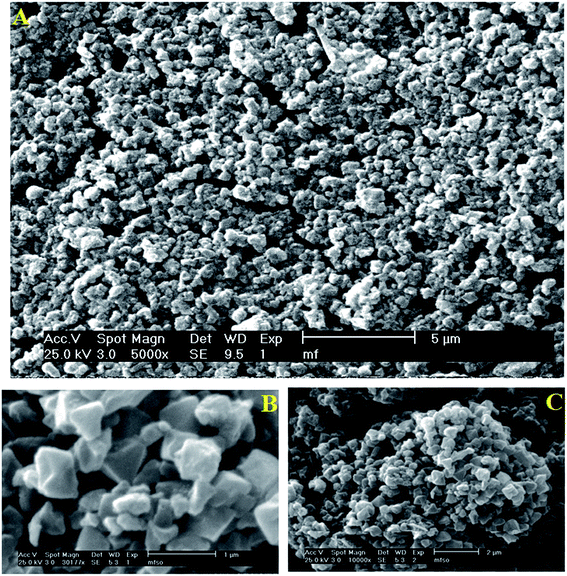
The SEM analysis was used to study the morphology and the structure of the magnetic MIL-101-SO3H nanocatalyst. The SEM images show an excellent dispersity of the Fe3O4 nanoparticles that incorporated into the MIL-101 pores (Fig. 3). Moreover, the energy dispersive spectroscopy (EDS) analysis confirmed the existence of C, O, Cr, S, and Fe in the magnetic MIL-101-SO3H nanocatalyst (Fig. 4).
The N2 adsorption–desorption isotherms for MIL-101 and magnetic MIL-101 are shown in the ESI,† and Table 1 lists the specific surface area and total pore volume of MIL-101, magnetic MIL-101 and magnetic MIL-101-SO3H. As expected, specific surface area and total pore volume were decreased after functionalization, using magnetic nanoparticles and incorporation of SO3H groups into the structure of MIL-101. The decrease of specific surface area and total pore volume indicates that magnetic nanoparticles were unambiguously encapsulated within the pores of MIL-101.
| Catalyst | Surface area (m2 g−1) | Total pore volume (mL g−1) |
|---|---|---|
| MIL-101 | 1835 | 0.87 |
| Magnetic MIL-101 | 647 | 0.33 |
| Magnetic MIL-101-SO3H | 453 | 0.21 |
The presence of Lewis acid CrIII sites and also SO3H groups as Brønsted acid sites, encourage us to evaluate the catalytic properties of magnetic MIL-101-SO3H nanocatalyst for the synthesis of 1,3,5-triphenylbenzene from acetophenone in toluene (Table 2). The reaction in the absence of the magnetic MIL-101-SO3H nanocatalyst did not result in the desired product (Table 2, entry 1). When the reaction was carried out in the presence of pristine MIL-101 (5%), the product was obtained in 30% within 3 h (Table 2, entry 2). The yield of product was increased to 35% in the presence of the magnetic MIL-101 (Table 2, entry 3). These observations indicate that CrIII sites in MIL-101 and the active sites of magnetic nanoparticle as a Lewis acid have a synergic effect for catalyzing the reaction. As the amount of the magnetic MIL-101-SO3H was increased, the reaction went to completion at 100 °C (Table 2, entries 4–8). This indicates the Brønsted acid sites increase the catalytic activity of magnetic MIL-101 in the reaction. In order to determine the best solvent, various solvents such as H2O, DMF, CH3CN, toluene, and dichloromethane were tested. The results showed that the yield of the reaction in toluene was higher than either other solvents or solvent-free conditions (Table 2, entries 9–13). After screening different temperatures, 100 °C was obtained as the best temperature for the reaction. The optimized conditions for the synthesis of the 1,3,5-triphenylbenzene were 30 mg of the magnetic MIL-101-SO3H in toluene as a solvent at 100 °C.
| Entry | Catalyst (mg) | Solvent | Temperature (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: acetophenone (3.00 mmol), catalyst, solvent (5 mL), 3 h.b Isolated yield. | ||||
| 1 | None | Toluene | 100 | 0 |
| 2 | MIL-101 (30) | Toluene | 100 | 30 |
| 3 | Magnetic MIL-101 (30) | Toluene | 100 | 35 |
| 4 | Magnetic MIL-101-SO3H (6) | Toluene | 100 | 45 |
| 5 | Magnetic MIL-101-SO3H (12) | Toluene | 100 | 60 |
| 6 | Magnetic MIL-101-SO3H (18) | Toluene | 100 | 70 |
| 7 | Magnetic MIL-101-SO3H (24) | Toluene | 100 | 80 |
| 8 | Magnetic MIL-101-SO3H (30) | Toluene | 100 | 90 |
| 9 | Magnetic MIL-101-SO3H (30) | Neat | 100 | 80 |
| 10 | Magnetic MIL-101-SO3H (30) | H2O | 100 | 0 |
| 11 | Magnetic MIL-101-SO3H (30) | DMF | 100 | 25 |
| 12 | Magnetic MIL-101-SO3H (30) | CH3CN | 82 | 15 |
| 13 | Magnetic MIL-101-SO3H (30) | Dichloromethane | 39 | 40 |
| 14 | Magnetic MIL-101-SO3H (30) | Toluene | 110 | 90 |
| 15 | Magnetic MIL-101-SO3H (30) | Toluene | 90 | 70 |
With the optimized conditions established, the synthesis of different 1,3,5-triarylbenzene derivatives was examined in the presence of magnetic MIL-101-SO3H in toluene. As shown in Table 3, acetophenone with a wide array of functional groups on the benzene ring such as bromo, chloro, fluoro, and methyl were employed to synthesis of 1,3,5-triarylbenzenes.
| Entry | Ar | Time (h) | Yieldb (%) | Product | Mp (°C)/reference |
|---|---|---|---|---|---|
| a Reaction conditions: phenyl methyl ketone (3.00 mmol), catalyst (30 mg), toluene (5 mL) at 100 °C.b Isolated yield. | |||||
| 1 | Phenyl | 3 | 90 | 2a | 170–172 (ref. 30) |
| 2 | p-CH3C6H4 | 3.5 | 85 | 2b | 179–181 (ref. 30) |
| 3 | p-BrC6H4 | 4 | 80 | 2c | 262–264 (ref. 30) |
| 4 | p-ClC6H4 | 4 | 80 | 2d | 248–250 (ref. 30) |
| 5 | p-FC6H4 | 4 | 80 | 2e | 238–240 (ref. 30) |
| 6 | p-MeOC6H4 | 4 | 75 | 2f | 140–142 (ref. 30) |
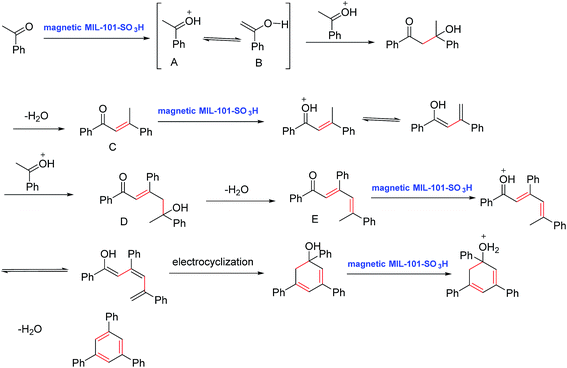
Based on the previous report,33 a plausible mechanism was proposed in Scheme 1. At first, acetophenone was protonated in the presence of magnetic MIL-101-SO3H nanocatalyst and enol form of acetophenone was generated. A reaction between protonated acetophenone (A) and (B) was followed by dehydration of α,β-unsaturated carbonyl compound (C), and then (C) activated in the presence of catalyst and subsequently was reacted with another protonated acetophenone molecule to produce compound (D). After dehydrogenation of D and prototropic shift, compound (E) generated the electrocyclization and dehydrogenation of it produced 1,3,5-triphenylbenzene.
Encouraged by the above discovery, we further investigated the possibility of using magnetic MIL-101-SO3H nanocatalyst for the synthesis of 2,4,6-triaryl pyridines via the reaction of acetophenone, benzaldehyde and ammonium acetate under solvent-free conditions. After screening different conditions, the best reaction conditions were 30 mg nanocatalyst under solvent-free conditions at 110 °C (Table 4).
| Entry | Catalyst (mg) | Temperature | Yieldb % |
|---|---|---|---|
| a Reaction conditions: acetophenone (2.1 mmol), benzaldehyde (1.00 mmol), ammonium acetate (2.00 mmol), catalyst, 110 °C.b Isolated yield. | |||
| 1 | — | 120 | 15 |
| 2 | MIL-101 (30) | 110 | 25 |
| 3 | Magnetic MIL-101 (30) | 110 | 30 |
| 4 | Magnetic MIL-101 SO3H nanocatalyst (6) | 110 | 55 |
| 5 | Magnetic MIL-101 SO3H nanocatalyst (12) | 110 | 65 |
| 6 | Magnetic MIL-101 SO3H nanocatalyst (18) | 110 | 75 |
| 7 | Magnetic MIL-101 SO3H nanocatalyst (24) | 110 | 85 |
| 8 | Magnetic MIL-101 SO3H nanocatalyst (30) | 110 | 95 |
With the optimized reaction conditions in hand, we tested the scope of the reaction for the synthesis of different 2,4,6-triaryl pyridine derivatives using acetophenones and benzaldehydes with a wide array of functional groups on the aromatic ring. All products were obtained in good to excellent yields (Table 5).
| Entry | R1 | R2 | Time (h) | Yieldb (%) | Product | Mp (°C)/reference |
|---|---|---|---|---|---|---|
| a Reaction conditions: phenyl methyl ketone (3.00 mmol), catalyst (30 mg), toluene (5 mL) 110 °C.b Isolated yield. | ||||||
| 1 | H | H | 2 | 90 | 4a | 135–137 (ref. 37) |
| 2 | H | 4-CH3 | 2.5 | 85 | 4b | 119–121 (ref. 37) |
| 3 | H | 4-Cl | 2.5 | 85 | ac | 125–127 (ref. 37) |
| 4 | 4-CH3 | H | 3 | 80 | 4d | 154–156 (ref. 42) |
| 5 | 4-CH3 | 4-CH3 | 3 | 80 | 4e | 175–177 (ref. 42) |
| 6 | 4-Cl | H | 3 | 85 | 4f | 186–188 (ref. 42) |
| 7 | 4-Cl | 4-OCH3 | 5 | 80 | 4g | 190–192 (ref. 42) |
Based on the previous reports,45,46 a proposed reaction pathway for the synthesis of 2,4,6-triaryl pyridine is shown in Scheme 2. Initially, acetophenone (A) in the presence of catalyst is converted to its enol form and consequently, gives nucleophilic addition to the benzaldehyde (B) to generate Aldol condensation product (C). After that, enamine (D) forms from the reaction of acetophenone and ammonia source, which attack to the α,β unsaturated carbonyl compound (C) in a Micheal addition to produce intermediate (E). Then cyclization lead to dihydropyridine (F) and finally aerobic oxidation generated 2,4,6-triphenylpyridine. As illustrated in Scheme 2, magnetic MIL-101-SO3H nanocatalyst plays an important role to accelerate this transformation.
The recyclability of the magnetic MIL-101-SO3H nanocatalyst was surveyed for the synthesis of the 1,3,5-triarylbenzenes and 2,4,6-triaryl pyridines under the optimized conditions. After the reaction time, the nanocatalyst was separated by an external magnet, washed, dried and reused in the next run. It was observed that in the next three consecutive uses of the magnetic MIL-101-SO3H nanocatalyst, the catalytic activity did not significantly decrease (Fig. 5).
 | ||
| Fig. 5 Recycle of the magnetic MIL-101-SO3H nanocatalyst for the synthesis of the 1,3,5-triarylbenzenes. | ||
4. Conclusion
The magnetic MIL-101-SO3H nanocatalyst was prepared by a uniform distribution of Fe3O4 nanoparticles within the pores of MIL-101. The structure of the catalyst was confirmed by the XRD, FT-IR, EDS and the SEM. The new nanocatalyst successfully was used for the synthesis of the 1,3,5-triarylbenzenes and 2,4,6-triaryl pyridines with fairly good yields. The magnetic MIL-101-SO3H nanocatalyst showed advantages such as recyclability, magnetic separability, good chemical stability, low solubility in organic solvents, and high surface area. Further work to prepare new catalysts based on MOFs for organic transformation is currently investigation in our laboratory.Acknowledgements
We gratefully acknowledge financial support from the Catalyst Center of Excellence (CCE) of Shahid Beheshti University and the Iran National Elites Foundation (INEF).References
- S. Qiu, M. Xue and G. Zhu, Chem. Soc. Rev., 2014, 43, 6116–6140 RSC.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed.
- A. H. Chughtai, N. Ahmad, H. A. Younus, A. Laypkov and F. Verpoort, Chem. Soc. Rev., 2015, 44, 6804–6849 RSC.
- H. Fei and S. M. Cohen, J. Am. Chem. Soc., 2015, 137, 2191–2194 CrossRef CAS PubMed.
- B. Wang, P. Yang, Z.-W. Ge and C.-P. Li, Inorg. Chem. Commun., 2015, 61, 13–15 CrossRef CAS.
- A. Dhakshinamoorthy, A. M. Asiri and H. Garcia, Chem. Soc. Rev., 2015, 44, 1922–1947 RSC.
- C. Bai, A. Li, X. Yao, H. Liu and Y. Li, Green Chem., 2016, 18, 1061–1069 RSC.
- H. Fei, J. Shin, Y. S. Meng, M. Adelhardt, J. Sutter, K. Meyer and S. M. Cohen, J. Am. Chem. Soc., 2014, 136, 4965–4973 CrossRef CAS PubMed.
- J. Tang, M. Yang, M. Yang, J. Wang, W. Dong and G. Wang, New J. Chem., 2015, 39, 4919–4923 RSC.
- O. I. Lebedev, F. Millange, C. Serre, G. Van Tendeloo and G. Férey, Chem. Mater., 2005, 17, 6525–6527 CrossRef CAS.
- Q. Liu, L. Ning, S. Zheng, M. Tao, Y. Shi and Y. He, Sci. Rep., 2013, 3, 2916 Search PubMed.
- A. Gomez-Paricio, A. Santiago-Portillo, S. Navalon, P. Concepcion, M. Alvaro and H. Garcia, Green Chem., 2016, 18, 508–515 RSC.
- A. Santiago-Portillo, S. Navalón, F. G. Cirujano, F. X. L. i. Xamena, M. Alvaro and H. Garcia, ACS Catal., 2015, 5, 3216–3224 CrossRef CAS.
- E. Kockrick, T. Lescouet, E. V. Kudrik, A. B. Sorokin and D. Farrusseng, Chem. Commun., 2011, 47, 1562–1564 RSC.
- W. Salomon, C. Roch-Marchal, P. Mialane, P. Rouschmeyer, C. Serre, M. Haouas, F. Taulelle, S. Yang, L. Ruhlmann and A. Dolbecq, Chem. Commun., 2015, 51, 2972–2975 RSC.
- M. Saikia and L. Saikia, RSC Adv., 2016, 6, 14937–14947 RSC.
- Q. Yang, Y.-Z. Chen, Z. U. Wang, Q. Xu and H.-L. Jiang, Chem. Commun., 2015, 51, 10419–10422 RSC.
- Y.-Z. Chen, Y.-X. Zhou, H. Wang, J. Lu, T. Uchida, X. Qiang, S.-H. Yu and H.-L. Jiang, ACS Catal., 2015, 5, 2062–2069 CrossRef CAS.
- P. Deria, W. Bury, I. Hod, C.-W. Kung, O. Karagiaridi, J. T. Hupp and O. K. Farha, Inorg. Chem., 2015, 54, 2185–2192 CrossRef CAS PubMed.
- J. Chen, R. Liu, H. Gao, L. Chen and D. Ye, J. Mater. Chem. A, 2014, 2, 7205–7213 CAS.
- M. G. Goesten, J. Juan-Alcañiz, E. V. Ramos-Fernandez, K. B. Sai Sankar Gupta, E. Stavitski, H. van Bekkum, J. Gascon and F. Kapteijn, J. Catal., 2011, 281, 177–187 CrossRef CAS.
- B. Li, Y. Zhang, D. Ma, L. Li, G. Li, G. Li, Z. Shi and S. Feng, Chem. Commun., 2012, 48, 6151–6153 RSC.
- Y. X. Zhou, Y. Z. Chen, Y. Hu, G. Huang, S. H. Yu and H. L. Jiang, Chem.–Eur. J., 2014, 20, 14976–14980 CrossRef CAS PubMed.
- Y. Qi, Y. Luan, X. Peng, M. Yang, J. Hou and G. Wang, Eur. J. Inorg. Chem., 2015, 2015, 5099–5105 CrossRef CAS.
- B. Li, K. Leng, Y. Zhang, J. J. Dynes, J. Wang, Y. Hu, D. Ma, Z. Shi, L. Zhu and D. Zhang, J. Am. Chem. Soc., 2015, 137, 4243–4248 CrossRef CAS PubMed.
- M. He, J. Zhou, J. Chen, F. Zheng, D. Wang, R. Shi, Z. Guo, H. Wang and Q. Chen, J. Mater. Chem. B, 2015, 3, 9033–9042 RSC.
- Y.-F. Huang, Y.-Q. Wang, Q.-S. Zhao, Y. Li and J.-M. Zhang, RSC Adv., 2014, 4, 47921–47924 RSC.
- M. Saikia, D. Bhuyan and L. Saikia, New J. Chem., 2015, 39, 64–67 RSC.
- Z. Jiang and Y. Li, J. Taiwan Inst. Chem. Eng., 2016, 59, 373–379 CrossRef CAS.
- Y. Zhao, J. Li, C. Li, K. Yin, D. Ye and X. Jia, Green Chem., 2010, 12, 1370–1372 RSC.
- F. Ono, Y. Ishikura, Y. Tada, M. Endo and T. Sato, Synlett, 2008, 2008, 2365–2367 CrossRef.
- Z. Li, W.-H. Sun, X. Jin and C. Shao, Synlett, 2001, 2001, 1947–1949 CrossRef.
- R. Tayebee and M. Jarrahi, RSC Adv., 2015, 5, 21206–21214 RSC.
- C. Allais, J.-M. Grassot, J. Rodriguez and T. Constantieux, Chem. Rev., 2014, 114, 10829–10868 CrossRef CAS PubMed.
- M. Adib, N. Ayashi and P. Mirzaei, Synlett, 2016, 27, 417–421 CrossRef CAS.
- P. V. Shinde, V. B. Labade, J. B. Gujar, B. B. Shingate and M. S. Shingare, Tetrahedron Lett., 2012, 53, 1523–1527 CrossRef CAS.
- H. Huang, X. Ji, W. Wu, L. Huang and H. Jiang, J. Org. Chem., 2013, 78, 3774–3782 CrossRef CAS PubMed.
- J. Safari, Z. Zarnegar and M. Borujeni, Chem. Pap., 2013, 67, 688–695 CAS.
- J. Safari, S. Gandomi-Ravandi and M. Borujeni, J. Chem. Sci., 2013, 125, 1063–1070 CrossRef CAS.
- A. Shaabani, M. B. Boroujeni and M. H. Sangachin, J. Chem. Sci., 2015, 127, 1927–1935 CrossRef CAS.
- A. Shaabani, M. Borjian Boroujeni and M. S. Laeini, Appl. Organomet. Chem., 2016, 30, 154–159 CrossRef CAS.
- A. Shaabani, M. Borjian Boroujeni and M. S. Laeini, RSC Adv., 2016, 6, 27706–27713 RSC.
- A. Shaabani, Z. Hezarkhani and M. K. Nejad, RSC Adv., 2016, 6, 30247–30257 RSC.
- L. Bromberg, Y. Diao, H. Wu, S. A. Speakman and T. A. Hatton, Chem. Mater., 2012, 24, 1664–1675 CrossRef CAS.
- E. Tabrizian, A. Amoozadeh, S. Rahmani, E. Imanifar, S. Azhari and M. Malmir, Chin. Chem. Lett., 2015, 26, 1278–1282 CrossRef CAS.
- A. Davoodnia, M. Bakavoli, R. Moloudi, N. Tavakoli-Hoseini and M. Khashi, Monatsh. Chem., 2010, 141, 867–870 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra24574a |
| This journal is © The Royal Society of Chemistry 2016 |