Self assembly and hydrogelation of spermine functionalized aromatic peptidomimetics against planktonic and sessile methicillin resistant S. aureus†
Abstract
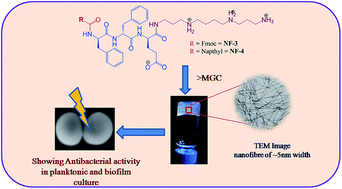
Peptide amphipathicity is a common characteristic of self assembling peptide nanomaterials and host defense cationic antimicrobial peptides. In the present work, we utilized amphipathicity of ultra short aromatic sequences with spermine as a cationic C-terminal tag for designing of antibacterial hydrogels against multidrug resistant bacteria. These designed peptidomimetics acquired single walled-nanofibres and nanotubes that formed hydrogels at higher concentrations between 0.5–1% w/v at physiological pH. In vitro antibacterial studies of the nanofibres exhibited potent activity (MIC: 14.2–227.2 μg mL−1) against Gram positive and Gram negative bacterial strains including clinically relevant pathogens such as methicillin resistant S. aureus (MRSA) and S. epidermidis. Bactericidal kinetic and scanning electron microscopy of peptidomimetics treated MRSA confirms the membrane active mode of action. These analogues also displayed a strong antibiofilm activity at the MIC level and demonstrated reduced viability of mature MRSA by >50% biofilms at 0.5–2% w/v hydrogels. Moreover, the nanofibres were neither cytotoxic nor hemolytic in nature. With selective modes of interaction of the designed nanostructure with bacteria these hydrogels could be potentially therapeutic against MRSA wound infections and also as implantable biomaterials.

 Please wait while we load your content...
Please wait while we load your content...