Novel incorporation of mesoporous NiCo2O4 into thermoplastic polyurethane for enhancing its fire safety
Abstract
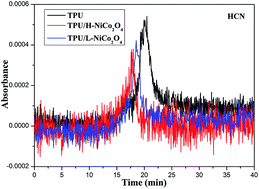
In this work, two kinds of NiCo2O4 particles are synthesized and their structures are confirmed from X-ray diffraction patterns and Fourier transform infrared spectra. The excellent dispersion of the particles in thermoplastic polyurethane (TPU) is confirmed by transmission electron microscopy. Char residue yield of TPU is enhanced obviously after the incorporation of NiCo2O4, suggesting a catalyzing carbonization effect. Toxic gases released from decomposition of the polymer, such as HCN, are fatal to humans, so it is of great significance to evaluate the toxic gases from TPU composites by thermogravimetric analysis/infrared spectrometry. The release of HCN is inhibited effectively by NiCo2O4, and this can be attributed to the gas adsorbing and barrier effects of the mesoporous particles. In addition, the amount of flammable gases is also reduced markedly. Moreover, the peak heat release rate and total heat release of TPU are decreased appreciably by NiCo2O4 with a higher specific surface area.

 Please wait while we load your content...
Please wait while we load your content...