Theoretical study on the reaction mechanism in the UV radiation cross-linking process of polyethylene
Hui Zhang*a,
Yan Shanga,
Mingxia Li*b,
Hong Zhaoa,
Xuan Wanga and
Baozhong Han*a
aKey Laboratory of Engineering Dielectrics and Its Application of Ministry of Education, College of Chemical and Environmental Engineering, Harbin University of Science and Technology, Harbin 150080, People's Republic of China. E-mail: hust_zhanghui11@hotmail.com; hbzhlj@163.com; Fax: +86-451-86392708; Fax: +86-451-86391659
bKey Laboratory of Functional Inorganic Material Chemistry of Education Ministry, Laboratory of Physical Chemistry, School of Chemistry and Materials Science, Heilongjiang University, Harbin 150080, People's Republic of China. E-mail: limingxiaxxq@sina.com; Fax: +86-451-86604331
First published on 8th November 2016
Abstract
A theoretical investigation on the benzophenone-initiated UV radiation cross-linking reactions of polyethylene is accomplished at B3LYP/6-311+G(d,p) level for high-voltage cable insulation materials. The reaction potential energies of 9 reaction channels are identified in the T1 state. The HOMO–LUMO energy gaps, ionization potentials, and electron affinities of the polyethylene, photoinitiator, voltage stabilizer, antioxidant, and by-products in the polyethylene insulation composite products are obtained. The results show that the by-products, photoinitiator, voltage stabilizer, and antioxidant can effectively increase the electrical breakdown strength. In addition, aromatic ketone voltage stabilizer and hindered phenol antioxidants of the studied molecule can be grafted to the polyethylene chain easily during the polyethylene UV radiation cross-linking process, and they have excellent compatibility with the polymer matrix. The investigation is expected to provide reliable information for optimizing the polyethylene UV radiation cross-linking process and for the development of insulation material for high-voltage cable for use at voltages exceeding 500 kV in real applications.
1. Introduction
Cross-linked polyethylene (XLPE) has been widely used for high-voltage cable insulation materials because it has excellent electrical and mechanical properties and better thermal and environmental cracking resistance than polyethylene. There are many disadvantages in the traditional process of polyethylene peroxide cross-linking, such as slow production speed, high energy consumption and precross-linking of the material on the surface of the extrusion die. The ultra-violet (UV) radiation cross-linking process may become a candidate for high-voltage cable insulation cross-linking.1–6 UV energy can easily penetrate the insulation wall and induce cross-linking inside the material with the help of a photoinitiator. The advantages of UV cross-linking compared with the traditional method include fast processing speed and energy saving, and the production is not thermo-sensitive any more. The radiation zone is only 2 m long compared with as long as 36 m for the peroxide reaction process. The energy consumption is only tens of kilowatts when using a mercury lamp and UV light-emitting diode (LED) hybrid system. However, hundreds of kilowatts is necessary for the peroxide reaction process. Experimental investigations show that the rate of the UV radiation cross-linking reaction is not only influenced by the power and radiation spectrum of the UV lamp used in the radiation system, but the type and content of photoinitiator system mixed in the polyethylene matrix.4–6 On the other hand, it is necessary to study the effect of UV radiation cross-linking byproducts in bulk on insulating properties, because it is known that aromatic molecular structures may have a “voltage stabilizing” effect on the insulation. Initiation of electrical treeing resulting in partial discharge and insulation failure under high and divergent electric field is associated with the chemical structure of XLPE material in power cables.7,8 Research revealing the reaction mechanism of UV radiation cross-linking polyethylene at molecular level is not very clear so far. The rated voltage of XLPE insulated power cable is limited to 500 kV even though the XLPE compound is manufactured with super-clean technology. Elucidating possible chemical reactions during cross-linking of polyethylene plays a crucial role in developing insulation materials for high-voltage cables. Some organic polycyclic aromatic compounds and compounds with benzophenone-like structures can increase the resistance to electrical treeing effectively,9–14 serving as so-called voltage stabilizers. This may be the next solution for XLPE compounds for insulating power cables using in excess of 500 kV. Recently, our research group employed both experimental and computational methods to study the effect of acetophenone as voltage stabilizer. The addition of acetophenone leads to a 50% increase of the alternating current (AC) electrical breakdown strength of XLPE.15 By the theoretical studies, in 2013 we primarily elucidated the mechanisms of aromatic carbonyl compounds as voltage stabilizers for increasing the electrical breakdown strength of XLPE.16–18 However, acetophenone easily migrates out of the polymeric matrix, leading to worse performance of polyethylene insulation materials. Benzil-type compounds with a larger alkoxy chain were evaluated as voltage stabilizers in a super-clean XLPE by Jarvid and his co-workers.13,14 As a result, the electrical treeing inception level was effectively raised, and the compatibility with polyethylene matrix was also significantly improved. This inspired us to consider whether the excellent compatibility of voltage stabilizers with the polyethylene matrix can improve the electrical breakdown strength of XLPE, namely, if the voltage stabilizer molecule could be grafted to polyethylene molecule chain to yield inactive products during the UV radiation cross-linking process, the resulting XLPE insulation materials could possess long-lasting insulation performance.A spectrum of electronic transitions by symmetry are both spin and orbital forbidden. Such transitions are only allowed through the coupling of nuclear and electronic motion. Normally, the species undergo single electron excitations, the first excited states are triplets.19,20 The benzophenone intersystem crossing (ISC) from S1 (n, π*) to T1 (n, π*) states, for which direct transition is forbidden by El-Sayed rules, was thoroughly reinvestigated by Aloïse and co-workers.21 They proposed that the benzophenone intermediate species is a T1 (n, π*) triplet. Granucci and co-workers22 presented a simulation of the photodynamics of benzophenone for the first 20 ps after n → π* excitation; both the dynamic and spin–orbit couplings were taken into account to clarify the mechanism of the S1 → T1 decay. They proposed that the main intersystem crossing channel is due to S1 → T1 transitions. An oscillation with about the same frequency was observed by Spighi and co-workers.23 The sensitized phosphorescence excitation spectra of jet-cooled benzophenone have been measured by Ito and co-workers.24 The lowest excited singlet state is Sl (n, π*), originating from Sl (n, π*) ← S0 transition of 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180 cm−1 (3.25 eV). Additionally, the origin of the first triplet state T1 (n, π*) ← S0 appears at 24
180 cm−1 (3.25 eV). Additionally, the origin of the first triplet state T1 (n, π*) ← S0 appears at 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 224 cm−1 (3.00 eV). Benzophenone is very weakly fluorescent, but highly phosphorescent in isolated molecular condition. Fang25 has theoretically discovered a minimum energy crossing point among the three potential energy surfaces (S1, T1, and T2) that appears to be common to a wide variety of aromatic carbonyl compounds. The existence of the S1/T2/T1 intersection results in the S1 → T1 transition via the T2 state. The T2 state functions as a relay and enables the S1 → T1 intersystem crossing to take place with a high rate. This is the reason why the lifetime of the S1 state for aromatic ketones is much shorter than that for aliphatic ketones, and aromatic carbonyl compounds are highly phosphorescent. Therefore, in this work the investigation of benzophenone-initiated chemical reactions during the UV radiation cross-linking process has been made only for the lowest triplet state.
224 cm−1 (3.00 eV). Benzophenone is very weakly fluorescent, but highly phosphorescent in isolated molecular condition. Fang25 has theoretically discovered a minimum energy crossing point among the three potential energy surfaces (S1, T1, and T2) that appears to be common to a wide variety of aromatic carbonyl compounds. The existence of the S1/T2/T1 intersection results in the S1 → T1 transition via the T2 state. The T2 state functions as a relay and enables the S1 → T1 intersystem crossing to take place with a high rate. This is the reason why the lifetime of the S1 state for aromatic ketones is much shorter than that for aliphatic ketones, and aromatic carbonyl compounds are highly phosphorescent. Therefore, in this work the investigation of benzophenone-initiated chemical reactions during the UV radiation cross-linking process has been made only for the lowest triplet state.
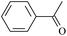
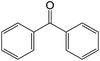
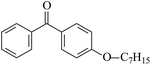
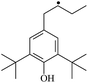
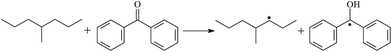
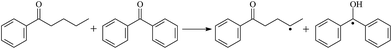
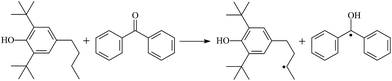
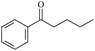
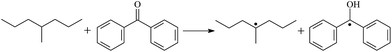
The possibility of voltage stabilizer grafting to the polyethylene molecule chain in the UV radiation cross-linking process has been calculated using density functional theory (DFT).26 The main materials for preparing XLPE generally comprise low-density polyethylene (LDPE), benzophenone (as photoinitiator), hindered phenol antioxidant (used to improve the thermal and oxygen aging properties of polyethylene), voltage stabilizer, and triallyl isocyanurate (TAIC, as multi-functional crosslinker). All these materials are mixed and then extruded to form the insulation wall of the cable on the conduction core at 180 °C. In this work, three molecules, 4-methylheptane (Pe), valerophenone (Vp), and 2,6-di-t-butyl-4-n-butylphenol (Bp), were selected as model molecules of cross-linkable polyethylene, voltage stabilizer, and antioxidant, respectively. The molecular formulae, molecular names, and corresponding abbreviations of the studied molecules are listed in Table 1. During the UV radiation cross-linking process, the cross-linkable polyethylene compound undergoes a lot of complicated reactions. First, benzophenone in the ground state is transformed into the singlet excited state by absorbing the UV emitted from optical system energy and is then transformed into the triplet excited state through intersystem crossing (ISC); then the triplet excited state of benzophenone can undergo hydrogen abstraction reaction with the cross-linkable polyethylene molecule chain, the voltage stabilizer molecule chain, antioxidant molecule chain, or the triallyl isocyanurate molecule chain, leading to the generation of the corresponding radicals; finally, these corresponding free radicals will react with each other to produce inactive products. The polyethylene molecule chain radicals can react not only with other polyethylene molecule chain radicals to form the macromolecular cross-linked polyethylene net structure, but also with molecular radicals of the voltage stabilizer, the antioxidant, or the TAIC to yield inert products, which leads to the grafting of voltage stabilizer and antioxidant to the polyethylene chain, so that the compatibility between polymer matrix and additives has been improved. Benzopinacol is by-product.
In this paper, we aimed at providing a systematic theoretical investigation of the possibility of the benzophenone-initiated reaction and the dominant reaction channel during the polyethylene UV radiation cross-linking process in the presence of voltage stabilizer. The UV radiation cross-linking reaction mechanism would be useful for the rational molecular design of additives and selection of the optimal process of the UV radiation cross-linking for real applications.
2. Computational methods
The equilibrium geometries of all the stationary points of the neutral and ion states of the studied molecules were optimized in the ground state using the B3LYP27–30 function with the 6-311+G(d,p) basis set, and the frequencies were also calculated by the same method in this work, except for the stationary points of 9 reaction channels in the triplet state. The B3LYP//6-311+G(d,p) level was confirmed to be suitable for the current study owing to the computational values of the adiabatic ionization potentials IP(a) and the electron affinities EA(a) at this level being in very good agreement with the corresponding experimental values in our previous paper.31 On the basis of these calculations, the energy gap (Eg) between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), the ionization potential (IP), and the electron affinity (EA) were obtained. The minimum energy path (MEP) was obtained by intrinsic reaction coordinate (IRC) theory with a gradient step-size of 0.05 (amu)1/2 bohr. Then, the first and second energy derivatives were obtained to calculate the curvature of the reaction path and the generalized vibrational frequencies along the reaction path. All the electronic structure calculations were performed using the GAUSSIAN09 program package.32 The relevant schematic formulae can be defined as follows: IP(v) = E+(M) − E(M); IP(a) = E+(M+) − E(M); EA(v) = E(M) − E−(M); EA(a) = E(M) − E−(M−), where E+(M+), E−(M−), and E(M) represent the energies of the cation, anion and neutral species, respectively, in their lowest energy geometries, while E+(M) and E(M+) refer to the energies of cation and neutral species with the geometries of neutral and cation, respectively; E−(M) and E(M−) are the energies of anion and neutral species with the geometries of neutral and anion, respectively, and v and a represent vertical energy based on the geometry of the neutral molecule and adiabatic energy from the optimized structure for both the neutral and charged molecule, respectively. A schematic description of geometric coordinate modifications and energy changes is shown in Fig. 1.3. Results and discussion
3.1. Stationary point geometries
The optimized geometric structures in the first triplet state T1 of the reactants, transition states, and products of 9 photoinitiator-initiated reactions at the B3LYP/6-311+G(d,p) level are presented in Fig. 2; other optimized equilibrium geometries of the stationary points in its ground state are also presented in Fig. 2. Optimized bond lengths of breaking and forming bonds for the 9 transition states, the corresponding reactant C–H bonds, and the product O–H bond, and calculated harmonic vibrational frequencies are listed in Table 2. Summaries of the chemical reaction equations of the possible photoinitiator-initiated reactions in the upper cross-linking production processes are included. All the transition states were confirmed by normal-mode analysis to have only one imaginary frequency, corresponding to the stretching modes of the coupling breaking and forming bonds. The other harmonic vibrational frequencies were confirmed by normal-mode analysis to have real frequency. The transition states (TSs) are abbreviated to the corresponding TS; reaction channels are represented by R. In Table 2, it can be seen that the transition state structures in the T1 state of the 9 studied hydrogen abstraction reactions have a common character: the elongation of the C–H breaking bonds in model molecules Pe, Vp, and Bp is less than that of the corresponding forming O–H bond in equilibrium Bzoh, indicating that these hydrogen abstraction reactions are all reaction-like, i.e., the reaction pathways will proceed via “early” transition states for an exothermic reaction, which is consistent with Hammond's postulate.333.2. Energies: frontier MOs, IPs, and EAs
Ionization potential and electron affinity energy of a molecule are important parameters to estimate the ability for it to be reduced and oxidized, respectively. In Table 3, the calculated values of the vertical and adiabatic IPs, EAs at the B3LYP/6-311+G(d,p) level and the corresponding experimental data34 (in parentheses) are listed, as well as the calculated HOMO–LUMO energy gaps (Eg). The varied trends of the ionization potentials and electron affinities are similar to those of the negative values of the corresponding HOMO and LUMO energies, respectively. The ability of a molecule to accept a hot electron is closely related to its LUMO. In Table 3, where there are σ electrons in a carbon chain, the value of Eg depends on the energy difference between orbitals σ and σ* (σ → σ*); Eg decreases with increasing carbon chain; the values of Eg (Vp, 5.19 eV) and Eg (Bzoc, 4.59 eV) are lower than those of Ap and Bzo. The introduction of phenyl or heteroatom groups into the molecule is propitious for electronic dissociation because of the high HOMO energy levels and small ionization potentials in terms of Koopmans' theorem; the value of Eg is the energy gap of π → π*. Thus, Eg (Bz, 4.90 eV) < Eg (Ap, 5.20 eV) < Eg (Pe, 8.38 eV), and Eg (Bzo, 4.61 eV) < Eg (Bz0). And π–π–π conjugation effects between carbonyl groups and benzene rings are formed, and a p–π conjugation effect is also formed when an –OR group is linked to the benzene ring in Bzo. The conjugated system in Bzo is larger than that of Bz, and as a result, it can also be concluded that Eg (Bzo) < Eg (Bz). The results are in accordance with the conclusion of Jarvid, Englund, and their co-workers,12–14 that the introduction of carbonyl and alkoxy into benzophenone molecules can effectively decrease the Eg value and these groups possess excellent compatibility with the polymer matrix. The energy of electronic transition is smaller when Eg decreases. In addition, the –OR groups in Bzo or Bzoc will exhibit inductive electron-donating effects, which means the electron densities of the benzene rings in Bzo or Bzoc are larger than that of Bz, and the ability to accept electrons is weaker. For instance, EA(a) (Bzo, 0.59 eV) < EA(a) (Bz, 0.73 eV) and EA(a) (Bzoc, 0.57 eV) < EA(a) (Bz). As carbonyl is an electron-withdrawing group, whereas –OH is an electron-donating group, the electron density on the benzene ring in Bp is larger than that in Vp, resulting in the ability to accept electrons being weaker in Bp than in Vp; EA (Bp, −0.73) < EA (Vp, 0.33). Similarly, EA (PBz, −0.03) < EA (Bz, 0.73).According to the production process of UV radiation cross-linking polyethylene insulation material for high-voltage cable, the by-product, photoinitiator, multi-functional crosslinker, voltage stabilizer, and antioxidant are usually adulterated in the polyethylene insulation composite product by, for example, the studied model molecules Bz, TAIC, Vp, Bp, and PBz. These molecules with carbonyl and/or benzene ring(s) possess larger EA(a) than Pe and smaller IP(v) values than Pe owing to their lower LUMO and higher HOMO energy levels. They possess lower barriers to accepting electrons. These conjugated aromatic carbonyl and/or benzene ring molecules possess stronger capability of trapping electrons than that of an aliphatic chain. They also possess lower IP(v) than Pe, and give rise to collision ionization before the polyethylene chain has been ionized, and so degradation of the polymer matrix can be prevented when these conjugated aromatic carbonyl or benzene ring molecules are doped into the polyethylene compound, which is consistent with the suggestion of Ashcraft et al.7
The carrier (electrons) may appear in XLPE-insulated high-voltage cable because of the local high electric field. The initial electrons that gain enough kinetic energy, known as “hot electrons”, can cause dielectric breakdown. The above-mentioned aromatic carbonyl or benzene ring molecules in XLPE insulation composite product can trap the hot electrons and decrease the kinetic energy of the hot electrons, so that the hot electrons do not have not enough energy to break the C–C bonds of XLPE. In addition, these molecules can also decrease Eg with decreasing transition energy, accomplish electronic transition to dissipate hot electron energy, and prevent degradation of the polymer matrix. As a result, they can effectively inhibit the initiation and propagation of electrical treeing in XLPE and simultaneously increase the electrical breakdown strength that XLPE can endure.
3.3. Energetics
The reaction enthalpies at 298 K (ΔH0298) and the potential barrier heights (ΔETS) with zero-point energy (ZPE) corrections in the T1 state obtained at the B3LYP/6-311+G(d,p) level are listed in Table 4, as well as the relative breaking bond dissociation energies (Do298). Breaking bond dissociation energies are closely related to the corresponding reaction potential barrier heights. The calculated result shows that the bond dissociation energy of the H atom in the hydroxyl of the antioxidant Bp (3.12 eV) is lower than that of H atoms in alkyl H-Bp4 (3.56 eV; or Bp3 4.06 eV, Bp2 4.05 eV). This means that antioxidants are often used to kill free radicals in XLPE insulation materials for high-voltage cables. Here, we aimed at investigating the possibility of the radical formation of Vp and Bp compared with Pe during the production process of UV radiation cross-linking polyethylene insulation material for high-voltage cables. The calculated ΔET1–S0 (the relative energy margins between S0 and T1 states) at the QCISD(T)//B3LYP level of acetophenone is 74.92 kcal mol−1, from our previous work,17 showing good consistency with the experimental reported value of 73.74 kcal mol−1.24 The calculated ΔET1–S0 at the QCISD(T)/6-311+G(3df,2p)//B3LYP/6-311+G(d,p) level of benzophenone is 2.95 eV in this work, in good agreement with the experimental value 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 224 cm−1 (3.00 eV) reported by Ito and co-workers.24 During the UV radiation cross-linking process, benzophenone is excited from the ground state S0 into the singlet excited state S1 and then transforms into the triplet excited state T1 through ISC; benzophenone in the T1 state would initiate a hydrogen abstraction reaction with Pe, Vp, or Bp, forming the corresponding radicals. The reaction potential barrier of forming Pe2 is close to that of forming Vp2 and Bp2. This means that it is possible to form Vp2 and Bp2 when forming Pe2, that is to say, the polyethylene molecule chain radical Pe2 can not only react with other Pe2 radicals to form the XLPE, but also with the voltage stabilizer Vp2 and the antioxidant Bp2 radicals to yield inactive products, resulting in the voltage stabilizer and antioxidant being able to graft to the polyethylene chain in the UV radiation cross-linking process. There, the reaction channels RVp2 and RBp2 are easier than RPe2 owing to the larger electronegativity of O than of C and the aromatic ketone in Vp2 exhibiting a conjugation effect. A similar case is also found in the reaction of Bp. The potential barrier to reaction channel RBp4 is smaller than that of the corresponding reaction channel RPe4, because the breaking bond dissociation energy in Bp4 (3.56 eV) is smaller than that in Pe4 (3.91 eV). Therefore, the H in Bp4 can dissociate easily and lead to more facile Bp4 radical formation with a lower energy barrier. The 9 reaction channels listed in Table 4 are exothermic reactions; the reaction channels RVp4 and RBp4 are more exothermic than the others, and as a result, the former reaction channels are more thermodynamically favorable with increasing temperature up to 180 °C. Vp may be effective as a voltage stabilizer in real application for increasing the breakdown strength of insulating XLPE material as it can be bonded to the polyethylene chain in the UV radiation cross-linking process. Further work to account for the TAIC reaction mechanism of accelerating the UV radiation cross-linking polyethylene process for high-voltage insulation cable is under way. New experimental and theoretical efforts are required in order to obtain a definitive mechanism.
224 cm−1 (3.00 eV) reported by Ito and co-workers.24 During the UV radiation cross-linking process, benzophenone is excited from the ground state S0 into the singlet excited state S1 and then transforms into the triplet excited state T1 through ISC; benzophenone in the T1 state would initiate a hydrogen abstraction reaction with Pe, Vp, or Bp, forming the corresponding radicals. The reaction potential barrier of forming Pe2 is close to that of forming Vp2 and Bp2. This means that it is possible to form Vp2 and Bp2 when forming Pe2, that is to say, the polyethylene molecule chain radical Pe2 can not only react with other Pe2 radicals to form the XLPE, but also with the voltage stabilizer Vp2 and the antioxidant Bp2 radicals to yield inactive products, resulting in the voltage stabilizer and antioxidant being able to graft to the polyethylene chain in the UV radiation cross-linking process. There, the reaction channels RVp2 and RBp2 are easier than RPe2 owing to the larger electronegativity of O than of C and the aromatic ketone in Vp2 exhibiting a conjugation effect. A similar case is also found in the reaction of Bp. The potential barrier to reaction channel RBp4 is smaller than that of the corresponding reaction channel RPe4, because the breaking bond dissociation energy in Bp4 (3.56 eV) is smaller than that in Pe4 (3.91 eV). Therefore, the H in Bp4 can dissociate easily and lead to more facile Bp4 radical formation with a lower energy barrier. The 9 reaction channels listed in Table 4 are exothermic reactions; the reaction channels RVp4 and RBp4 are more exothermic than the others, and as a result, the former reaction channels are more thermodynamically favorable with increasing temperature up to 180 °C. Vp may be effective as a voltage stabilizer in real application for increasing the breakdown strength of insulating XLPE material as it can be bonded to the polyethylene chain in the UV radiation cross-linking process. Further work to account for the TAIC reaction mechanism of accelerating the UV radiation cross-linking polyethylene process for high-voltage insulation cable is under way. New experimental and theoretical efforts are required in order to obtain a definitive mechanism.
4. Conclusion
A systematic theoretical study on the benzophenone-initiated reaction mechanisms in the polyethylene UV radiation cross-linking process has been carried out at the atomic and molecular levels. Aromatic ketone voltage stabilizer Vp and hindered phenol antioxidant Bp molecules can be bonded to the polyethylene chain during the polyethylene UV radiation cross-linking process. The adulterant molecules benzophenone, voltage stabilizer, antioxidant, and by-products in the XLPE insulation composite product with carbonyl or phenyl groups can effectively prevent hot electrons bombarding C–C bonds of the XLPE matrix under the local high electric field. The suggested mechanism for increasing the breakdown strength of XLPE may provide reliable information to optimize the UV radiation cross-linking process, to select photoinitiator and antioxidant, and to design the perfect voltage stabilizer for real applications.Acknowledgements
We thank Professor Tierui Zhang (Key Laboratory of Photochemical Conversion and Optoelectronic Materials, Technical Institute of Physics and Chemistry (TIPC), Chinese Academy of Sciences (CAS), Beijing 100190, PR China) for his fruitful discussions and checking English. This work was supported by the National Basic Research Program of China (2012CB723308), the National Natural Science Foundation of China (51337002, 21201059 and 50977019), a Doctoral Foundation from the Ministry of Education of China (20112303110005), and a Science Foundation for Distinguished Young Scholar of Heilongjiang Province (JC201206).References
- B. Qu and B. Rånby, Photocross-linking of Low-density Polyethylene. I. Kinetics and Reaction Parameters, J. Appl. Polym. Sci., 1993, 48, 701–709 CrossRef CAS.
- B. Qu, W. Bao, Q. Wu and W. Shi, Recent Developments on Photoinitiated Crosslinking of Polyethylene and Its Applications for Manufacturing Insulated Wire and Cable, Int. Conf. Prop. Appl. Dielectr. Mater., 2009, 33–36 Search PubMed.
- Y. Qing and B. Rånby, Photoinitiated Crosslinking of Low Density Polyethylene. IV: Continuous Extrusion Application, Polym. Eng. Sci., 1992, 32, 831–835 CAS.
- Q. Wu and B. Qu, Photoinitiating Characteristics of Benzophenone Derivatives as New Initiators in the Photocrosslinking of Polyethylene, Polym. Eng. Sci., 2001, 41, 1220–1226 CAS.
- Y. Qing, W. Xu and B. Rånby, Photoinitiated Crosslinking of Low Density Polyethylene I: Reaction and Kinetics, Polym. Eng. Sci., 1991, 31, 1561–1566 Search PubMed.
- B. Qu, W. Shi and B. Rånby, Studies of Photocrosslinking of Polyethylene in the Melt, Polym. Mater. Sci. Eng., 1990, 6, 37–43 CrossRef CAS.
- A. C. Ashcraft, R. M. Eichhorn and R. G. Shaw, Laboratory Studies of Treeing in Solid Dielectrics and Voltage Stabilization of Polyethylene, IEEE Int. Symp. Electr. Insul., 1976, 6 Search PubMed.
- M. Jarvid, A. Johansson, V. Englund, A. Lundin, S. Gubanski, C. Müller and M. R. Andersson, High Electron Affinity: A Guiding Criterion for Voltage Stabilizer Design, J. Mater. Chem. A, 2015, 3, 7273–7286 CAS.
- Y. Yamano and H. Endoh, Increase in Breakdown Strength of PE Film by Additives of Azocompounds, IEEE Trans. Dielectr. Electr. Insul., 1998, 5, 270–275 CrossRef CAS.
- Y. Yamano, Roles of Polycyclic Compounds in Increasing Breakdown Strength of LDPE Film, IEEE Trans. Dielectr. Electr. Insul., 2006, 13, 773–781 CrossRef CAS.
- Y. Yamano and M. Iizuka, IEEE Trans. Dielectr. Electr. Insul., 2009, 16, 189–198 CrossRef CAS.
- V. Englund, R. Huuva and S. M. Gubanski, IEEE Trans. Dielectr. Electr. Insul., 2009, 16, 1455–1460 CrossRef CAS.
- M. Jarvid, A. Johansson, V. Englund, S. Gubanski and M. R. Andersson, Electrical Tree Inhibition by Voltage Stabilizers, Annu. Rep.–Conf. Electr. Insul. Dielectr. Phenom., 2012, 605–608 CAS.
- M. Jarvid, A. Johansson, J. M. Bjuggren, H. Wutzel, V. Englund, S. Gubanski, C. Müller and M. R. Andersson, Tailored Side-Chain Architecture of Benzil Voltage Stabilizers for Enhanced Dielectric Strength of Cross-Linked Polyethylene, J. Polym. Sci., Part B: Polym. Phys., 2014, 52, 1047–1054 CrossRef CAS.
- H. Zhang, Y. Shang, H. Zhao, B. Z. Han and Z. S. Li, Study of the Effect of Valence Bond Isomerizations on Electrical Breakdown by Adding Acetophenone to Polyethylene as Voltage Stabilizers, Comput. Theor. Chem., 2015, 1062, 99–104 CrossRef CAS.
- H. Zhang, Y. Shang, H. Zhao, B. Z. Han and Z. S. Li, Mechanisms on Inhibition of Polyethylene Electrical Tree Aging: A Theoretical Study, J. Mol. Model., 2013, 19, 3035–3044 CrossRef CAS PubMed.
- H. Zhang, Y. Shang, H. Zhao, B. Z. Han and Z. S. Li, Mechanisms on Electrical Breakdown Strength Increment of Polyethylene by Acetophenone and its Analogues Addition: A Theoretical Study, J. Mol. Model., 2013, 19, 4477–4485 CrossRef CAS PubMed.
- H. Zhang, Y. Shang, X. Wang, H. Zhao, B. Z. Han and Z. S. Li, Mechanisms on Electrical Breakdown Strength Increment of Polyethylene by Aromatic Carbonyl Compounds Addition: A Theoretical Study, J. Mol. Model., 2013, 19, 5429–5438 CrossRef CAS PubMed.
- Ó. Rubio-Pons, O. Loboda, B. Minaev, B. Schimmelpfennig, O. Vahtras and H. Ågren, CASSCF Calculations of Triplet-state Properties. Applications to Benzene Derivatives, Mol. Phys., 2003, 101, 2103–2114 CrossRef.
- B. F. Minaev, S. Knuts, H. Ågren and O. Vahtras, The Vibronically Induced Phosphorescence in Benzene, Chem. Phys., 1993, 175, 245–254 CrossRef CAS.
- S. Aloïse, C. Ruckebusch, L. Blanchet, J. Réhault, G. Buntinx and J. P. Huvenne, The Benzophenone S1 (n, π*) → T1 (n, π*) States Intersystem Crossing Reinvestigated by Ultrafast Absorption Spectroscopy and Multivariate Curve Resolution, J. Phys. Chem. A, 2008, 112, 224–231 CrossRef PubMed.
- L. Favero, G. Granucci and M. Persicob, Surface Hopping Investigation of Benzophenone Excited State Dynamics, Phys. Chem. Chem. Phys., 2016, 18, 10499–10506 RSC.
- G. Spighi, M. A. Gaveau, J. M. Mestdagh, L. Poisson and B. Soep, Phys. Chem. Chem. Phys., 2014, 16, 9610–9618 RSC.
- N. Ohmori, T. Suzuki and M. Ito, Why Does Intersystem Crossing Occur in Isolated Molecules of Benzaldehyde, Acetophenone, and Benzophenone?, J. Phys. Chem., 1988, 92, 1086–1093 CrossRef CAS.
- W. H. Fang, Ab Initio Determination of Dark Structures in Radiationless Transitions for Aromatic Carbonyl Compounds, Acc. Chem. Res., 2008, 41, 452–457 CrossRef CAS PubMed.
- R. G. Parr and W. Yang, Density-functional Theory of Atoms and Molecules, Oxford University Press, New York, 1989 Search PubMed.
- T. N. Truong, W. T. Duncan and R. L. Bell, Chemical Applications of Density-Functional Theory, American Chemical Society, Washington, DC, 1996, p. 85 Search PubMed.
- C. Lee, W. Yang and R. G. Parr, Development of the Colle–Salvetti Correlation Energy Formula into a Functional of the Electron Density, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS.
- B. Miehlich, A. Savin, H. Stoll and H. Preuss, Results Obtained with the Correlation Energy Density Functionals of Becke and Lee, Yang and Parr, Chem. Phys. Lett., 1989, 157, 200–206 CrossRef CAS.
- A. D. Becke, Density-functional Thermochemistry. III. The Role of Exact Exchange, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- H. Zhang, Y. Shang, M. X. Li, H. Zhao, X. Wang and B. Z. Han, Theoretical Study on the Radical Reaction Mechanism in the Cross-linking Process of Polyethylene, RSC Adv., 2015, 5, 90343–90353 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Revision A.02, Gaussian, Inc, Wallingford CT, 2009 Search PubMed.
- G. S. Hammond, A Correlation of Reaction Rates, J. Am. Chem. Soc., 1955, 77, 334–338 CrossRef CAS.
- S. G. Lias, R. D. Levin, S. A. Kafafi and J. E. Bartmess, in NIST ChemistryWebBook, NIST Standard Reference Database Number 69, 2016 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |