Co-Delivery of angiostatin and curcumin by a biodegradable polymersome for antiangiogenic therapy
Yue Caoa,
Yan Lic,
Yin Wuc,
Wenliang Lic,
Chunlei Yuc,
Yanxin Huangb,
Luguo Sun *b,
Yongli Bao*c and
Yuxin Li*a
*b,
Yongli Bao*c and
Yuxin Li*a
aNational Engineering Laboratory for Druggable Gene and Protein Screening, Northeast Normal University, Jingyue Street 2555, Changchun 130117, P. R. China. E-mail: liyx486@nenu.edu.cn; Fax: +86-431-89165917; Tel: +86-431-89165917
bInstitute of Genetics and Cytology, Northeast Normal University, Renmin Street 5268, Changchun 130024, P. R. China. E-mail: sunlg388@nenu.edu.cn; Fax: +86-431-89165922; Tel: +86-431-89165922
cSchool of Life Sciences, Northeast Normal University, Renmin Street 5268, Changchun 130024, P. R. China. E-mail: baoyl800@nenu.edu.cn; Fax: +86-431-89165920; Tel: +86-431-89165920
First published on 26th October 2016
Abstract
Co-Encapsulated angiostatin (AS) and curcumin (Cur) in poly(ethylene glycol)-b-poly(ε-caprolactone) polymersomes (PEG–PCL-PMs) were prepared by film hydration method. The size and zeta potential of the prepared PEG–PCL-PMs were about 153 nm and −0.22 mV, respectively. Transmission electron microscopy was employed to confirm the polymersomal structure of PEG–PCL-PMs. In vitro drug release profiles showed a sustained release of angiostatin and curcumin from the dual-drug loaded polymersomes under physiological conditions (pH 7.4). In addition, confocal laser scanning microscopy and flow cytometry showed that drug-loaded polymersomes were delivered efficiently into the cytoplasm and nuclei of human microvascular endothelial cell line (HMEC-1) and then released active drugs in cells. Dual drug-loaded polymersomes (AS–Cur-PMs) remarkably inhibited proliferation and migration of HMEC-1 cells compared to those treated with the single drug. The chick chorioallantoic membrane assay showed that AS–Cur-PMs inhibited vessel sprouting in the chicken chorioallantoic membrane. Our data demonstrates a synergistic effect of angiostatin and curcumin in inhibiting angiogenesis, both in vitro and in vivo.
1. Introduction
The growth of solid tumors is predicated on the generation of new blood vessels for nutrition and oxygen supply.1 Therefore, antiangiogenic therapy has been proposed as a promising strategy for treatment of various neoplasms.2 A number of antiangiogenic agents from hydrophilic endogenous inhibitors to hydrophobic chemotherapeutic agents have been explored. Among angiogenic inhibitors, angiostatin is a proteolytic fragment of plasminogen, which was originally isolated and purified from serum and urine of tumor-bearing animals.3 Angiostatin was shown to restrain the growth of transplanted human breast, colon, pancreatic and prostate cancer cells in mice through inhibition of endothelial cell proliferation and migration.4 However, its short half-life, molecular instability, and high cost limit its clinical applications.5 Therefore, it is worthwhile to develop an appropriate delivery system, which can improve the bioavailability of angiostatin.In the past few years, nanocarriers have been widely investigated for drug-delivery (including in cancer therapy) because of their ability to improve drug bio-distribution and stability, prolong half-life, and preferential accumulation in tumor tissues via the enhanced permeation and retention effect (EPR).6–8
Recent work suggested that curcumin, extracted from the turmeric of Curcuma longa,9 has a remarkable anti-carcinogenic activity against several types of tumors, including, skin, breast, and neurological cancers.10–14 The broad spectrum of anticancer activity of curcumin may be due in part to inhibition of angiogenesis.15–18 Curcumin has lower intrinsic toxicity among the chemotherapeutic agents. Oral administration of curcumin at a dose of 12 g per day was shown to be well tolerated.15 However, low water-solubility, poor stability, and rapid systemic elimination are some of its shortcomings.19,20
Combination therapy is a good choice for cancer treatment, because it can address multiple mechanisms.21 Previous studies have demonstrated that angiostatin combined with other cancer therapeutics including radiotherapy, chemotherapy and immunotherapy, provide better therapeutic effect.22–25
Polymersomes (PMs), a type of self-assembled polymeric vesicles, represent a novel class of nanocarriers.26,27 PMs encapsulate hydrophilic drugs in their aqueous cores and simultaneously integrate hydrophobic drugs in their membrane.28–32 Hence, PMs are very useful for delivering combination therapy to enhance the therapeutic efficacy of anticancer drugs.
In this study, poly(ethylene glycol)-b-poly(ε-caprolactone) (PEG–PCL) diblock copolymers were designed and prepared to assemble polymersomes for the combinatorial delivery of angiostatin and curcumin. Polymersomes were characterized using transmission electron microscope (TEM), dynamic light scattering (DLS), static light scattering (SLS) and zeta potential. In vitro cytotoxicity of dual-drug loaded polymersomes was evaluated using the HMEC-1. Anti-migration and anti-angiogenesis efficacy were analyzed by scratch assay and the chick chorioallantoic membrane (CAM) assay, respectively.
2. Materials and methods
2.1 Materials
Methoxyl poly(ethylene glycol) (mPEG, Mn = 5 kDa), ε-caprolactone (ε-CL), stannous octoate, fetal bovine serum (FBS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium-bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Angiostatin kringles 1 to 3 (K1–3) were obtained from our laboratory (Northeast Normal University, China). Curcumin was ordered from Aladdin (Shanghai, China). HMEC-1 cell line was obtained from National Institute for the Control of Pharmaceutical and Biological Products. BCA™ Protein Assay Kit and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Beyotime (Shanghai, China). Other chemicals used were of analytical grade. Specific-pathogen-free (SPF) Leghorn fertile chicken eggs were ordered from a local hatchery (Changchun, China).2.2 Characterization
Morphological examination of polymersomes was performed using a transmission electron microscopy JEOL 1010. The size and size distribution of polymersomes were determined by a WyattQELS dynamic light scattering (DLS) instrument. Static light scattering (SLS) experiments was performed on an ALV goniometer. Zeta potentials (ζ-potential) of the PMs were measured using a 90Plus particle size analyzer. The molecular weight and polydispersity index (PDI) of the copolymer was determined using a Waters 1515 gel permeation chromatograph instrument system (GPC). The critical aggregation concentration (CAC) of the polymersome was measured by fluorescence measurements in the presence of pyrene molecules. The fluorescence spectra were obtained with a PerkinElmer LS50B luminescence spectrometer. Confocal laser scanning microscope (CLSM) images were captured on a Carl Zeiss LSM 780 confocal microscope.2.3 Synthesis of mPEG-b-PCL
Poly(ethylene glycol)-b-poly(ε-caprolactone) (PEG–PCL) was synthesized by ring-opening polymerization of ε-CL using mPEG as the initiator in the presence of stannous octoate as a catalyst.33 The molecular weight and composition of mPEG-b-PCL were determined by 1H NMR in CDCl3.2.4 Formation of blank polymersomes
Polymersomes were prepared using the film hydration method.34,35 Briefly, 100 mg PEG–PCL diblock copolymer was dissolved in 20 mL dichloromethane (DCM). Then, the solvent was slowly removed by a rotary evaporator to form a thin film. To this, 20 mL of MES buffer (20 mM, pH 5.4) was added. Finally, the mixture was stirred for 12 h at 60 °C. The formed polymersomes were further purified by density gradient centrifugation method.362.5 Determination of critical aggregation concentration
The critical aggregation concentration of the copolymer was determined using pyrene as a fluorescence probe. The initial copolymers were dissolved in MES buffer. Pyrene in acetone (3 × 10−5 mol L−1) was evaporated in vials. Then, the above fully stirred aqueous polymer solutions were added. The fluorescence spectra were performed at an excitation wavelength of 390 nm and the emission fluorescence at 336 and 339 nm at room temperature were monitored.2.6 Preparation of drug-loaded polymersomes and determination of loading efficiency
Angiostatin were loaded onto polymersomes by film hydration method. The polymersomes were prepared as previously described except that MES solutions (pH 5.4, 20 mM) of angiostatin were applied. Free angiostatin was removed by dialysis against the same MES for 12 h at room temperature, with change of media every three hours. To obtain dual-drug loaded polymersomes (AS–Cur-PMs), curcumin solution in DMSO was added to the above solution at a curcumin to copolymer feed ratio of 10 wt% and then stirred for 3 h at 37 °C. Free curcumin was removed by dialysis (MWCO 3500) against distilled water for 24 h. Based on film hydration method, single drug formulations (AS-PMs, Cur-PMs) were synthesized.To determine angiostatin loading content and loading efficiency, angiostatin-loaded polymersomes were disrupted by adding three times the volume of DCM, which led to complete release of loaded angiostatin. The amount of loaded angiostatin was determined by BCA assays according to the manufacturers' protocol.
The amount of curcumin was determined by fluorescence measurements at 425 nm after dissolving the curcumin-loaded polymersome solution in 3-fold DCM. A standard curve was established on fluorometry with known concentrations Cur in DCM/H2O (3/1 v/v). Each experiment was conducted in triplicate. The drug loading content (DLC%) and the drug loading efficiency (DLE%) were calculated by the following equations:
| DLC (%) = (amount of drug in polymersome/amount of drug loaded polymersome) × 100% |
| DLE (%) = (amount of drug in polymersome/amount of drug in feed) × 100% |
2.7 In vitro drug release
In vitro drug release of angiostatin and curcumin from AS–Cur-PMs was performed in triplicate in PBS (pH 7.4, 20 mM). 100 mg of freeze-dried sample was suspended in 10 mL of PBS in a centrifuge tube and the tube was kept in a 37 °C water bath shaker with continuous shaking at 120 rpm. At desired time intervals, this tube was centrifuged at 2000 rpm for 10 min. The supernatant was taken out and the tube replenished with an equal volume of fresh buffer. The obtained supernatants were separated into two tubes and kept at −70 °C. One was used to measure the released angiostatin on BCA assays following the procedure of angiostatin load test; and the other was used to measure the released curcumin by fluorescence measurement following the procedure of curcumin load test.2.8 Intracellular drug release
2.9 Inhibition of cell viability assay
The relative inhibition of viability of HMEC-1 by polymersomes was evaluated in vitro by MTT assay. Generally, the cells were cultured in a 96-well plate at 6 × 103 cells per well in 200 μL of DMEM medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin/streptomycin (100 units per mL) at 37 °C and 5% CO2 for 24 h. Varying concentrations of blank PMs and drug-loaded PMs (AS-PMs, Cur-PMs and AS–Cur-PMs) were added. MTT assay was performed at 24, 48 and 72 h of incubation. The absorbance of the solution was measured on a Bio-Rad 680 microplate reader at 570 nm. Each experiment was performed for three times.2.10 Scratch assay
Since migration is considered to be a decisive step for progress of cancer metastasis,37 the influence of four types of polymersomes (PMs, AS-PMs, Cur-PMs and AS–Cur-PMs) on inhibition of cell migration was evaluated. Scratch assay was performed using a protocol described elsewhere.38 HMEC-1 cells were seeded onto 6-well plates supplemented with 2 mL of growth medium and incubated to form a confluent monolayer. A scratch was made on cell monolayer using a sterile 200 mL pipette tip and cells were washed several times with PBS to remove detached cells. After that, fresh growth medium with PMs was added and the cells incubated for 24 h. Microscopic images were acquired by OLYMPUS phase contrast microscope (10 × 20).2.11 Chick embryo chorioallantoic membrane assay
The CAM assay was performed using the improved CAM technique described elsewhere.39 Fertilized chicken eggs were incubated at 37 °C for 7 days at a humidity level of 90%. A window (diameter 10 × 10 mm) was drilled in the eggshell over the air sac region and the chorioallantoic membranes exposed. Sterilized filter paper discs loaded with PMs, AS-PMs, Cur-PMs and AS–Cur-PMs were placed in the CAM. The eggs were resealed by Scotch tape and returned to the incubator. After incubation for 48 h, digitized CAM images were recorded using a digital camera.3. Results and discussion
3.1 Characterization of diblock copolymers
The mPEG-b-PCL copolymers were synthesized via ring-opening polymerization of ε-CL in anhydrous conditions. Fig. 1A exhibits the 1H NMR spectrum of mPEG–PCL. The peak at 3.61 ppm (peak a) corresponds to the methylene unit in the PEG segment. The peaks of the PCL segment at 4.06 ppm (peak f), 2.30 ppm (peak b), 1.64 ppm (peaks c and e) and 1.38 ppm (peak d) were also identified in the 1H-NMR spectrum. The Mn value of PCL was 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600, which was estimated by comparing the integrals of signals at 4.06 ppm and 3.61 ppm. By GPC analysis the copolymer gives a Mn = 16
600, which was estimated by comparing the integrals of signals at 4.06 ppm and 3.61 ppm. By GPC analysis the copolymer gives a Mn = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 g mol−1 with a polydispersity index (PDI) of 1.48. GPC further confirmed that these block copolymers had controlled molecular weights.
700 g mol−1 with a polydispersity index (PDI) of 1.48. GPC further confirmed that these block copolymers had controlled molecular weights.
 | ||
| Fig. 1 (A) 1H NMR spectrum (400 MHz, CDCl3) of mPEG5k–PCL12k, the property of blank polymersomes were examined by TEM (B) and DLS (C), (D) the CAC of polymersomes. | ||
3.2 Characterization of blank polymersomes
Due to a rather low weight fraction of hydrophilic blocks (fphil = 29 wt%), formation of polymersomes is preferred.40–43 TEM confirmed that PEG5k–PCL12k formed a hollow vesicular structure with an average diameter of about 140 nm (Fig. 1B). The hydrodynamic radii (Rh) measured on DLS was approximately 76.6 nm (Fig. 1C). The smaller size on TEM analysis is on account of the dehydration of the polymersomes during TEM sample preparation. The gyration radius (Rg) determined by SLS was 81.9 nm. The ρ-value (ρ = Rg/Rh) of 1.07 indicated a hollow sphere structure.44In addition, the zeta potential measurements demonstrated that the surface charge of PEG–PCL polymersomes (−0.22 mV) was close to neutral, which augments drug stability and prolongs its circulation in blood.45 The critical aggregation concentration (CAC) determined using pyrene for fluorescence displayed that these PEG–PCL polymersomes had low CAC of 0.00722 mg mL−1 (Fig. 1D).
3.3 Loading and in vitro drug release
Experiments were conducted to evaluate the capacity of PEG–PCL polymersomes for drug entrapment. The DLC of AS-loaded PM and Cur-loaded PM was 5.9% and 5.0%, respectively. DLE was 63.7% and 54.2%, respectively. In dual-drug loaded PM, the DLC and DLE were 5.7% and 62.7% for angiostatin, and 4.37% and 48.1% for curcumin, respectively. The loading efficiency of AS was higher than that of Cur, which is attributable to the large aqueous core in the polymersomes (Fig. 2).In vitro release behavior of AS and Cur from AS–Cur-PMs was investigated in PBS (pH 7.4) over a period of 72 h. Fig. 2 shows the release and slow leakage kinetics of water soluble AS and insoluble Cur. At 24 h, the release of AS and Cur were 50.7% and 59.8%, respectively, which was followed by a slow and steady release. At the end of the test, the amount of AS and Cur released were about 62.4% and 67.9%, respectively.
3.4 Cellular uptake and intracellular release
Taking advantage of the intrinsic fluorescence of curcumin, CLSM and flow cytometric analyses were performed to analyze the cellular uptake of curcumin in HMEC-1 cells. As shown in (Fig. 3), AS–Cur-PMs showed obvious fluorescence in cytoplasm and nuclei of cells. Additionally, stronger Cur fluorescence was observed in the cells incubated with AS–Cur-PMs for 4 h as compared to those treated for 2 h. The cellular uptake study demonstrated that PEG–PCL polymersomes successfully transport the drug into the cytoplasm and nuclei of HMEC-1 cells.3.5 Cellular proliferation inhibition
MTT assays were performed to evaluate relative ability of the various polymersomes (PMs, AS-PMs, Cur-PMs and AS–Cur-PMs) to inhibit the growth of endothelial cells (HMEC-1). As shown in Fig. 4, the inhibition of proliferation of endothelial cells by polymersomes was increased when cells were treated with AS–Cur-PMs. These results demonstrated that the angiostatin specially inhibited the proliferation of endothelial cells.46,47 Furthermore, the combination of angiostatin and curcumin showed synergistic inhibitory effect on endothelial cell proliferation.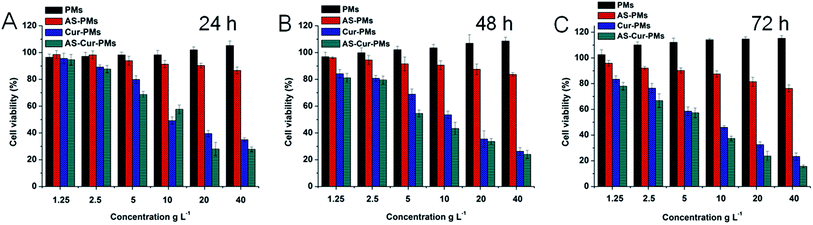 | ||
| Fig. 4 Inhibition of cell viability of blank and drug-loaded polymersomes to HMEC-1 cells after incubation for 24 h (A), 48 h (B), and 72 h (C). | ||
3.6 Scratch assay
In vitro scratch assay was performed in order to investigate the migration potential of HMEC-1 cells. This assay mimics the cell migration progression in vivo. In the assay, HMEC-1 monolayers were scratched as described, and treated with blank and drug-loaded PMs. When comparing the images taken at the time of initial scratching (0 h) with those taken 24 h later, many HMEC-1 cells had migrated into the denuded areas in the blank polymersome group (Fig. 5). The scratch closure in the dual-drug loaded polymersome treatment groups was significantly lower than that in the single-drug loaded polymersome treated group. These results demonstrate the potential for combined use of angiostatin and curcumin in inhibiting HMEC-1 migration in vitro.3.7 CAM assay
Inhibition of angiogenesis is an important strategy for arresting the growth of tumors. CAM assay is a rapid and reproducible method to study angiogenesis.48 The CAM assay was performed with 20 embryos in each group. As shown in Fig. 6, the inhibition of AS-PMs, Cur-PMs and AS–Cur-PMs inhibited the formation of new blood vessels and branches reaching 46.1%, 56.3% and 76.1% respectively. Our results demonstrated the synergistic effect of co-encapsulated AS and Cur in inhibiting angiogenesis in vivo. | ||
| Fig. 6 Inhibition of angiogenesis by PMs (A), AS-PMs (B), Cur-PMs (C) and AS–Cur-PMs (D) on the CAMs. (E) Quantification of the number of CAM. | ||
4. Conclusion
In this paper, we report the encapsulation of two drugs with different properties (AS and Cur, hydrophilic and hydrophobic molecules, respectively) into PEG–PCL polymersomes. The behavior of dual-drugs loaded polymersomes were studied, both in vitro and in vivo. The results showed that the dual-drug loaded system (AS–Cur-PMs) can inhibit endothelial cell proliferation and migration more effectively as compared to use of single-drug loaded polymersome (AS-PMs, Cur-PMs). The ability of AS–Cur-PMs to inhibit the generation of new blood capillaries indicates its antiangiogenic potential. To conclude, co-delivery of angiostatin and curcumin by polymersomes is envisioned as a promising strategy for synergistic enhancement of antiangiogenic activity and thus has further potential in cancer treatment.Acknowledgements
This study was supported by grants from the following foundations: the National Natural Science Foundation of China (No. 81272919, 81272242, 81502284, 51403031), the Fundamental Research Funds for the Central Universities, the Research Foundation of JiLin Provincial Science & Technology Development (No. 20150101188JC, 20150309003YY, 20140203008YY, 20140520049JH 20130201008ZY).References
- J. Folkman, Cancer Res., 1983, 36, 201–208 CAS.
- C. Y. Gong, S. Y. Deng and Q. J. Wu, Biomaterials, 2013, 34, 1413–1432 CrossRef CAS PubMed.
- M. S. O'Reilly, L. Holmgren and Y. Shing, Cell, 1994, 79, 315–328 CrossRef.
- L. Claesson-Welsh, M. Welsh, N. Ito, B. Anand-Apte, S. Soker, B. Zetter, M. S. O'Reilly and J. Folkman, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 5579–5583 CrossRef CAS.
- C. Ramachandran and W. You, Breast Cancer Res. Treat., 1999, 54, 269–278 CrossRef CAS PubMed.
- J. S. Lee and J. Feijen, J. Controlled Release, 2012, 161, 473–483 CrossRef CAS PubMed.
- O. C. Farokhzad and R. Langer, ACS Nano, 2009, 3, 16–20 CrossRef CAS PubMed.
- Y. Kim, M. Tewari, J. D. Pajerowski, S. Cai, S. Sen and J. Williams, J. Controlled Release, 2009, 134(2), 132–140 CrossRef CAS PubMed.
- A. E. Gururaj, M. Belakavadi and D. A. Venkatesh, Biochem. Biophys. Res. Commun., 2002, 297, 934–942 CrossRef CAS PubMed.
- P. Anand, C. Sundaram, S. Jhurani, A. B. Kunnumakkara and B. B. Aggarwal, Cancer Lett., 2008, 267, 133–164 CrossRef CAS PubMed.
- B. B. Aggarwal, A. Kumar and A. C. Bharti, Anticancer Res., 2003, 23, 363–398 CAS.
- A. Banerji, J. Chakrabarti and A. Mitra, Cancer Lett., 2004, 211, 235–242 CrossRef CAS PubMed.
- J. Ravindran, S. Prasad and B. B. Aggarwal, AAPS J., 2009, 11, 495–510 CrossRef CAS PubMed.
- J. L. Arbiser, N. Klauber, M. T. Huang, C. Fisher, E. Flynn and H. R. Byers, Mol. Med., 1998, 4, 376–383 CAS.
- Z. M. Shao, Z. Z. Shen and C. H. Liu, Int. J. Cancer, 2002, 98, 234–240 CrossRef CAS PubMed.
- D. Thalloor, A. K. Singh and G. S. Sidhu, Cell Growth Differ., 1998, 9, 305–312 Search PubMed.
- Y. G. Lin, A. B. Kunnumakkara and A. Nair, Clin. Cancer Res., 2007, 13, 3423–3430 CrossRef CAS PubMed.
- P. Anand, A. B. Kunnumakkara, R. A. Newman and B. B. Aggarwal, Mol. Pharm., 2007, 4807–4818 Search PubMed.
- R. K. Gangwar, G. B. Tomar, V. A. Dhumale, S. Zinjarde, R. B. Sharma and S. Datar, J. Agric. Food Chem., 2013, 61, 9632–9637 CAS.
- H. Bermudez, A. K. Brannan, D. A. Hammer, F. S. Bates and D. E. Discher, Macromolecules, 2002, 35, 8203–8208 CrossRef CAS.
- H. J. Mauceri, N. N. Hanna and M. A. Beckett, Nature, 1998, 394(6690), 287–291 CrossRef CAS PubMed.
- H. J. Mauceri, S. Seetharam, M. A. Beckett and R. Heimann, Cancer Chemother. Pharmacol., 2002, 50(5), 412–418 CrossRef CAS PubMed.
- K. Eriksson, P. Magnusson and J. Dixelius, FEBS Lett., 2003, 536, 19–24 CrossRef CAS PubMed.
- S. Gyorffy, K. Palmer, T. J. Podor, M. Hitt and J. Gauldie, J. Immunol., 2001, 166(10), 6212–6217 CrossRef CAS.
- V. P. Torchilin and A. N. Lukyanov, Drug Discovery Today, 2003, 8(6), 259–266 CrossRef CAS PubMed.
- J. Gaitzsch, D. Appelhans, D. Gräfe, P. Schwille and B. Voit, Chem. Commun., 2011, 47, 3466–3468 RSC.
- D. Grafe, J. Gaitzsch, D. Appelhans and B. Voit, Nanoscale, 2014, 6, 10752–10761 RSC.
- K. Kita-Tokarczyk, J. Grumelard, T. Haefele and W. Meier, Polymer, 2005, 3540–3563 CrossRef CAS.
- P. Tanner, S. Egli, V. Balasubramanian, O. Onaca, C. G. Palivan and W. Meier, FEBS Lett., 2011, 1699–1760 CrossRef CAS PubMed.
- L. Messager, J. Gaitzsch, L. Chierico and G. Battaglia, Curr. Opin. Pharmacol., 2014, 18, 104–111 CrossRef CAS PubMed.
- J. Gaitzsch, X. Huang and B. Voit, Chem. Rev., 2016, 116, 1053–1093 CrossRef CAS PubMed.
- X. Y. Zhang, M. Lomora, T. Einfalt, W. Meier, N. Klein, D. Schneider and C. G. Palivan, Biomaterials, 2016, 89, 79–88 CrossRef CAS PubMed.
- Y. Na, Y. He, X. Shuai, Y. Kikkawa, Y. Doi and Y. Inoue, Biomacromolecules, 2002, 3, 1179–1186 CrossRef CAS PubMed.
- P. P. Ghoroghchian, P. R. Frail and K. Susumu, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 2922–2927 CrossRef CAS PubMed.
- G. Battaglia and A. Ryan, Nat. Mater., 2005, 4, 869–876 CrossRef CAS PubMed.
- J. D. Robertson, L. Rizzello, M. Avila-Olias, J. Gaitzsch, C. Contini, M. S. Magoń, S. A. Renshaw and G. Battaglia, Sci. Rep., 2016, 6, 27494 CrossRef CAS PubMed.
- A. M. Goodwin, Microvasc. Res., 2007, 74, 172–183 CrossRef CAS PubMed.
- S. C. Ricardo, M. G. Vera and B. Fátima, J. Funct. Foods, 2014, 11, 160–171 CrossRef.
- G. He, J. Luo, T. Zhang, H. Chen and C. Sun, Acta Sci. Nat. Univ. Sunyatseni, 2003, 42, 126–128 Search PubMed.
- A. Blanazs, S. P. Armes and A. J. Ryan, Macromol. Rapid Commun., 2009, 30(4–5), 267–277 CrossRef CAS PubMed.
- M. Avila-Olias, C. Pegoraro, G. Battaglia and I. Canton, Ther. Delivery, 2013, 4, 27–43 CrossRef CAS PubMed.
- G. Battaglia and A. J. Ryan, J. Am. Chem. Soc., 2005, 127, 8575–8764 Search PubMed.
- G. Battaglia, J. Phys. Chem. B, 2006, 110, 10272–10279 CrossRef CAS PubMed.
- O. Stauch, R. Schubert, G. Savin and W. Burchard, Biomacromolecules, 2002, 3, 565–578 CrossRef CAS PubMed.
- S. Honary and F. Zahir, Trop. J. Pharm. Res., 2013, 12, 265–273 Search PubMed.
- B. J. Annutti, S. T. Gately and M. E. Quevedo, Cancer Res., 1997, 57, 5277–5280 Search PubMed.
- W. H. Hou, T. Fang and L. X. Xue, Protein Expression Purif., 2006, 47, 93–98 CrossRef CAS PubMed.
- M. Richardson and G. Singh, Curr. Drug Targets: Cardiovasc. & Haematol. Disord., 2003, 3, 155–185 CAS.
| This journal is © The Royal Society of Chemistry 2016 |



