Efficient synthesis of novel 1,2,3-triazole-linked quinoxaline scaffold via copper-catalyzed click reactions†
Abstract
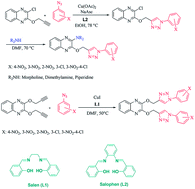
In this work, new derivatives of the 1,2,3-triazole-linked quinoxaline ring system are prepared by the reaction of 2-chloro-3-(prop-2-ynyloxy)quinoxaline or 2,3-bis(prop-2-ynyloxy)quinoxaline with aromatic azides via copper-catalyzed azide-alkyne cycloaddition reactions in the presence of the Schiff base ligands. These reaction procedures have the advantages of high-to-excellent yields, short reaction times, mild experimental conditions, and operational simplicity. The synthesized compounds were screened against the three bacterial strains Micrococcus luteus, Pseudomonas aeruginosa, and Bacillus subtilis. The anti-bacterial activity of 6b against P. aeruginosa was better than that for the standard drug (tetracycline).

 Please wait while we load your content...
Please wait while we load your content...