High-performance electrocatalyst for oxygen reduction reaction derived from copolymer networks and iron(II) acetate†
Mei Yanga,
Hongbiao Chen*a,
Duanguang Yanga,
Yong Gaoa and
Huaming Li*ab
aCollege of Chemistry, Xiangtan University, Xiangtan 411105, Hunan Province, P. R. China. E-mail: chhb606@163.com; lihuaming@xtu.edu.cn
bKey Laboratory of Polymeric Materials & Application Technology of Hunan Province, Key Laboratory of Advanced Functional Polymeric Materials of College of Hunan Province, Xiangtan University, Xiangtan 411105, Hunan Province, P. R. China
First published on 4th October 2016
Abstract
A novel non-precious-metal electrocatalyst for the oxygen reduction reaction (ORR) is prepared by the pyrolysis of copolymer networks (CPN) in the presence of iron(II) acetate. 2,4,6-Tripyrrol-1,3,5-triazine (TPT) and pyrrole (Py) are selected as the building blocks for the fabrication of a nitrogen-rich network copolymer that forms a self-supporting spherical structure and contains a high density of metal-coordination sites. The CPN is synthesized by a scalable, one-step Friedel–Crafts reaction of a TPT and Py mixture with dimethoxymethane. The as-prepared Fe–NTP/C-900 (pyrolyzed at 900 °C) exhibits high ORR activity in alkaline media with onset and half-wave potentials about 50 and 65 mV (vs. Ag/AgCl) higher than those of the Pt/C catalyst. Meanwhile, the Fe–NTP/C-900 catalyst shows relatively excellent ORR activity in acidic media with onset and half-wave potentials only 15 and 55 mV lower than those of the Pt/C catalyst. Overall, the as-synthesized Fe–NTP/C-900 is a potential candidate as a high activity, stable, and low-cost ORR catalyst for FCs.
Introduction
The sluggish kinetics of the oxygen reduction reaction (ORR) at the cathode is the current technological bottleneck in the energy conversion efficiency of fuel cells (FCs). So far, the best materials for catalysis of the ORR are platinum (Pt) and its alloys.1,2 However, Pt-based catalysts generally suffer from disadvantages such as high cost and scarcity, poor durability and being prone to methanol/CO poisoning, which hinder the development and long-term economic feasibility of FCs. To solve these problems, various non-precious-metal catalysts have been prepared and used as alternatives to Pt-based catalysts for the ORR. Among them, carbon-supported transition metal/nitrogen (M–N/C) materials have emerged as feasible substitutes, in terms of their ORR activities and stabilities in both alkaline and acidic media.3–7Numerous studies have shown that the ORR activity of the M–N/C catalysts has been greatly influenced by the types of transition-metal species and nitrogen-containing precursors. Up to present, only the M–N/C catalysts with Fe, Co, and Mn, have been found to possess excellent activity and durability.8–12 In the meantime, a series of N-containing polymers have been used in the preparation of M–N/C catalysts to replace the expensive macrocycles. Such N-containing polymers include polyacrylonitrile, polyimide, polypyrrole, polyaniline, poly(bis-2,6-diaminopyridinesulfoxide), polyporphine, formaldehyde-based resins (such as urea-formaldehyde, thiourea-formaldehyde, selenourea-formaldehyde, and melamine-formaldehyde resins), and N-containing heterocycle-metal coordination polymers.10,13–23 For the above-mentioned N-containing polymers, two main strategies including copyrolysis and direct pyrolysis have been applied to prepare M–N/C catalysts. The former method involves the physical blending of an N-rich polymer, a metal precursor, and a carbon support followed by copyrolysis at high temperature. In some cases, the N-containing polymer can be used as a self-supporting catalyst, hence dispensing with the need of carbon support. The latter approach involves the direct pyrolysis of N-containing heterocycle-metal coordination polymers without using transition-metal precursor or carbon support. Although this method prevents the migration and loss of metal in the pyrolysis process, makes it react smoothly with N and carbon to form active sites, hence enhancing the active site densities of the M–N/C catalysts, it is still quite difficult to synthesize such N-rich heterocycle-metal coordination polymers. In this regard, it is feasible to prepare the M–N/C catalysts by copyrolysis of N-rich polymer and metal precursor that can interact with each other.
In our previous article, we have systematically investigated the ORR activity of the Fe–N/C catalyst prepared by pyrolyzing the homopolymer of 2,4,6-tripyrrol-1,3,5-triazine (TPT) and Fe(OAc)2 mixture at 900 °C (Fe–NT/C-900). It was found that the Fe–NT/C-900 catalyst exhibited a high ORR activity with onset and half-wave potentials of 0.041 and −0.070 V (vs. Ag/AgCl), respectively, in alkaline media.24 In the present study, we describe another Fe–N/C catalyst for ORR using TPT-pyrrole (Py) copolymer network (CPN) and iron(II) acetate as the precursors. We select CPN for preparing Fe–N/C catalyst because copolymer synthesis offers the ability to alter the properties of a homopolymer in a desired direction by the introduction of an appropriately chosen second monomeric unit. That is to say, the desirable properties of two different homopolymers can be integrated into a single copolymer. Considering that the poly(TPT)-derived Fe–N/C catalyst possesses a high ORR activity and the Py can coordinate with transition metals,25 copolymerization of TPT and Py is expected to alter such CPN properties as nitrogen doping contents and bonding configurations, and metal-coordination sites as compared to pure poly(TPT), hence further enhancing the ORR activity of the CPN-derived catalyst. In the current case, the CPN was fabricated by a scalable, one-step Friedel–Crafts reaction of a low-cost cross-linker, formaldehyde dimethyl acetal (FDA), with TPT and Py comonomer. Such synthesis protocol can produce cost-effective CPN with relatively high surface area and the only by-product is MeOH. Unlike the linear polymers previously used for the M–N/C catalysts, the CPN can be employed as a self-supporting catalyst thus dispensing the need of a carbon support. After pyrolyzed at 900 °C, N/Fe-codoped porous carbon materials were produced in a moderate yield. Compared to commercial Pt/C catalyst, the as-synthesized Fe–NTP/C-900 (pyrolyzed at 900 °C) catalyst exhibits high ORR activity in alkaline media, and comparable ORR activity in acidic media. The as-synthesized Fe–NTP/C-900 is a potential candidate as a high activity and low-cost ORR catalyst for FCs.
Experimental section
1. Materials
TPT was prepared according to the procedure reported previously by our group26 (see ESI†). The CPN was prepared by a one-step Friedel–Crafts reaction of FDA with TPT and Py comonomer, the product is denoted as TPT-Py CPN. For comparison, pure poly(TPT) network (PTPT PN) and pure PPy network (PPy PN) were separately prepared under identical conditions (see ESI†). All other reagents were used as received.2. Synthesis of Fe–NTP/C-900, Fe–NT/C-900 and Fe–NP/C-900
Fe–NTP/C-900, Fe–NT/C-900 and Fe–NP/C-900 were synthesized as follows: 350 mg of TPT-Py CPN, PTPT PN, and PPy PN were separately mixed with Fe(OAc)2 (100 mg) in ethanol for 3 h. The resulting suspensions were evaporated to dryness and then separately placed into a ceramic tube and pyrolyzed at 900 °C for 1 h in Ar with a heating rate of 4 °C min−1. To remove the unreacted metallic iron and other iron compounds produced from agglomeration during pyrolysis, the composites were preleached in 0.5 M H2SO4 at 80 °C for 24 h. Finally, the composites were separately heat-treated for a second time at 900 °C for 1 h in Ar, and the products are denoted as Fe–NTP/C-900 for TPT-Py CPN, Fe–NT/C-900 for PTPT PN, and Fe–NP/C-900 for PPy PN, respectively. For comparison, pure TPT-Py CPN without Fe(OAc)2 was also heat-treated under optimal conditions and the obtained material is denoted as NTP/C-900. Their physical and electrochemical properties are listed in Table 1.| Samples | N content (at%) | Eoa (V) | E1/2b (V) | JLc (mA cm−2) | JKd (mA cm−2) | ne |
|---|---|---|---|---|---|---|
| a Onset potential.b Half-wave potential.c Limiting current density obtained at −0.8 V with the rotation speed of 1600 rpm.d Kinetic-limiting current density calculated at −0.35 V.e Electron transfer number calculated at −0.35 V based on the K–L equation; all samples were measured in O2 saturated 0.1 M KOH solution. | ||||||
| Pt/C | 0 | 0.001 | −0.120 | 5.41 | 21.1 | 4.00 |
| Fe–NTP/C-900 | 2.59 | 0.051 | −0.055 | 5.79 | 28.4 | 3.96 |
| Fe–NT/C-900 | 2.10 | 0.041 | −0.070 | 5.52 | 22.5 | 4.02 |
| Fe–NP/C-900 | 2.03 | −0.004 | −0.145 | 4.92 | 14.9 | 3.87 |
| NTP/C-900 | 1.86 | −0.001 | −0.127 | 5.30 | 15.6 | 3.70 |
3. Electrochemical measurements
All electrochemical tests were performed on a CHI760D electrochemical workstation (Shanghai Chenhua Co., China) with a standard three-electrode cell system equipped with gas flow controller at room temperature. A Pt wire and an Ag/AgCl (KCl-saturated) were used as the counter and reference electrodes, and 0.1 M KOH was used as the electrolyte, respectively. In 0.1 M HClO4 electrolyte, a graphite rod and an Ag/AgCl (KCl-saturated) were used as the counter and the reference electrodes, respectively. Glassy carbon electrode (GCE, 5 mm diameter, 0.196 cm2) coated with the as-prepared catalysts' ink was used as the working electrode. For ORR activity measurement, 4.0 mg of the as-prepared Fe–NTP/C catalysts or commercial 20 wt% Pt/C catalyst (Johnson Matthey, hereafter “Pt/C”) was dispersed in a 4.0 mL mixture of ethanol and Nafion (Aldrich, 5.0 wt%) with a volume ratio of 98.5/1.5. The mixture was ultrasonicated to form a homogeneous ink with a concentration of 1.0 mg mL−1. Then, 56 μL of the Fe–NTP/C catalysts ink (for 20 wt% Pt/C, the catalyst loading was 28 μL) was carefully deposited on the pre-cleaned glassy carbon electrode and allowed to dry, resulting in a Fe–NTP/C catalysts loading of 286 μg cm−2 (for 20 wt% Pt/C, the catalyst loading is 143 μg cm−2). ORR activity was evaluated by cyclic voltammetry (CV) and linear sweep voltammetry (LSV) techniques on rotating disk electrode (RDE, Pine Research Instrumentation) in O2 (or N2)-saturated alkaline (0.1 M KOH) and acidic (0.1 M HClO4) media. The working electrode was scanned at a rate of 10 mV s−1 with varying rotating speeds from 400 to 2000 rpm. RRDE experiment was also conducted to determine the 4e selectivity. The disk potential was cycled from −1 to 0.2 V (vs. Ag/AgCl) at a scan rate of 10 mV s−1, and the ring potential was set to 0.4 V and 1.1 V (vs. Ag/AgCl) in 0.1 M KOH and 0.1 M HClO4, respectively. Methanol tolerant and durability testing were conducted by chronoamperometric technique in at the bias potential of −0.25 V (vs. Ag/AgCl) and 0.45 V (vs. Ag/AgCl) in O2-saturated 0.1 M KOH and 0.1 M HClO4 electrolyte with a rotation rate of 1600 rpm, respectively.Results and discussion
1. Synthesis and characterization of Fe–NTP/C catalysts
In this study, TPT and Py were copolymerized by a one-step Friedel–Crafts reaction in 1,2-dichloroethane (DCE) using FeCl3 as the catalyst and FDA as the cross-linking agent, giving TPT-Py CPN (Scheme 1). The as-synthesized CPN can be employed as a self-supporting catalyst and the use of a carbon support is therefore excluded as mentioned previously.17,27,28 The Fe–NTP/C catalyst was then synthesized by the pyrolysis of TPT-Py CPN and Fe(OAc)2 mixture at 900 °C under flowing Ar. The resultant catalyst is denoted as Fe–NTP/C-x, where TP stands for the N-doped carbon derived from the TPT-Py CPN and x stands for the pyrolysis temperature. Considering the fact that the synthesis parameters, such as the molar ratio of TPT/Py for copolymerization, mass ratio of TPT-Py CPN/Fe(OAc)2 for pyrolysis, and pyrolysis temperature, play important roles in the regulation of the ORR performance of the final catalysts, a series of experiments were performed to screen the optimal synthesis conditions. Our experimental results demonstrate that the optimal synthesis conditions are: TPT/Py molar ratio = 1/3, TPT-Py CPN/Fe(OAc)2 mass ratio = 3.5/1, and pyrolysis temperature = 900 °C (Fig. S1–S4, see ESI†). We therefore focus our further study on the Fe–NTP/C-900 catalyst.Fig. 1a shows the SEM images of the TPT-Py CPN precursor. As can be seen, the TPT-Py CPN exhibits an irregularly spherical architecture with diameter ranging from 230 to 850 nm. In addition, aggregated particles can be clearly seen. After pyrolysis, the spherical morphology is still maintained, but the surface become rough and porous (Fig. 1b). Fig. 1c–f depict the TEM images of the as-prepared Fe–NTP/C-900 catalyst. Clearly, the porous texture has been successfully formed in the catalyst as demonstrated in Fig. 1c and d. Moreover, high-magnification TEM images (Fig. 1e and f) reveal that the presence of Fe nanoparticles encapsulated by graphitic nanoshells. The inset of Fig. 1f shows the d-spacing of the encapsulated Fe particles. Such metal-containing particles encapsulated in well-defined onion-like graphitic carbon nanoshells was previously observed with PANI–FeCo–C catalysts.10,27 The corresponding elemental mapping of C, O, N and Fe (Fig. 1g) reveals that both the four elements are uniform dispersed over the spherical architecture.
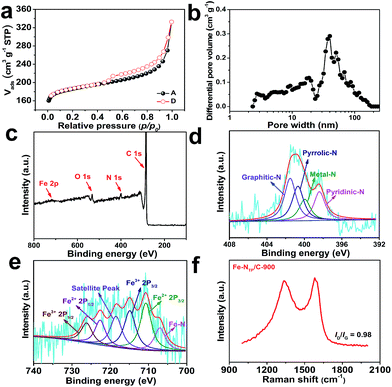
Fig. 2a shows the N2 adsorption/desorption isotherms for the Fe–NTP/C-900. According to the IUPAC classification, Fe–NTP/C-900 exhibits a typical type-IV isotherm with a type-H4 hysteresis loop at p/p0 = 0.45, suggesting the presence of large mesopores embedded in a matrix with interconnected channels of much smaller size.29,30 Fig. 2b shows the pore size distribution (PSD) for the Fe–NTP/C-900 catalyst calculated by density functional theory (DFT) method. As can be seen, the porosity of the Fe–NTP/C-900 catalyst is consisted of a large proportion of mesopores and a very small proportion of macropores. The specific surface area and total pore volume of the Fe–NTP/C-900 catalyst are 724 m2 g−1 and 0.514 cm3 g−1, respectively, which are substantially higher than those of its TPT-Py CPN precursor, i.e., 205 m2 g−1 and 0.336 cm3 g−1. Clearly, these pores are favorable to improve ORR activity of the Fe–NTP/C-900 catalyst.
The XPS survey spectrum of the Fe–NTP/C-900 catalyst (Fig. 2c) exhibits four kinds of elements, carbon (93.66%), nitrogen (2.59%), oxygen (3.59%), and iron (0.16%). However, the Fe content detected by the inductively coupled plasma atomic emission spectrometry (ICP-AES) analysis is 0.875% (Table S2, see ESI†), which is much larger than that detected by XPS, may be due to the fact that the Fe element mainly embedded in the graphitic carbon layers of the Fe–NTP/C-900 catalyst. This phenomenon is in good agreement with the HRTEM observations for this catalyst (Fig. 1e and f).
The N 1s peak of the Fe–NTP/C-900 catalyst (Fig. 2d) can be deconvoluted into four peaks: pyridinic-N (398.5 eV, 21.96%), Fe–N (399.9 eV, 16.78%), pyrrolic-N (400.7 eV, 27.22%), and graphitic-N (401.5 eV, 34.04%)31,32 (Table S6, see ESI†). Obviously, XPS analysis demonstrates the existence of Fe–Nx species in the Fe–NTP/C-900 catalyst, which has been confirmed as the major active sites for the ORR by Müllen and his coworkers.15 Moreover, Ferrando et al.33 has also demonstrated that pyridinic Fe–N (pyridinic Fe–N2 and Fe–N2+2) and pyrrolic Fe–N4 species are existed in the PANI–Fe–C catalyst prepared by pyrolyzing the mixture of carbon black, PANI and FeCl3. On the other hand, graphitic-N and pyridinic-N are also reported to play crucial roles in ORR in metal-free, N-doped carbon catalysts.34–36 Similarly, the Fe 2p spectrum can be deconvoluted into six peaks at 706.8, 710.7, 714.4, 718.2, 722.6, and 726.1 eV (Fig. 2e), which can be assigned to Fe–N, Fe2+ 2p3/2, Fe3+ 2p3/2, satellite peak, Fe2+ 2p1/2, and Fe3+ 2p1/2, respectively, indicating the coexistence of Fe(II) and Fe(III) species in the Fe–NTP/C-900 catalyst.32 These surface Fe ions can potentially serve as N-coordination sites for the formation of Fe–Nx species as indicated in the N 1s scan. In our case, the high ORR activity of Fe–NTP/C-900 catalyst may be attributed to the high contents of Fe–Nx species as well as the two kinds of ORR active N species (graphitic- and pyridinic-N).
The Raman spectrum of Fe–NTP/C-900 shown in Fig. 2f exhibits the ordered graphitic carbon (G band) at ca. 1580 cm−1 and defect band (D band) at ca. 1334 cm−1.37 An estimation of the total order related to the carbon structures can be given by the intensity ratio between Raman D and G bands, ID/IG. The ID/IG value of the Fe–NTP/C-900 is 0.98, indicating the existence of more defects in carbon framework.
2. ORR activity
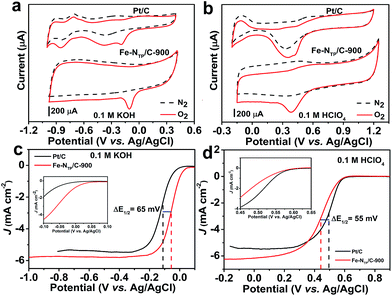
Cyclic voltammograms (CV) of the Fe–NTP/C-900 and Pt/C catalysts in N2- or O2-saturated alkaline (0.1 M KOH) and acidic (0.1 M HClO4) solutions were initially investigated to evaluate the ORR activity. As shown in Fig. 3a, no redox peaks are observed for the Fe–NTP/C-900 catalyst in N2-saturated alkaline solution. Conversely, a pair of broad redox peaks corresponding to the Fe(II)/Fe(III) redox couple are observed at 0.38 and 0.47 V for this catalyst in N2-saturated acidic electrolyte (Fig. 3b). In the presence of O2, well-defined ORR peak can be clearly observed for the Fe–NTP/C-900 catalyst in both alkaline and acidic electrolytes (Fig. 3a and b). In 0.1 M KOH solution, the ORR peak potential and current are −0.11 V and 325 μA for the Fe–NTP/C-900 catalyst, which are higher than those of the Pt/C catalyst, i.e., −0.13 V and 243 μA, respectively. However, the ORR peak potential and current shift to 0.39 V and 448 μA for the Fe–NTP/C-900 catalyst in 0.1 M HClO4 solution, which are close to those of the Pt/C catalyst, i.e., 0.38 V and 450 μA, respectively. Notably, a small oxidation peak also appears at 0.55 V in the acidic media, indicating that Fe doping in the carbon material can act as a mediator in the electron-transfer process for ORR.38Linear sweep voltammograms (LSVs) for the Fe–NTP/C-900 and Pt/C catalysts in O2-saturated alkaline and acidic solutions were then performed to further evaluate their ORR performance. All LSV curves present in the figure have been corrected by removing the background signals obtained in N2 saturated electrolyte. In the alkaline media, the ORR onset (Eo) and half-wave potentials (E1/2) for the Fe–NTP/C-900 catalyst are 0.051 V and −0.055 V, respectively, which are more positive than those of the Pt/C catalyst (Eo = 0.001 V, E1/2 = −0.120 V, inset of Fig. 3c). In the acid solution (Fig. 3d), the Eo and E1/2 values for the Fe–NTP/C-900 catalyst increase to 0.605 V and 0.445 V, respectively, which are more negative than those of the Pt/C catalyst (Eo = 0.620 V, E1/2 = 0.500 V). In addition, the limiting current densities (JL) of the Fe–NTP/C-900 catalyst are 5.79 and 6.24 mA cm−2 in alkaline and acidic solutions at a rotating speed of 1600 rpm, respectively, which are also higher than those of the Pt/C catalyst. The ORR Tafel slopes measured with the Fe–NTP/C-900 catalyst in the alkaline and acid electrolytes are ca. 72 and 80 mV per decade, respectively, which was comparable to that of Pt/C (ca. 77 mV per decade in alkaline electrolyte; ca. 70 mV per decade in acid electrolyte, Fig. S5, see ESI†). These results indicate that the ORR kinetics of the Fe–NTP/C-900 catalyst is similar to that of Pt/C catalyst in both alkaline and acid electrolytes.
For the Fe–N/C catalysts, both the Fe–Nx species and the N doping in the carbon matrix (graphitic- and pyridinic-N) have been generally regarded as the ORR active sites.39–43 In our case, the ORR activity of the Fe–NTP/C-900 catalyst is mainly attributed to the M–Nx species. In order to verify this, the as-synthesized TPT-Py CPN was directly subjected to pyrolyze under identical conditions after the Fe residues (Friedel–Crafts catalyst) were completely removed from the copolymer network. The pyrolyzed pure TPT-Py CPN catalyst (denoted as NTP/C-900) exhibits much poorer ORR activity than that of the Fe–NTP/C-900 catalyst in both alkaline and acid electrolytes, especially in acidic media (Fig. S6 and Table S4, see ESI†), indicating that the Fe–Nx species plays a vital role in the high ORR activity of the Fe–NTP/C-900 catalyst (see XPS analysis). As a further control experiment, the Fe–NT/C-900 and Fe–NP/C-900 catalysts derived from PTPT PN/Fe(OAc)2 and PPy PN/Fe(OAc)2 mixtures were also prepared under identical conditions and found to be inferior to the Fe–NTP/C-900 catalyst in ORR activity (Fig. S6 and Table S4, see ESI†). The excellent ORR activity of the Fe–NTP/C-900 catalyst is possibly due to the following reasons. Firstly, the incorporation of PPy into the copolymer network can enhance the final Fe loading of the resultant Fe–NTP/C-900 catalyst (0.875 wt%), because PPy can form PPy–iron–oxygen coordination complex with iron salts as demonstrated by previous study.44 In the current case, under the same initial Fe loading (9.17 wt%), the Fe–NP/C-900 catalyst prepared from the PPy PN and Fe(OAc)2 mixture indeed has the highest final Fe loading (2.789 wt%), while the Fe–NT/C-900 catalyst prepared from the PTPT PN and Fe(OAc)2 mixture has the lowest final Fe loading (0.595 wt%) (Table S5, Fig. S7, see ESI†). Secondly, the Fe–NTP/C-900 catalyst also has the highest ORR active N and Fe–Nx species contents among the three catalysts (Fig. S8 and S9, Table S6, see ESI†). Thirdly, the BET surface area and the pore size distribution for the three catalysts are somewhat different. It is obvious that the Fe–NTP/C-900 catalyst possesses the highest BET surface area together with a moderate pore size (Fig. S10–S12 and Table S7, see ESI†). Based on these observations, it is possible that the chemo physical properties, such as the N doping content and bonding configuration, Fe-coordination sites, and pore structure of the Fe–NTP/C materials can be roughly adjusted by the CPN precursor through copolymerization. However, further detailed experiments are still necessary in order to confirm this conclusion.
In order to verify the catalytic pathway and reaction kinetics of the ORR on Fe–NTP/C-900, LSVs on a RDE were further performed at different rotation speeds from 400 to 2000 rpm in O2-saturated alkaline and acidic solutions (Fig. 4a and b, Fig. S13†). The Koutecky–Levich (K–L) plots45 (J−1 vs. ω−1/2) were calculated from LSVs and compared at −0.35 V in O2-saturated alkaline solution and at 0.15 V in O2-saturated acidic solution (Fig. 4c and d). In addition, K–L plots at other potentials are shown in Fig. S14 (see ESI†). All K–L plots display good linearity regardless of the potentials, indicating first-order reaction kinetics with respect to the concentration of O2. Furthermore, the kinetic limiting current density (JK) values calculated from the intercepts of linear K–L plots in O2-saturated alkaline and acidic electrolytes are shown in Fig. 4e and f. The corresponding JK value of the Fe–NTP/C-900 catalyst is 28.4 mA cm−2 in the alkaline solution, higher than that of Pt/C catalyst (21.1 mA cm−2). In the acidic solution, the JK value of the Fe–NTP/C-900 catalyst reaches 69.6 mA cm−2, nearly 2 times higher than that of Pt/C catalyst (32.8 mA cm−2). This may be attributed to the high specific surface area and abundant mesoporous structure of the Fe–NTP/C-900 catalyst, which led to the rapid electron transfer. Considering that the loading for the Pt/C catalyst is less than that for the Fe–NTP/C-900 catalyst, their ORR current densities are also compared in terms of mass activity (Fig. S15 and S16 and Table S8, see ESI†).
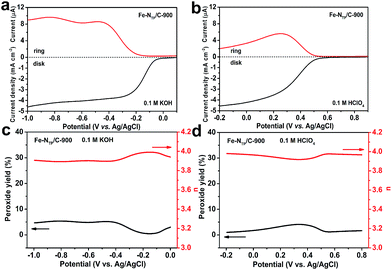
The n value of the Fe–NTP/C-900 was calculated to be ca. 3.96 ± 0.06 in the alkaline and acidic solutions, demonstrating high selectivity towards an overall 4e reduction mechanism occurring on the Fe–NTP/C-900 catalyst. To further verify this conclusion, rotating ring-disk electrode (RRDE) measurements technique was applied to determine the number of electrons transferred and the H2O2 generation rate of the Fe–NTP/C-900 catalyst. Fig. 5a and b showed the curves of the disk and ring currents vs. the potential at 900 rpm in alkaline and acidic solutions, respectively. The H2O2 yield (%) and the n curves (Fig. 5c and d) can be calculated from the disk currents (id) and the ring currents (ir). Obviously, the H2O2 yield increases with the ir, but showing an inverse behaviour between the id. This implies that the more active the catalyst is, the lower the current detected at the ring electrode. In the alkaline solution (Fig. 5c), the H2O2 yield is less than 5% and the average n value is 3.92 at all potentials within the range between 0.0 and −1.0 V. In the acidic solution (Fig. 5d), the H2O2 yield is less than 4% and the average n value is 3.96 at all potentials within the range between 0.8 and −0.2 V. The n value calculated from RRDE measurements is in agreement with the n value based on the K–L plots, implying that good selectivity toward the 4e pathway occurring on the Fe–NTP/C-900 catalyst. Based on the above analysis, it is clear that the ORR activity of the Fe–NTP/C-900 catalyst is significantly higher than those previously reported M–N/C catalysts (Table S10, see ESI†).
To investigate the MeOH crossover effects and durability of Fe–NTP/C-900, we carried out chronoamperometric measurements in O2-saturated alkaline and acidic solutions. As shown in Fig. 6a and b, the corresponding amperometric response for Fe–NTP/C-900 electrode remained almost unchanged after the addition of 3 M MeOH at 145 s into the testing cell in both alkaline and acidic electrolytes, showing a remarkably good tolerance of crossover effects, while Pt/C by contrast catalyzes both the ORR and MeOH oxidation reactions in the cathode.
The durability of Fe–NTP/C-900 and Pt/C has been assessed through the chronoamperometric measurements in O2-saturated alkaline and acidic solutions at a rotation rate of 1600 rpm for a period of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s (Fig. 6c and d). It is clear that Fe–NTP/C-900 is even much more stable than Pt/C, with the relative current density being as high as 97.2% and 90.5% after the 10
000 s (Fig. 6c and d). It is clear that Fe–NTP/C-900 is even much more stable than Pt/C, with the relative current density being as high as 97.2% and 90.5% after the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s test in alkaline and acidic electrolytes, respectively. These results indicate that Fe–NTP/C-900 is an ideal ORR electrocatalyst for practical application in FCs.
000 s test in alkaline and acidic electrolytes, respectively. These results indicate that Fe–NTP/C-900 is an ideal ORR electrocatalyst for practical application in FCs.
Conclusions
Here we have demonstrated a highly efficient M–N/C ORR electrocatalyst in both alkaline and acidic conditions by directly pyrolyzing copolymer networks and iron(II) acetate mixture in Ar. The copolymer networks were fabricated by a scalable, one-step Friedel–Crafts reaction of a low-cost cross-linker, formaldehyde dimethyl acetal, with 2,4,6-tripyrrol-1,3,5-triazine and pyrrole mixture. The structural and chemical properties of the resultant Fe–NTP/C catalysts were systematically investigated. The as-prepared Fe–NTP/C-900 showed excellent catalytic activity, long durability and high selectivity for ORR in alkaline medium. Moreover, the ORR on the Fe–NTP/C-900 catalyst in alkaline and acidic conditions follows the efficient 4e transfer pathway, demonstrating the complete reduction of O2 into H2O. Therefore, the as-synthesized Fe–NTP/C-900 is a potential candidate as a high activity and low-cost ORR catalyst for FCs.Acknowledgements
Financial support from Program for NSFC (51272219), RFDP (20124301110006), and the Construct Program of the Key Discipline in Hunan Province is greatly acknowledged.Notes and references
- Y. J. Wang, N. Zhao, B. Fang, H. Li, X. T. Bi and H. Wang, Chem. Rev., 2015, 115, 3433–3437 CrossRef CAS PubMed.
- Y. J. Wang, B. Fang, H. Li, X. T. Bi and H. Wang, Prog. Mater. Sci., 2016, 82, 445–498 CrossRef CAS.
- J. Maruyama and I. Abe, Chem. Commun., 2007, 2879–2881 RSC.
- X. Wang, X. Fan, H. Lin, H. Fu and J. Wang, et al., RSC Adv., 2016, 6, 37965–37973 RSC.
- D. H. Deng, L. Yu, X. Q. Chen, G. X. Wang and L. Jin, et al., Angew. Chem., Int. Ed., 2013, 52, 371–375 CrossRef CAS PubMed.
- P. Cheng, S. Li, R. Li, J. Yan and W. Yu, RSC Adv., 2015, 5, 107389–107395 RSC.
- K. P. Gong, F. Du, Z. H. Xia, M. Durstock and L. M. Dai, Science, 2009, 323, 760–764 CrossRef CAS PubMed.
- Z. Chen, D. Higgins, A. Yu, L. Zhang and J. Zhang, Energy Environ. Sci., 2011, 4, 3167–3192 CAS.
- F. Jaouen, E. Proietti, M. Lefevre, R. Chenitz and J. P. Dodelet, et al., Energy Environ. Sci., 2011, 4, 114–130 CAS.
- G. Wu, K. L. More, C. M. Johnston and P. Zelenay, Science, 2011, 332, 443–447 CrossRef CAS PubMed.
- L. Z. Gu, L. H. Jiang, J. T. Jin, J. Liu and G. Q. Sun, Carbon, 2015, 82, 572–578 CrossRef CAS.
- N. R. Sahraie, U. I. Kramm, J. Steinberg, Y. Zhang and A. Thomas, et al., Nat. Commun., 2015, 6, 8618 CrossRef CAS PubMed.
- R. Bashyam and P. Zelenay, Nature, 2006, 443, 63–66 CrossRef CAS PubMed.
- S. Gupta, D. Tryk, I. Bae, W. Aldred and E. Yeager, J. Appl. Electrochem., 1989, 19, 19–27 CrossRef CAS.
- H. W. Liang, W. Wei, Z. S. Wu, X. Feng and K. Müllen, J. Am. Chem. Soc., 2013, 135, 16002–16005 CrossRef CAS PubMed.
- G. Wu and P. Zelenay, Acc. Chem. Res., 2013, 46, 1878–1889 CrossRef PubMed.
- L. Lin, Q. Zhu and A. W. Xu, J. Am. Chem. Soc., 2014, 136, 11027–11033 CrossRef CAS PubMed.
- H. H. Yin, S. L. Liu, C. L. Zhang, J. C. Bao and Y. L. Zheng, et al., ACS Appl. Mater. Interfaces, 2014, 6, 2086–2094 CAS.
- Z. Xiang, Y. Xue, D. Cao, L. Huang and J. Chen, et al., Angew. Chem., Int. Ed., 2014, 53, 2433–2437 CrossRef CAS PubMed.
- L. L. Yang, Y. M. Su, W. M. Li and X. W. Kan, J. Phys. Chem. C, 2015, 119, 11311–11319 CAS.
- Y. Zhao, K. Watanabe and K. Hashimoto, J. Mater. Chem., 2012, 22, 12263–12267 RSC.
- V. Nallathambi, X. Li, J. W. Lee and B. N. Popov, ECS Trans., 2008, 16, 405–417 CAS.
- N. P. Subramanian, X. Li, V. Nallathambi, S. P. Kumaraguru, H. Colon-Mercado, G. Wu, J. W. Lee and B. N. Popov, J. Power Sources, 2009, 188, 38–44 CrossRef CAS.
- M. Yang, H. Chen, D. Yang, Y. Gao and H. Li, J. Power Sources, 2016, 307, 152–159 CrossRef CAS.
- M. O. Senge, Angew. Chem., Int. Ed. Engl., 1996, 35, 1923–1925 CrossRef CAS.
- M. Yang, D. G. Yang, H. B. Chen, Y. Gao and H. M. Li, J. Power Sources, 2015, 279, 28–35 CrossRef CAS.
- P. Zamani, D. Higgins, F. Hassan, G. Jiang and J. Wu, et al., Electrochim. Acta, 2014, 139, 111–116 CrossRef CAS.
- G. H. Wang, K. Z. Jiang, M. L. Xu, C. G. Min and B. H. Ma, et al., J. Power Sources, 2014, 266, 222–225 CrossRef CAS.
- K. S. Sing, Pure Appl. Chem., 1985, 57, 603–619 CrossRef CAS.
- J. C. Groen, L. A. A. Peffer and J. Pérez-Ramírez, Microporous Mesoporous Mater., 2003, 60, 1–17 CrossRef CAS.
- J. Wu, W. M. Li, D. Higgins and Z. G. Chen, J. Phys. Chem. C, 2011, 115, 18856–18862 CAS.
- R. L. Arechederra, K. Artyushkova, P. Atanassov and S. D. Minteer, ACS Appl. Mater. Interfaces, 2010, 2, 3295–3302 CAS.
- M. Ferrando, A. J. Kropf and D. J. Myers, J. Phys. Chem. C, 2012, 116, 16001–16013 Search PubMed.
- M. Lefevre, E. Proietti, F. Jaouen and J. P. Dodelet, Science, 2009, 324, 71–74 CrossRef CAS PubMed.
- Y. Zhao, K. Watanabe and K. Hashimoto, J. Am. Chem. Soc., 2012, 134, 19528–19531 CrossRef CAS PubMed.
- Y. Wang, X. Cui, Y. Li, L. Chen and H. Chen, et al., Carbon, 2014, 68, 232–239 CrossRef CAS.
- M. S. Dresselhaus, A. Jorio, M. Hofmann, G. Dresselhaus and R. Saito, Nano Lett., 2010, 10, 751–758 CrossRef CAS PubMed.
- R. Jiang and D. Chu, J. Power Sources, 2014, 245, 352–361 CrossRef CAS.
- N. Ramaswamy, U. Tylus, Q. Jia and S. Mukerjee, J. Am. Chem. Soc., 2010, 135, 15443–15449 CrossRef PubMed.
- M. Lefevre, J. P. Dodelet and P. Bertrand, J. Phys. Chem. B, 2002, 106, 8705–8713 CrossRef CAS.
- Y. Nabae, S. Moriya, K. Matsubayashi, S. M. Lyth and M. Malon, et al., Carbon, 2010, 48, 2613–2624 CrossRef CAS.
- R. Silva, D. Voiry, M. Chhowalla and T. Asefa, J. Am. Chem. Soc., 2013, 135, 7823–7826 CrossRef CAS PubMed.
- W. Li, J. Wu, D. C. Higgins, J. Y. Choi and Z. Chen, ACS Catal., 2012, 2, 2761–2768 CrossRef CAS.
- Y. L. Liang, Z. L. Tao and J. Chen, Adv. Energy Mater., 2012, 2, 742–769 CrossRef CAS.
- S. Y. Wang, D. Yu and L. Da, J. Am. Chem. Soc., 2011, 133, 5182–5185 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional detailed experimental information, characterization data, and structure properties for polymer network, electrochemical measurement results for Fe–NTP/Cs and Pt/C. See DOI: 10.1039/c6ra24314b |
| This journal is © The Royal Society of Chemistry 2016 |