Electrochemical determination of 2,4-dichlorophenol at β-cyclodextrin functionalized ionic liquid modified chemical sensor: voltammetric and amperometric studies
Abstract
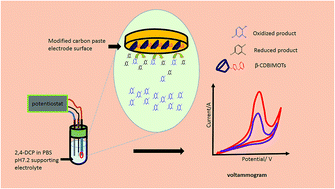
A highly effective approach was developed for the specific detection of 2,4-dichlorophenol (2,4-DCP) in real samples, based on a cyclodextrin functionalized ionic liquid modified carbon paste electrode (β-CD-BIMOTs/CPE). First, systematic optimization procedures were carried out for β-CD-BIMOTs/CPE by cyclic voltammetry (CV) and chrono-amperometry methods. Second, the optimized methods were applied for the determination of 2,4-DCP in environmental samples. A large increase in the peak currents was observed in CV and chrono-amperometry of 2,4-DCP when using β-CD-BIMOTs/CPE compared to native cyclodextrin modified carbon paste electrode (β-CD/CPE) and bare carbon paste electrode (CPE). A comparison of voltammetric behavior between CPE, β-CD/CPE and β-CD-BIMOTs/CPE indicated that the combination of ionic liquid and cyclodextrin in the composition of the carbon paste significantly improved the conductivity and compatibility of the sensor. The introduction of β-CD-BIMOTs as a modifier results in clearly enhanced sensitivity and selectivity towards 2,4-DCP over a wide concentration range of 4 μmol L−1 to 100 μmol L−1, with a detection limit of 1.2 μmol L−1. The interferences from foreign substances in the peak current response were also studied and caused only minor changes in the signals (<4.6%). Good repeatability was achieved because of the stability of the modified electrode. The proposed method was applied to the determination of 2,4-DCP in leachates from landfill, mineral water and lake water with recoveries of (85.46–117.68%), (98.72–107.91%) and (89.25–107.46%), respectively.

 Please wait while we load your content...
Please wait while we load your content...