Nitrogen-doped 3D porous carbons with iron carbide nanoparticles encapsulated in graphitic layers derived from functionalized MOF as an efficient noble-metal-free oxygen reduction electrocatalysts in both acidic and alkaline media†
Juanhong Xuea,
Ling Zhaob,
Zhiyu Doua,
Yan Yanga,
Yue Guana,
Zhen Zhua and
Lili Cui*a
aKey Laboratory of Applied Chemistry and Nanotechnology at Universities of Jilin Province, Department of Chemistry and Chemical Engineering, Changchun University of Science and Technology, Changchun 130022, China. E-mail: cuilili1127@gmail.com
bShenyang Rubber Research & Design Institute Company Limited, Shenyang, China
First published on 16th November 2016
Abstract
A novel kind of functionalized metal organic framework (F-MOF, Prussian blue/graphene oxide composite, PB/GO) is prepared by a simple method. The morphology characterization indicates that iron carbide nanoparticles supported on highly nitrogen-doped 3D porous carbons (Fe–C/NG) are formed after direct annealing of F-MOF under an Ar atmosphere. Fe–C/NG-10%-700-AL (10% represents the mass ratio between GO and iron salt; 700 stands for pyrolysis temperature; AL means acid leaching) exhibits excellent catalytic activity for the ORR (oxygen reduction reaction) in both acidic and alkaline media, together with superior durability, excellent methanol tolerance and a nearly four-electron pathway for the ORR. The excellent catalytic performance for the ORR on Fe–C/NG-10%-700-AL is attributed to the existence of iron carbide nanoparticles, highly doped N concentration, high surface area and pores of 3D graphene and the synergistic effect between multiple components.
1. Introduction
The catalyst in the oxygen reduction reaction (ORR) plays an important role in the industrial application of fuel cells. As is well known, Pt and its alloys have been considered as the most efficient ORR catalysts,1–3 but disadvantages of these catalysts, including the high cost, low tolerance to methanol and limited stability, restrict further commercial application of fuel cells. This urgent situation has triggered intense investigations on non-precious catalysts with improved catalytic activity, long-term durability, excellent surface properties, as well as low cost.In recent years, extensive efforts have been devoted to exploring non-precious metal or metal-free catalysts for ORR to replace Pt-based catalysts such as heteroatom doped carbon materials,4–8 and transition metal/nitrogen/carbon catalysts.9–13 In principle, the ORR behavior are governed by the intrinsic properties of catalyst associated with the type and the number of catalytic active sites, specific surface area, conductivity and mass transport in catalyst layer. Among them, the two vital factors are: (1) the type of active sites which are determined by chemical structure, and the synergetic effect between different components; (2) the exposure of effective sites and the mass transport of ORR-relevant species, which highly depend on the specific surface area and pore structure. Nevertheless, much progress has been gained, to rationally design and controllably synthesize the non-precious catalyst with proper pore size distribution, high surface area and high density of effective catalytic active sites still remain a significant challenge.
Recently, an agreement has been reached that nitrogen-doped carbon nanostructures (NCNs) exhibit comparable ORR activity to that of commercial Pt/C in alkaline fuel cells,14–16 but these catalysts display poor activity towards ORR in acid medium, which greatly limits their application in proton exchange membrane fuel cells (PEMFCs). So increasing efforts have been devoted to developing a family of iron carbide based catalysts that show catalytic activity similar to Pt-based catalysts in both acidic and alkaline solutions.17–19
Lately, metal–organic frameworks (MOFs) composed of highly ordered metal ions or clusters and organic ligands, have attracted extensive interests due to their tunable properties of functionality, pore sizes, and surface areas.20,21 Especially, the porous carbon, metal/carbon and metal oxide/carbon materials have been gained derived from MOFs.22–24 Prussian blues (PBs) and its analogues, a branch of MOFs, are attractive candidates owing to their unique magnetic, electric properties25–28 and its wide applications such as electrochemical supercapacitor,29 energy storage30 and structure-based drug design.31 Porous carbon materials supporting iron carbide nanoparticles as ORR catalysts are obtained by annealing of PB nanoparticles, which exhibits excellent ORR catalytic activity in alkaline media, but little research is conducted in acidic solution.32–34 It is, therefore, highly inspiring to develop stable and efficient ORR catalysts derived from PB to make them suitable for both alkaline and acidic solutions.
Herein, in order to promote the commercial development of PEMFC, we report a facile and environmentally friendly way to synthesize a new type of nitrogen-doped 3D graphene supporting iron carbide nanoparticles as ORR catalyst which exhibits enhanced catalytic activity with respects to onset potential, half-wave potential and limiting-diffusion current in both acidic and alkaline media.
2. Experimental section
2.1 Chemicals and reagents
Graphite powder was purchased from Sinopharm Chemical Reagent Co., Ltd. Pt/C (20 wt% Pt on Vulcan XC-72) was bought from Alfa Aesar. All other reagents were of analytical grade and used without further purification.2.2 Preparation of Fe–C/NG-M-T-AL catalyst
GO was synthesized using a modified Hummers method.35 PB/GO was synthesized by adopting a similar method with a little modification.36 The schematic synthesis route of Fe–C/NG-M-T-AL was illustrated in Scheme 1. Firstly, 0.065 g FeCl3·6H2O, 0.085 g K3Fe(CN)6 and 0.372 g KCl were dissolved in 50 mL pH = 1.5 HCl aqueous solution. Then different volume of GO aqueous solution (2 mg mL−1) was slowly added in the mixture under stirring. After 12 h, the product was centrifuged and washed with deionized water and ethanol for several times. Finally the composite was dried in vacuum at 45 °C for 12 h. The obtained dark green precipitation was denoted as PB/GO-M, where M represents the mass ratio of GO to ferric salt. Subsequently, heat-treatment was performed in a tubular furnace at a heating rate of 5 °C min−1 under Ar atmosphere and the PB/GO-M pyrolyzed at different temperatures was denoted as Fe–C/NG-M-T, where T represents pyrolysis temperature. To remove the unstable Fe-based nanoparticles, the black Fe–C/NG-T was washed with 1 mol L−1 HCl by vibration for 24 h. After acid leaching, the catalysts were denoted as Fe–C/NG-M-T-AL.2.3 Preparation of Fe–C/NG-M-T-AL catalysts modified electrode
Firstly, Fe–C/NG-M-T-AL catalyst was dispersed in ethanol to form a 1 mg mL−1 solution by ultrasonic vibration for 2 h. Following, the glassy carbon electrode was polished with 0.3 and 0.05 μm alumina powder successively, then ultrasonically flushed with deionized water and ethanol for several times, subsequently, blow-dried by nitrogen. At last, 55 μL of the catalyst dispersion was evenly dropped onto the surface of the freshly polished electrode, which gave rise to a loading of catalyst 0.28 mg cm−2. Then 1.5 μL of 0.5% Nafion solution was dripped to protect the pasted catalyst from loss in the electrolyte solution. The Pt/C modified electrode was prepared with the same procedure as Fe–C/NG-M-T-AL modified electrode as well as the same catalyst loading.2.4 Characterization
Morphologies and structures of samples were investigated by scanning electron microscopy (SEM, JEOL JSM-6701F electron microscope operating at 5 kV) and transmission electron microscopy (TEM, JEOL-2010 transmission electron microscope operating at 200 kV). Nitrogen sorption isotherms were measured at 77 K using a Micromeritics ASAP 2020 surface area and porosity analyzer. X-ray diffraction (XRD) patterns were collected on a powder X-ray diffractometer at 40 kV and 15 mA using Co-Kα radiation (RIGAK, D/MAX2550VB/PC). Raman spectra analysis was conducted on a LabRAM HR Evolution Raman spectrometer at 532 nm. X-ray-photoelectron spectroscopy (XPS) spectra was obtained on an ESCLAB 250 spectrometer with a monochromatized Al Kα X-ray source.2.5 Electrochemical measurements
Electrochemical tests were performed on a CHI 660D electrochemical workstation (Shanghai CHENHUA company) with a traditional three-electrode cell at room temperature incorporating a Pt wire as the counter electrode, a saturated calomel electrode (SCE) as the reference electrode and the modified GC electrode (d = 3 mm) as the working electrode.The ORR performance was tested in O2-saturated 0.5 M H2SO4 or 0.1 M KOH at room temperature. Cyclic voltammetry (CV) was performed on a CHI 660D electrochemical workstation (Shanghai CHENHUA company) at the scan rate of 100 mV s−1 in a N2- or O2-saturated electrolyte solution.
The rotating disk electrode (RDE, d = 5 mm), current–time chronoamperometric response (i–t), methanol tolerance experiment and rotating ring disk electrode (RRDE, ddisk = 5.61 mm) were conducted on a Pine Instrument Company AF-MSRCE modulator speed rotator on a CHI660E electrochemical workstation (CH Instruments, Shanghai CHENHUA company) employing a standard three-electrode system at a scanning rate of 10 mV s−1 in an O2-saturated electrolyte solution.
The linear relationships between j−1 and ω−0.5 at various potentials for the ORR on Fe–C/NG-M-T-AL and Pt/C were also determined by the Koutecky–Levich equation given below:
| 1/J = 1/JK + 1/JL = 1/nFKC0 + 1/Bω1/2 |
| B = 0.62nFC0(D0)2/3ν−1/6 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mol−1), ω is the angular velocity of the disk (ω = 2πN, N is the linear rotation speed), C0 is the bulk concentration of O2 dissolved in the electrolyte, D0 is the O2 diffusion coefficient, ν is the kinematic viscosity of the electrolyte, (C0 = 1.2 × 10−3 mol L−1, D0 = 1.9 × 10−5 cm2 s−1, ν = 0.01 cm2 s−1 for both 0.5 M H2SO4 and 0.1 M KOH solutions).
485 C mol−1), ω is the angular velocity of the disk (ω = 2πN, N is the linear rotation speed), C0 is the bulk concentration of O2 dissolved in the electrolyte, D0 is the O2 diffusion coefficient, ν is the kinematic viscosity of the electrolyte, (C0 = 1.2 × 10−3 mol L−1, D0 = 1.9 × 10−5 cm2 s−1, ν = 0.01 cm2 s−1 for both 0.5 M H2SO4 and 0.1 M KOH solutions).
The ring electrode was held at 0.25 V vs. SCE to detect the yield of hydrogen peroxide species in RRDE experiments with the H2O2 collection coefficient of 0.37. The ORR catalytic activity between Fe–C/NG-700-AL and the commercial Pt/C were compared by the percentage of H2O2 species (eqn (1)) and the electron transfer number (eqn (2)) as the following:
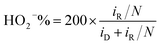 | (1) |
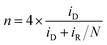 | (2) |
All potentials were converted to values versus the reversible hydrogen electrode (RHE) based on: ERHE = ESCE + 0.998 V (0.1 M KOH solution),37 and ERHE = ESCE + 0.273 V (0.5 M H2SO4 solution)38 and all electrochemical tests were carried out at room temperature.
3. Results and discussion
3.1 Characterization of Fe–C/NG-10%-700-AL
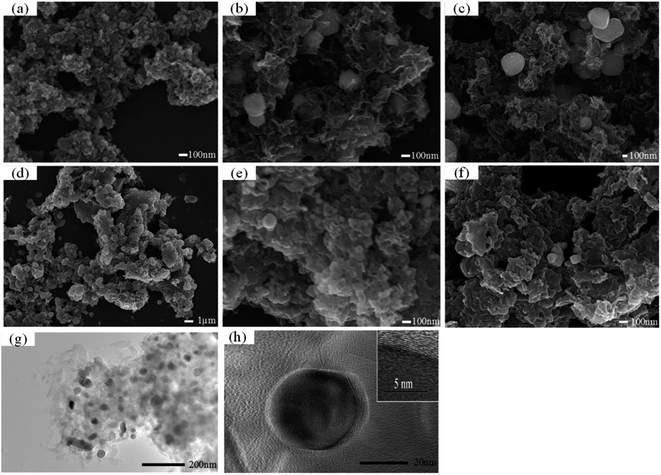
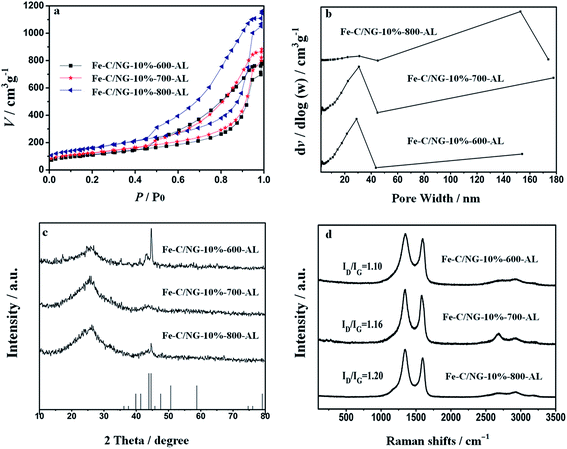
In the previous reports, PB could be fabricated by directly mixing Fe3+ and (Fe(CN)6)3− in the presence of reducing agents such as L-ascorbic acid.39 In this work, PB/GO is prepared by directly mixing Fe3+ and (Fe(CN)6)3− at the acidic condition in the presence of reducing agent GO. The SEM image of PB/GO-10% precursor material is illustrated in Fig. 1a. Due to the strong interaction between PB and GO, PB nanocubes with a side length of about 40 nm are closely dispersed on the surface of GO, which is also demonstrated in TEM image as shown in Fig. 1b. The precursor, PB/GO, serving as a novel kind of F-MOF which possesses rich nitrogen and iron source is directly carbonized under Ar flow at different temperatures. As exhibited in Fig. 2a–c, the iron carbide nanoparticles supported on 3D graphene have formed after pyrolysis. Moreover, the Fe–C/NG obtained at different pyrolysis temperatures show a similar morphology with the diameter of nanoparticles ranging from 100 to 500 nm enclosed in graphitic layers, indicating iron carbide nanoparticles as the catalytic active sites and porous 3D graphene have been successfully obtained derived from F-MOF. Furthermore, as observed in Fig. 2d–f, the morphologies of Fe–C/NG-M-T-AL essentially remain unchanged compared with Fe–C/NG-M-T. The morphology and structure of the Fe–C/NG-10%-700-AL is further investigated by TEM. The typical TEM image in Fig. 2g shows spherical nanoparticles distribute on the surface of graphene layers. HRTEM image (Fig. 2h) of Fe–C/NG-10%-700 further shows iron carbide nanoparticles are entirely wrapped by the carbon layers, which is in good agreement with the results of SEM images. Additionally, SEM images of Fe–C/NG-M-800-AL are shown in Fig. S1.†To probe the specific surface area and pore size of the Fe–C/NG-10%-T-AL samples, nitrogen sorption–desorption experiments were performed. A combination of type IV and type II isotherms,40 indicating the presence of mesopores and macropores is observed in all samples of Fe–C/NG-10%-T-AL (Fig. 3a). As shown in Table 1, the BET surfaces area and pore volume both increase with the raising of pyrolysis temperature. Moreover, the pore size distributions analyzed by BJH method (Fig. 3b) show a narrow distribution of mesopores centered at about 29.2 nm and a broad distribution of macropores ranging from 50 to 150 nm. Mass transfer and the exposure of active sites largely depends on their pore size distribution and surface area. Universally, the meso- and macroporosity are favorable for the efficient diffusion of the ORR-related species (O2, H+, OH−, and H2O).41 In addition, the larger surface area is expected to provide more active sites for the enhancement of ORR performance.
| Samples | BET surfaces area/m2 g−1 | Pore size/nm | Pore volume/cm3 g−1 |
|---|---|---|---|
| Fe–C/NG-10%-600-AL | 403.6720 | 11.5587 | 1.2357 |
| Fe–C/NG-10%-700-AL | 448.8386 | 11.7935 | 1.3884 |
| Fe–C/NG-10%-800-AL | 581.1298 | 11.8193 | 1.8231 |
The crystalline structure of Fe–C/NG-M-T-AL is studied by X-ray diffraction (XRD) (Fig. 3c and S2†), similar diffraction curves are observed. The characteristic peak at ca. 44.6° correspond to the (201) plane of Fe5C2 (PDF card no. 20-0508). The hump at about 26.2° becomes stronger and broader (Fig. S2†) with increasing the mass ratio of GO to ferric salt, which may be due to the overlapping diffraction peaks of the reduced and unreduced graphitic carbon shell (002). The Raman spectra of Fe–C/NG-10%-T-AL are measured to further estimate the carbon structures and the degree of defects. As shown in Fig. 3d, the pronounced D band locates at 1353 cm−1 and G band at 1599 cm−1, which are in accordance with the sp3 defective sites and an ordered graphitic carbon atoms, respectively.42 The value of ID/IG increases from 1.10 to 1.19 with the pyrolysis temperature from 600 to 800 °C, indicating that more defect sites are obtained in Fe–C/NG-10%-800-AL. Additionally, 2D and D + D′ bands of graphite are characterized by two small peaks located at 2675 cm−1 and 2915 cm−1, respectively, implying the multilayer architecture instead of a single layer of graphene.43
X-ray photoelectron spectroscopy (XPS) is carried out to elucidate the surface elements and the changes of nitrogen bonding configurations in the Fe–C/NG-10%-T-AL catalysts with the annealing temperatures. The measured spectra reveal the existence of C, O, N and Fe as shown in Fig. 4a. The Fe 2p spectra for the PB/GO can be divided into several peaks. The peaks located at 712.8 and 721.4 eV, which are associated with Fe3+ in Fe4III[FeII(CN)6]3.44 The residual peak at 708.5 eV shows the presence of Fe 2p3/2 (Fe2+) in Fe4III[FeII(CN)6]3.45 Notably, no obvious Fe 2p signal could be detected in Fe–C/NG-10%-T-AL samples due to the iron carbide nanoparticles surrounded by graphitic layers, which is reasonable considering that PB/GO has decomposed completely (Fig. 4b). As shown in Fig. 4c and S3,† the high-resolution N 1s of Fe–C/NG-10%-T-AL spectra are deconvoluted into four peaks, corresponding with pyridinic N (398.3 ± 0.18 eV), pyrrolic N (399.7 ± 0.22 eV), graphitic N (400.7 ± 0.28 eV) and oxidized N (403.1 ± 0.62 eV).46–50 The percentage of N species depending on pyrolyzing temperature is listed in Fig. 4d and Table S1,† which shows that the highest amount of pyridinic N (1.23 at%) and graphitic N (0.89 at%) on the Fe–C/NG-10%-700-AL which contribute to improving the ORR performance.51,52
3.2 The catalytic performance on Fe–C/NG-10%-700-AL in alkaline medium
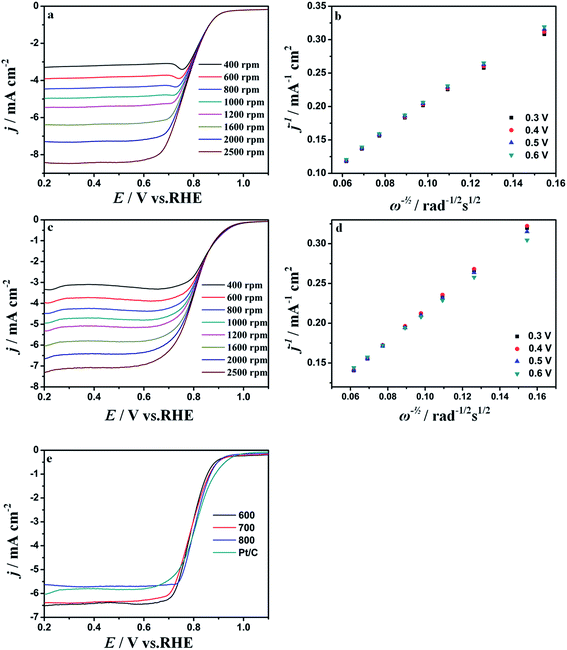
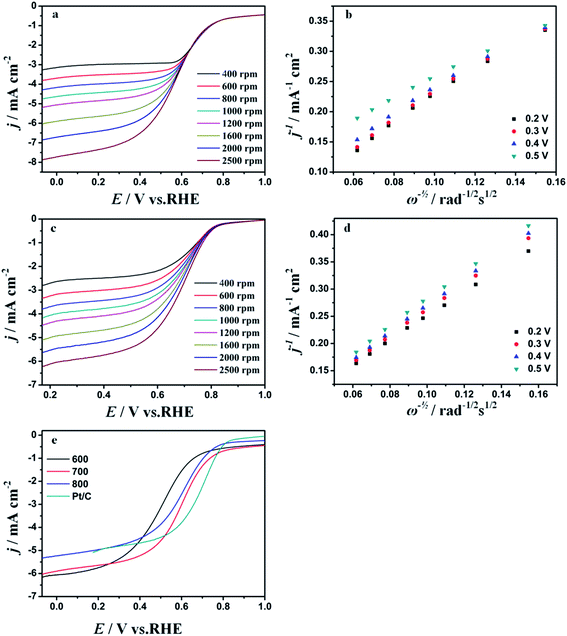
In order to demonstrate the electrocatalytic activity for Fe–C/NG-10%-T-AL, linear sweep voltammetry (LSV) measurements are carried on a rotating disk electrode (RDE) in O2-saturated 0.1 M KOH solution. The electrode potential is reported with respect to the reversible hydrogen electrode (RHE). The Fe–C/NG-10%-700-AL catalyst exhibits high onset potential (0.940 V) and the half-wave potential (0.793 V), which are similar to those of Pt/C catalyst, respectively. Furthermore, it shows the better ORR performance than other Fe–C/NG-10%-T-AL and Fe–C/NG-M-T-AL samples with the same catalyst loading (Fig. 5e and S4†). Simultaneously, Fe–C/NG-10%-700-AL exhibits similar advantages compared with other ORR catalysts containing iron carbide active species reported recently, the corresponding comparisons are given in Table S2.† RDE tests are carried out to study the reaction kinetics of the Fe–C/NG-10%-700-AL and the commercial Pt/C (Fig. 5a and c). The current density exhibits an increase with rotating rates from 400 to 2500 rpm ascribed to the shorten diffusion distance of electrolytes.53 The linear relationships between j−1 and ω−0.5 at various potentials are exhibited by the Koutecky–Levich (K–L) equation (Fig. 5b and d). The good linearity of these K–L plots and their similar slopes in the potential range of 0.3–0.6 V vs. RHE demonstrate a first-order ORR kinetics as to the concentration of dissolved O2 and suggest a similar electron transfer number for ORR on Fe–C/NG-10%-700-AL and Pt/C in the selected potential range, respectively.To analyze the kinetic properties of ORR by using the prepared electrocatalysts, the Tafel slopes are obtained from the linear plots of LSVs at 1600 rpm. Then, the excellent ORR activity of Fe–C/NG-10%-700-AL is further evaluated by the Tafel plots of the over potential vs. log (current density) with its linear portions at low overpotential. For the Tafel plots, the kinetic current was calculated from the mass-transport correction of the RDE using the following equation:
| JK = (J × JL)/(J − JL) | (3) |
The much smaller Tafel slope (Fig. 6a, −94 mV per decade) corresponds to a more favorable ORR kinetics over Fe–C/NG-10%-700-AL compared with that of the Pt/C (−103 mV per decade).
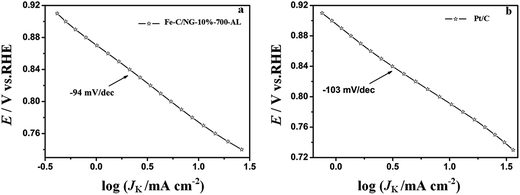 | ||
| Fig. 6 (a) Tafel plots for Fe–C/NG-10%-700-AL and (b) Pt/C-20% extracted from the data in panel Fig. 5a and c, respectively. | ||
To gain additional insight into the ORR process on Fe–C/NG-10%-700-AL, we employ a rotating ring-disk electrode (RRDE) technique with which the amount of hydrogen peroxide species generated at the disk electrode could be accurately detected as shown in Fig. 7a. On the basis of the ring and disk currents, the electron transfer number and the measured H2O2 yield at fixed potentials on Fe–C/NG-10%-700-AL and Pt/C are displayed in Fig. 7b and c, respectively. The output of H2O2 on both Fe–C/NG-10%-700-AL and Pt/C is below 6%, indicating hydroperoxide is scarcely measured. The observed hydrogen peroxide yield depends on the thickness of the catalyst layer. Hydrogen peroxide formed in thick catalyst layers such as that used in the present study may be reduced when traveling to the catalyst layer surface and thus undetected.
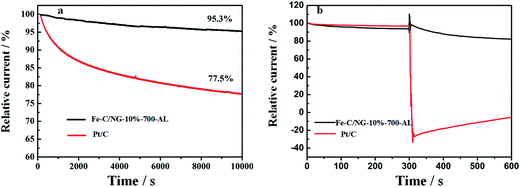
The stability of Fe–C/NG-10%-700-AL catalyst is evaluated by current–time chronoamperometry at a constant voltage of 0.708 V vs. RHE in O2-saturated 0.1 M KOH solution at a rotating speed of 1600 rpm. As shown in Fig. 8a, after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s, the commercial Pt/C suffer from 22.5% loss of the original current density, however, the composite could retain 95.3% of the initial current, suggesting the Fe–C/NG-10%-700-AL exhibits distinguished catalytic stability. Furthermore, the methanol crossover effect is estimated by observing the current density change after adding 3 M methanol at 300 s. A remarkable decrease in the ORR current density is observed for the commercial Pt/C catalyst (Fig. 8b). On the contrary, no noticeable change happens on the Fe–C/NG-10%-700-AL catalyst. These results convey to us that Fe–C/NG-10%-700-AL catalyst not only enjoys a superior durability toward ORR, but also exhibits great tolerance to methanol.
000 s, the commercial Pt/C suffer from 22.5% loss of the original current density, however, the composite could retain 95.3% of the initial current, suggesting the Fe–C/NG-10%-700-AL exhibits distinguished catalytic stability. Furthermore, the methanol crossover effect is estimated by observing the current density change after adding 3 M methanol at 300 s. A remarkable decrease in the ORR current density is observed for the commercial Pt/C catalyst (Fig. 8b). On the contrary, no noticeable change happens on the Fe–C/NG-10%-700-AL catalyst. These results convey to us that Fe–C/NG-10%-700-AL catalyst not only enjoys a superior durability toward ORR, but also exhibits great tolerance to methanol.
3.3 The catalytic performance on Fe–C/NG-10%-700-AL in acid medium
The Fe–C/NG-10%-700-AL catalyst also exhibits excellent ORR activity in acidic medium. LSVs are performed on the RDE with rotating rates from 400 to 2500 rpm in 0.5 M H2SO4 electrolyte saturated with O2 (Fig. 9a and c). The onset and half-wave potential are 0.828 and 0.597 V vs. RHE, respectively in O2-saturated 0.5 M H2SO4 solution which are roughly 0.016 and 0.074 V lower than those of the commercial Pt/C catalyst, respectively as shown in Fig. 8e. The K–L plots of both Fe–C/NG-10%-700-AL and Pt/C show good linearity with a similar slope at different potentials (Fig. 9b and d). Also, Fe–C/NG-10%-700-AL catalyst exhibits similar catalytic performance compared with other non-noble metal ORR catalysts as illustrated in Table S3.†Based on the above-mentioned results, the Fe–C/NG-10%-700-AL displays the superiorly electrocatalytic activity for ORR in both alkaline and acidic media stemming from the fact that the catalyst possesses the highest quantity of effective active sites (pyridinic N, graphitic N and Fe5C2, as shown in Table S1†), the high specific surface area (448.8 cm2 g−1) and unique porosity architecture.
Kinetic currents derived from the mass transport corrected ORR currents according to eqn (3) (Fig. 10a and b) show a Tafel slope of −242 and −210 mV per decade for Fe–C/NG-10%-700-AL and Pt/C at high overpotentials, respectively, while at low overpotentials the corresponding slopes are −186, −112 mV per decade, respectively. The Tafel slopes on Fe–C/NG-10%-700-AL are larger than those of Pt/C, indicative of a lower activity of our as-prepared catalyst, which agrees with the results of LSV test.
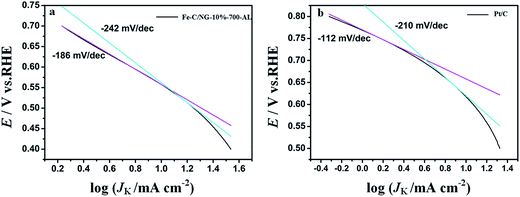 | ||
| Fig. 10 (a) Tafel plots for Fe–C/NG-10%-700-AL and (b) Pt/C-20% extracted from the data in panel Fig. 9a and c, respectively. | ||
To further investigate the ORR performance of Fe–C/NG-10%-700-AL, the RRDE test is carried out (Fig. 11a). The electron transfer number is determined to be about 4.0, indicating a 4e process in acid medium as shown in Fig. 11b. And the production of H2O2 species is lower than 7%, which is close to that of the commercial Pt/C within the potential range from 0.20 to 0.60 V (Fig. 11c).
The methanol crossover effect and stability toward ORR should be taken into consideration, when the catalytic performance is quantified and compared. The stability is evaluated on both Fe–C/NG-10%-700-AL and Pt/C (Fig. 12a). For Fe–C/NG-10%-700-AL catalyst, the current density decreases by 20.4% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s, and the value for Pt/C catalyst is 57.9%. The methanol crossover effects of the Fe–C/NG-10%-700-AL and Pt/C catalyst are estimated in O2-saturated acid medium as shown in Fig. 12b. When methanol added, the current density of Fe–C/NG-10%-700-AL has a little change, however, for the case of Pt/C the current density suffers from 50.7% decrease. The above results indicate that Fe–C/NG-10%-700-AL has good methanol tolerance and stability as a cathode catalyst in fuel cells.
000 s, and the value for Pt/C catalyst is 57.9%. The methanol crossover effects of the Fe–C/NG-10%-700-AL and Pt/C catalyst are estimated in O2-saturated acid medium as shown in Fig. 12b. When methanol added, the current density of Fe–C/NG-10%-700-AL has a little change, however, for the case of Pt/C the current density suffers from 50.7% decrease. The above results indicate that Fe–C/NG-10%-700-AL has good methanol tolerance and stability as a cathode catalyst in fuel cells.
4. Conclusion
In summary, we have demonstrated a facile and cost-effective method to fabricate a novel kind of porous carbon architectures functionalized by iron and nitrogen. The influence of pyrolysis temperature on the ORR activity has been investigated in detail. The surface area and the overall quantity of doped N on Fe–C/NG-10%-700-AL could be tailored by tuning pyrolysis temperature. Benefited from the high specific surface area (448.8 cm2 g−1), unique porosity architecture and high active site density, the Fe–C/NG-10%-700-AL exhibits superior activity, stability and methanol tolerance for ORR in both acidic and alkaline media. Consequently, the Fe–C/NG-10%-700-AL catalyst with low cost and superior electrocatalytic performance holds great promise to become an ideal candidate for the replacement of the commercial Pt/C as cathode catalyst for PEMFCs and other systems involving ORR in both acidic and alkaline solutions.Acknowledgements
The work has been supported by the Natural Science Foundation of China (No. 21603017 and 21273024) and Natural Science Foundation of Jilin Province, China (No. 20160101298JC).References
- X. J. Zhou, J. L. Qiao, L. Yang and J. J. Zhang, Adv. Energy Mater., 2014, 4, 1289–1295 Search PubMed.
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245–4269 CrossRef CAS PubMed.
- X. W. Yu and S. Y. Ye, J. Power Sources, 2007, 172, 145–154 CrossRef CAS.
- T. Palaniselvam, M. O. Valappil, R. Illathvalappil and S. Kurungot, Energy Environ. Sci., 2014, 7, 1059–1067 CAS.
- H. P. Cong, P. Wang, M. Gong and S. H. Yu, Nano Energy, 2014, 3, 55–63 CrossRef CAS.
- J. S. Zhang, A. Byeon and J. W. Lee, Chem. Commun., 2014, 50, 6349–6352 RSC.
- S. K. Ramasahayam, U. B. Nasini, V. Bairi, A. U. Shaikh and T. Viswanathan, RSC Adv., 2014, 4, 6306–6313 RSC.
- J. Y. Baek, I. Y. Jeon and J. B. Baek, J. Mater. Chem. A, 2014, 2, 8690–8695 CAS.
- Q. X. Lai, Q. Su, Q. W. Gao, Y. Y. Liang, Y. X. Wang, Z. Yang, X. G. Zhang, J. P. He and H. Tong, ACS Appl. Mater. Interfaces, 2015, 7, 18170–18178 CAS.
- Y. Hou, Z. H. Wen, S. M. Cui, S. Q. Ci, S. Mao and J. H. Chen, Adv. Funct. Mater., 2015, 25, 872–882 CrossRef CAS.
- J. Wei, Y. X. Hu, Y. Liang, B. Kong, J. Zhang, J. C. Song, Q. L. Bao, G. P. Simon, S. P. Jiang and H. T. Wang, Adv. Funct. Mater., 2015, 25, 5768–5777 CrossRef CAS.
- F. Jaouen and J.-P. Dodelet, J. Phys. Chem. C, 2009, 113, 15422–15432 CAS.
- M. Lefèvre, E. Proietti, F. Jaouen and J.-P. Dodelet, Science, 2009, 324, 71–74 CrossRef PubMed.
- Z. S. Wu, L. Chen, J. Z. Liu, K. Parvez, H. W. Liang, J. Shu, H. Sachdev, R. Graf, X. L. Feng and K. Müllen, Adv. Mater., 2014, 26, 1450–1455 CrossRef CAS PubMed.
- R. Ning, C. J. Ge, Q. Liu, J. Q. Tian, A. M. Asiri, K. A. Alamry, C. M. Li and X. P. Sun, Carbon, 2014, 78, 60–69 CrossRef CAS.
- Z. S. Wu, S. B. Yang, Y. Sun, K. Parvez, X. L. Feng and K. Mullen, J. Am. Chem. Soc., 2012, 134, 9082–9085 CrossRef CAS PubMed.
- Y. Hu, J. O. Jensen, W. Zhang, L. N. Cleemann, W. Xing, N. J. Bjerrum and Q. F. Li, Angew. Chem., Int. Ed., 2014, 53, 3675–3679 CrossRef CAS PubMed.
- G. Y. Zhong, H. J. Wang, H. Yu and F. Peng, J. Power Sources, 2015, 286, 495–503 CrossRef CAS.
- W. X. Yang, X. J. Liu, X. Y. Yue, J. B. Jia and S. J. Guo, J. Am. Chem. Soc., 2015, 137, 1436–1439 CrossRef CAS PubMed.
- Y. Cheng, A. Kondo, H. Noguchi, H. Kajiro, K. Urita, T. Ohba, K. Kaneko and H. Kanoh, Langmuir, 2009, 25, 4510–4513 CrossRef CAS PubMed.
- E. Jeong, W. R. Lee, D. W. Ryu, Y. Kim, W. J. Phang, E. K. Koh and C. S. Hong, Chem. Commun., 2013, 49, 2329–2331 RSC.
- R. Babu, A. C. Kathalikkattil, R. Roshan, J. Tharun, D. W. Kim and D. W. Park, Green Chem., 2016, 18, 232–242 RSC.
- B. Singco, L. H. Liu, Y. T. Chen, Y. H. Shih, H. Y. Huang and C. H. Lin, Microporous Mesoporous Mater., 2016, 223, 254–260 CrossRef CAS.
- J. Y. Han, D. P. Wang, Y. H. Du, S. B. Xi, J. D. Hong, Z. Chen, T. H. Zhou and R. Xu, J. Mater. Chem. A, 2015, 3, 20607–20613 CAS.
- O. Sato, T. Iyoda, A. Fujishima and K. Hashimoto, Science, 1996, 272, 704–705 CAS.
- T. Uemura and S. Kitagawa, J. Am. Chem. Soc., 2003, 125, 7814–7815 CrossRef CAS PubMed.
- S. Vaucher, J. Fielden, M. Li, E. Dujardin and S. Mann, Nano Lett., 2002, 2, 225–229 CrossRef CAS.
- P. H. Zhou, D. S. Xue, H. Q. Luo and X. G. Chen, Nano Lett., 2002, 2, 845–847 CrossRef CAS.
- J. Chen, K. L. Huang, S. Q. Liu and X. Hu, J. Power Sources, 2009, 186, 565–569 CrossRef CAS.
- L. Hu, P. Zhang, Q. W. Chen, H. Zhong, X. Y. Hu, X. R. Zheng, Y. Wang and N. Yan, Cryst. Growth Des., 2012, 12, 2257–2264 CAS.
- K. Wichapong, M. Lawson, S. Pianwanit, S. Kokpol and W. Sippl, J. Chem. Inf. Model., 2010, 50, 1574–1588 CrossRef CAS PubMed.
- Y. Hou, T. H. Huang, Z. H. Wen, S. Mao, S. M. Cui and J. H. Chen, Adv. Energy Mater., 2014, 4, 1220–1225 Search PubMed.
- M. B. Zakaria, RSC Adv., 2016, 6, 10341–11035 RSC.
- B. K. Barman and K. K. Nanda, Green Chem., 2016, 18, 427–432 RSC.
- G. X. Wang, X. P. Shen, B. Wang, J. Yao and J. Park, Carbon, 2009, 47, 1359–1364 CrossRef CAS.
- E. Jin, X. F. Lu, L. L. Cui, D. M. Chao and C. Wang, Electrochim. Acta, 2010, 55, 7230–7234 CrossRef CAS.
- M. L. Xiao, J. B. Zhu, L. G. Feng, C. P. Liu and W. Xing, Adv. Mater., 2015, 27, 2521–2527 CrossRef CAS PubMed.
- Y. G. Li, H. L. Wang, L. M. Xie, Y. Y. Liang, G. S. Hong and H. J. Dai, J. Am. Chem. Soc., 2011, 133, 7296–7299 CrossRef CAS PubMed.
- L. Chen, X. J. Wang, X. T. Zhang and H. M. Zhang, J. Mater. Chem., 2012, 22, 22090–22096 RSC.
- K. S. Xia, X. L. Tian, S. X. Fei and K. You, Int. J. Hydrogen Energy, 2014, 39, 11047–11054 CrossRef CAS.
- H. W. Liang, X. Zhuang, S. Brüller, X. Feng and K. Müllen, Nat. Commun., 2014, 5, 4973 CrossRef CAS PubMed.
- L. Y. Feng, Y. G. Chen and L. Chen, ACS Nano, 2011, 5, 9611–9618 CrossRef CAS PubMed.
- J. S. Li, S. L. Li, Y. J. Tang, M. Han, Z. H. Dai, J. C. Bao and Y. Q. Lan, Chem. Commun., 2015, 51, 2710–2713 RSC.
- L. Zhang, A. D. Zhang, D. Du and Y. H. Lin, Nanoscale, 2012, 4, 4674–4679 RSC.
- L. Wang, Y. J. Ye, H. Z. Zhu, Y. H. Song, S. J. He, F. G. Xu and H. Q. Hou, Nanotechnology, 2012, 23, 455502 CrossRef PubMed.
- P. H. Matter, L. Zhang and U. S. Ozkan, J. Catal., 2006, 239, 83–96 CrossRef CAS.
- S. Maldonado and K. J. Stevenson, J. Phys. Chem. B, 2005, 109, 4707–4716 CrossRef CAS PubMed.
- D. H. Long, W. Li, L. C. Ling, J. Miyawaki, I. Mochida and S. H. Yoon, Langmuir, 2010, 26, 16096–16102 CrossRef CAS PubMed.
- H. R. Byon, J. Suntivich and Y. Shao-Horn, Chem. Mater., 2011, 23, 3421–3428 CrossRef CAS.
- Y. Y. Shao, X. Q. Wang, M. Engelhard, C. M. Wang, S. Dai, J. Liu, Z. G. Yang and Y. H. Lin, J. Power Sources, 2010, 195, 4375–4379 CrossRef CAS.
- K. L. Ai, Y. L. Liu, C. P. Ruan, L. H. Lu and G. M. Lu, Adv. Mater., 2013, 25, 998–1003 CrossRef CAS PubMed.
- L. F. Lai, J. R. Potts, D. Zhan, L. Wang, C. K. Poh, C. H. Tang, H. Gong, Z. X. Shen, J. Y. Lin and R. S. Ruoff, Energy Environ. Sci., 2012, 5, 7936–7942 CAS.
- J. Liang, Y. Zheng, J. Chen, J. Liu, D. Hulicova-Jurcakova, M. Jaroniec and S. Z. Qiao, Angew. Chem., Int. Ed., 2012, 51, 3892–3896 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra24299e |
| This journal is © The Royal Society of Chemistry 2016 |