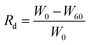BMSCs-laden gelatin/sodium alginate/carboxymethyl chitosan hydrogel for 3D bioprinting†
Jie Huang‡
a,
Han Fu‡ad,
Zhiying Wanga,
Qingyuan Mengb,
Sumei Liub,
Heran Wangc,
Xiongfei Zhengc,
Jianwu Dai*ab and
Zhijun Zhang*a
aKey Laboratory of Nano-Bio Interface, Division of Nanobiomedicine, CAS Center for Excellence in Nanoscience, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou 215123, Jiangsu, P. R. China. E-mail: jwdai@genetics.ac.cn; zjzhang2007@sinano.ac.cn
bState Key Laboratory of Molecular Developmental Biology, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing 100190, P. R. China
cState Key Laboratory of Robotics, Shenyang Institute of Automation, Chinese Academy of Sciences, Shenyang 110016, P. R. China
dUniversity of Chinese Academy of Sciences, 19(A) Yuquan Road, Beijing, 100039, P. R. China
First published on 8th November 2016
Abstract
Three-dimensional (3D) bioprinting technology offers the possibility to deliver, in a defined and organized manner, scaffolding materials and living cells, and therefore holds much promise for tissue engineering and regenerative medicine. In this work, we explored the possibility of gelatin/sodium alginate/carboxymethyl chitosan (Gel/SA/CMCS) hydrogel combining with bone mesenchymal stem cells (BMSCs) for 3D bioprinting. Compared to the commonly used 3D bioprinting materials such as gelatin/sodium alginate (Gel/SA) hydrogel, the Gel/SA/CMCS hydrogel we developed shows excellent equilibrium water content, good mechanical properties, antibacterial activity, and a slow degradation rate. With this kind of material, we generated a scaffold with homogenous cell distribution. Cell viability after 3D printing showed that over 85% of the printed cells were viable at 0 and 2 days of culturing. Our work demonstrates the feasibility of mixing Gel/SA/CMCS hydrogel with stem cells for 3D bioprinting.
Introduction
Tissue engineering is most promising in alleviating organ shortages while minimizing side effects and maximizing the patient's quality of life.1 The majority of strategies currently used in tissue engineering involve employing a scaffold, which is used to organize cells in a three-dimensional (3D) structure to direct the growth and formation of the desired tissue.2 Nowadays, 3D bioprinting approaches utilizing layer-by-layer additive fabrication technology have recently been employed to produce a 3D structure that can exhibit high resolution geometric and mechanical complexity.3,4 3D bioprinting is an attractive biocompatible rapid prototyping technique, which can follow computer-assisted design to build a complex bioactive tissue construct with desired pattern, shape and size, and biological functions. Unlike other rapid prototyping techniques (e.g. stereolithography and selective laser sintering), 3D bioprinting can simultaneously deposit live cells, growth factors along with biomaterial scaffolds at precisely controlled locations to mimic the native tissue architecture.5 Moreover, by changing certain parameters in the 3D printing settings, it is possible to tune the porosity of printed scaffolds. And porosity is an important parameter that can affect cell survival and activity, because proper porosity provides a high contact area between the implant and the surrounding fluids, thus facilitating oxygen and nutrient supply to encapsulated cells.6In 3D bioprinting, hydrogels are most often used as a cell carrier, owing to their high physicochemical and mechanical tenability7,8 and permeability to nutrients and waste for encapsulated cells.9,10 The hydrogels have to meet a number of requirements in order to be suitable for 3D bioprinting.11 First, the rheological properties of the hydrogels must enable the rapid deposition of strands, ensuring the integrity configuration of the scaffold during printing. Second, the hydrogels must have adequate mechanical properties to allow the construct to retain its shape after printing. Third, the hydrogels must be cytocompatible and allow for proliferation and differentiation of the encapsulated cells.
There are many reports on 3D bioprinting utilizing living cells and hydrogel scaffolds such as gelatin,12 collagen,13 silk fibroin,14 calcium alginate,15 and chitosan.16 Among them, alginate, which could be cross linked by Ca2+, is easy to fabricate 3D structures for its relatively high stiffness. Sluijter et al. have demonstrated that hCMPCs can be printed and cultured in alginate scaffolds and that culture conditions did not modify growth and commitment of the cells.17 Butcher et al. used the alginate/gelatin complex hydrogel to fabricate living heterogeneous aortic valve conduits by 3D bioprinting.18 They found that the gelatin could accelerate the gel time of alginate/gelatin hydrogel at 4 °C. However, the morphology of the hydrogels composed of alginate/gelatin was not very ideal. Chitosan, a natural polymer, is a highly versatile biomaterial. Contrary to many synthetic polymers, chitosan has a hydrophilic surface that promotes cell adhesion and proliferation and its degradation products are non-toxic. More importantly, the hydrogel based on the mixture of the alginate with chitosan has many advantages for 3D scaffolds. Alginate–chitosan scaffolds can be constructed by ionical bonding between the amine group of chitosan and the carboxyl group of alginate, which results in improved mechanical strength compared to chitosan or alginate alone.19 Additional attributes of alginate/chitosan complex-based hydrogels include good biocompatibility, controllable biodegradability, lasting structural stability, and minimal immunogenicity.20,21
Mesenchymal stem cells isolated from bone marrow (BMSCs) represent an attractive cell source for 3D bioprinting owing to their accessibility and inherent ability to differentiate into many kinds of cell types.22 In the present study, the hydrogel consisting of gelatin (Gel), sodium alginate (SA), and chitosan (CS) was generated and BMSCs was encapsulated within the hybrid hydrogel for 3D bioprinting (Scheme 1). Here, gelatin expedited gelation at 15 °C during printing process, sodium alginate cross linked with Ca2+ to maintain the morphology of the 3D scaffolds, and chitosan improved the mechanical strength of the complex hydrogel. Moreover, to improve the aqueous solubility of chitosan, we modified the chitosan with carboxymethyl groups. We found that the gelatin/sodium alginate/carboxymethyl chitosan (Gel/SA/CMCS) hydrogel shows better performance, such as excellent equilibrium water content, good mechanical properties, antibacterial effect, and slow degradation rate than that of sodium alginate/gelatin (Gel/SA) hydrogel. The cells in Gel/SA/CMCS hydrogel showed good viability at 0 and 2 days after printing, suggesting that Gel/SA/CMCS hydrogel is a suitable candidate for 3D bioprinting.
 | ||
| Scheme 1 Schematic illustration of combined use of gelatin, sodium alginate, and chitosan for 3D bioprinting. | ||
Materials and methods
Materials
Sodium alginate and gelatin from porcine skin were purchased from Sigma. Chitosan was purchased from Weifang Sea Source Biological Products Limited Corporation. Calcium chloride (CaCl2) and other reagents were purchased from China National Medicine Corporation and used as received. The water purified by a three-stage Millipore Milli-Q plus 185-purification system was used throughout all experiments.Synthesis of carboxymethyl chitosan
Carboxymethyl chitosan was prepared by the method of Liu et al.23 Chitosan (5 g) and sodium hydroxide (4 g) were added into 80% isopropanol solution to swell and alkalize for 2.5 h. Monochloroacetic acid (6 g) dissolved in isopropanol (20 mL) was then added into the reaction mixture dropwise for 30 min at 4 °C. After that, the mixture was reacted for 4 h at 50 °C, then stopped by adding hydrochloric acid to adjust the pH of the solution to 7. The solid product was filtered and rinsed with 70–90% ethyl alcohol to desalt and dewater, and vacuum dried at room temperature.Preparation of the hydrogels
Gelatin was dissolved in water at concentration of 0.1 g mL−1 under constant stirring at 50 °C. Then, certain amount of alginate (the final concentration is 0.01 g mL−1) and carboxymethyl chitosan (the final concentration is 0.001 g mL−1) were dissolved into the gelatin solution at the same temperature. The resultant Gel/SA/CMCS hydrogel discs were then ionically cross linked by 2% CaCl2 solution for 1 min, 5 min and 9 min, respectively. Moreover, Gel/SA hydrogel was prepared in the same way but without carboxymethyl chitosan.Swelling of the hydrogels
Equilibrium water content (M), a measure of swellability of hydrogel, was estimated by immersing dried hydrogel samples (Gel/SA/CMCS hydrogel and Gel/SA hydrogel) to swell in PBS at 37 °C for 7 days. M was defined according to the following equation:where Ws and Wd refer to the weight of the swollen sample and the dried sample, respectively.24
Mechanical testing of the hydrogels
In this work, the tensile testing of the hydrogel was performed. Prior to mechanical testing by a material testing machine (H5K-S, Hounsfield, UK), all frozen dried samples were cut into small strips, and then the width, length, and thickness were measured with a micrometer, and three strips of each scaffold were chosen for the mechanical test. The load cell is 10 kN, and the strain rate is 100 mm min−1. Stress and strain were calculated according to the following equations:where σ, ε, P, w, d, l and l0 stand for stress, strain, load, mat width, mat length, extension length, and gauge length, respectively. Here, the values of w and d are the initial dimensions, so the stress values reported are engineering stress. Young's modulus can be calculated from stress–strain curves.25,26
Degradation behavior of the hydrogels
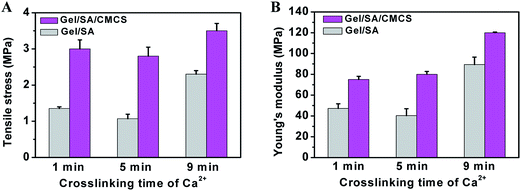
The degradation ratio of the hydrogels was measured after 60 days of incubation. Gel/SA/CMCS and Gel/SA hydrogels, in triplicate, were incubated in physiological saline solution at 37 °C, respectively. The dried samples were weighed after being frozen and lyophilized. The degradation ratio (Rd) of the hydrogels was calculated according to the following equation:where W0 and W60 represent the initial dried weight and the dried weight of the sample at day 60, respectively.
Antibacterial activity of the hydrogels
The hydrogel samples were evaluated for antibacterial activity according to a previously established protocol.27 In brief, an Escherichia coli culture was streaked onto a nutrient agar Petri dish. Hydrogel samples were then placed to the inoculated Petri dish and incubated for 1 day at 37 °C in the incubation chamber. The optical density (OD) at 260 nm was recorded on a UV spectrophotometer (Eppendorf, German) at RT.Cell isolation, culturing and seeding
The animal surgeries were approved by Institute of Genetics and Developmental Biology-Institutional Animal Care and Use Committee (IGDB-IACUC), Chinese Academy of Sciences, in accordance with NIH guidelines for the care and use of laboratory animals (NIH Publication no. 85-23 Rev. 1985). Bone mesenchymal stem cells (BMSCs) were harvested from bone marrow of the femurs and tibias of male adult Sprague-Dawley rats aged 4 week-old as described previously.28 BMSCs were cultured in DMEM/F12 basic media containing 10% fetal bovine serum, 100 U mL−1 penicillin, 100 U mL−1 streptomycin, 100 mmol mL−1 non-essential amino acids, and 100 mmol mL−1 sodium pyruvate in a humidified atmosphere of 5% CO2 at 37 °C. The 3–5 passage BMSCs were used in the following experiments.3D bioprinting
A customized microextrusion-based 3D printer (Oganp 1800lite, Shenyang Institute of Automation, Chinese Academy of Sciences) equipped with z-axis-controlled ink reservoirs was used in our study. First, the architectural information for spatial patterns of the hydrogel was designed by the CAD/CAM tool and imported into a user-friendly software interface allowing on-demand printing. Subsequently, the BMSCs-laden Gel/SA/CMCS hydrogel precursor (2 × 106 cell per mL) with a homogeneous plotting temperature controlled by a two-stage syringe heating mantle was printed through the nozzle orifice (210 μm in diameter) onto a substrate, using a mechanical dispensing system at speeds of 1–10 mm s−1, and then cooled down at 15 °C. The pressure is ranging from 20 to 60 psi. Finally, the printed objects were treated with 2% CaCl2 solution for 5 min, washed with PBS, and then incubated at 37 °C.Morphology of the hydrogels
Morphology of the hydrogel without cells was examined on a HITACHI S-3000N scanning electron microscope (SEM). All hydrogel samples were sputter coated with a 10 nm gold film before SEM observation.Live/dead cell staining
Cell viability was evaluated by a double-staining procedure using fluorescein diacetate (FDA) and propidium iodide (PI) at 0 day and 2 days, respectively. In brief, the BMSCs-laden hydrogel scaffolds were washed with PBS and then incubated with FDA (5 μg mL−1) and PI (10 μg mL−1) in PBS for 5 min at 37 °C. After incubation, the samples were washed with PBS twice and photographed using a Leica TCS SP5 confocal microscope (Leica Microsystems, Germany). Cell viability was calculated via the FDA/PI staining images, in which the green and red fluorescence corresponded to the viable cells staining with FDA and the dead cells staining with PI, respectively.Statistical analysis
All experiments were performed at least three times and the data were expressed as the mean ± standard deviation.Results and discussion
Water content, mechanical properties and degradation of the hydrogels
The FTIR spectrum of CMCS is showed in Fig. 1. The characteristic peaks at 2873 cm−1, 1594 cm−1, 1157 cm−1, and 1091 cm−1, can be attributed to the C–H stretching, N–H bending, bridge-O-stretching, and C–O stretching modes of chitosan, respectively.29 And the two peaks at 1425 cm−1 and 1627 cm−1 can be assigned to the symmetric and asymmetric stretching modes of COO− group, respectively, indicating the successful binding of carboxymethyl groups to chitosan.29The equilibrium water content of the hydrogel, which is one of the most important properties for biomaterials, correlates with the infrastructure of biomaterials.30 The equilibrium water content of the hydrogels with or without CMCS was calculated (Table 1). For the crosslinking time 1 min, 5 min and 9 min, the equilibrium water content of Gel/SA hydrogel was determined to be 87.48%, 83.57% and 82.85%, respectively. The longer the crosslinking time, the higher the crosslinking density of the Gel/SA hydrogel is, and the lower the equilibrium water content of Gel/SA hydrogel becomes. However, the equilibrium water content of Gel/SA/CMCS hydrogel is a little higher than that of Gel/SA hydrogel, increases to 88.31% when the crosslinking time is 9 min, which is more suitable for cell culture.31 That means the addition of CMCS could increase the equilibrium water content of hydrogel.
| Hydrogel composition | Crosslinking time of Ca2+ | Equilibrium water content (%) |
|---|---|---|
| Gel/SA | 1 min | 87.48% |
| Gel/SA | 5 min | 83.57% |
| Gel/SA | 9 min | 82.85% |
| Gel/SA/CMCS | 1 min | 93.77% |
| Gel/SA/CMCS | 5 min | 88.97% |
| Gel/SA/CMCS | 9 min | 88.31% |
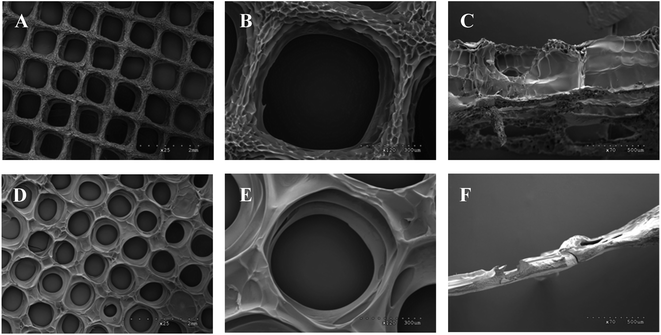
We then studied and compared the mechanical properties of Gel/SA and Gel/SA/CMCS hydrogels. The experiment revealed that tensile and Young's modulus of the Gel/SA hydrogel and Gel/SA/CMCS hydrogel could be regulated by changing the crosslinking time of Ca2+ (Fig. 2). Increasing the crosslinking time of Ca2+ facilitated the generation of calcium alginate, leading to significant enhancement of the Young's modulus of the hydrogels. In addition, the tensile and Young's modulus of Gel/SA/CMCS hydrogel are much higher than that of Gel/SA hydrogel. The amine group of chitosan was ionically bonded to the carboxyl group of alginate, which results to the improvement of mechanical strength of Gel/SA/CMCS hydrogel,19 indicating that CMCS could improve the mechanical property of the hydrogel.
 | ||
| Fig. 2 (A) Tensile stress and (B) Young's modulus of Gel/SA/CMCS and Gel/SA hydrogels with different crosslinking time of Ca2+, respectively. | ||
A favorable and biodegradable scaffold provides structural support for initial cell growth and then would gradually degrade after new tissue formation.32,33 Thus, we investigated the degradation behavior of the Gel/SA hydrogel and Gel/SA/CMCS hydrogel. As shown in Fig. 3, with the increase of the crosslinking time of Ca2+, the degradation rate of the hydrogels decreased. Moreover, at the same crosslinking time, Gel/SA/CMCS hydrogels display less degradation rate than that of Gel/SA hydrogel after 60 days of incubation in vitro, indicating that CMCS could improve the stability of the hydrogel.
 | ||
| Fig. 3 Relative degradation rate of Gel/SA/CMCS and Gel/SA hydrogels after incubation in physiological environment for 60 d, respectively. | ||
Furthermore, Gel/SA/CMCS hydrogel displayed better antibacterial activity than Gel/SA hydrogel (Fig. 4) due to the antibacterial property of CMCS.34 This is beneficial to their biomedical applications.
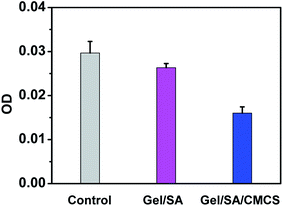 | ||
| Fig. 4 The antibacterial effect of Gel/SA and Gel/SA/CMCS hydrogels against Escherichia coli, with the blank control during the test. | ||
Morphology characterization of the hydrogels
3D bioprinting provides a flexible automated on-demand platform to fabricate complex living architectures.22 Several techniques are applied in 3D bioprinting, such as valve-based printing and inkjet-based printing.35–37 Among them, the microextrusion bioprinters controlled by the extrusion of a material robotically with a microextrusion nozzle has been widely used in 3D bioprinter development. The stage or microextrusion head moves along x and y axis, in this way a layer is deposited as a foundation for the next layer when the extrusion head rises along z axis.Our customized microextrusion bioprinter consists of a material-handling and dispensing stage equipped with a temperature-controlled system (Fig. 5). BMSCs-laden Gel/SA/CMCS hydrogel precursor or Gel/SA hydrogel precursor can be placed in the cell-dispensing channel for printing (Fig. 5A and B). Electromechanical valve operates the dispenser that is mounted on a three-axis robotic stage for the micrometer resolution. In the printing process (Fig. 5C), the solution was dispensed and printed layer by layer controlled by the CAD/CAM software. Then the hydrogel was cross linked at 15 °C. After that, the hydrogel was cross linked again by CaCl2 for 5 min. Some key parameters, including applied pressure (20–60 psi), temperature (4–37 °C), plotting speed (1–10 mm s−1), plotting infill (0.7–1.2 mm), plotting height (0.01–0.09 mm) and nozzle orifice (180–210 μm), were studied and optimized during 3D bioprinting.
 | ||
| Fig. 5 Fabrication of 3D scaffolds using a customized 3D printer. (A) 3D printer equipped with refrigeration. (B) Printing nozzle. (C) Schematic of the printing and crosslinking processes. | ||
SEM images further displayed the microstructure of Gel/SA/CMCS hydrogel printed as the designed pattern (Fig. 6A), whereas the magnified illustrations showed the layer-by-layer microstructure (Fig. 6B). The section microstructure shows that the Gel/SA/CMCS hydrogel has a highly porous structure (Fig. 6C), providing a sustainable cell culture environment for BMSCs renewal in culture media. However, the microstructure of Gel/SA hydrogel without CMCS shows that the framework is easy to collapse, leading to the deformation and shrinkage of the pores (Fig. 6D–F). This is mainly due to that the Gel/SA/CMCS hydrogel exhibited better mechanical property than of the Gel/SA hydrogel. In order to mimic native tissue microenvironment and regulate the cell behavior, the pore size and shape are important for 3D scaffolds. The oxygen tension, hydrostatic pressure and nutrient gradients may be affected by the pore sizes, leading to alter the different extracellular matrix deposition of the cells.38,39 Moreover, the pore structures with an increased curvature and surface area are more beneficial to cell proliferation and initial tissue formation, which can be explained by variations in actin network organization.40,41 Therefore, CMCS could keep the perfect morphology and large pores of the scaffold during 3D bioprinting process.
Printed cell-laden hydrogel scaffolds
For extrusion bioprinting, materials should have an appropriate yield stress, viscosity and crosslinking velocity to print a defined structure. In addition, the materials should provide a biocompatible environment as well as an adequate mechanical support for cell viability and function.42 In our work, we employed a complex hydrogel consisting of three natural biomaterials, gelatin, alginate and carboxymethyl chitosan. Here, the gelatin provides the appropriate viscous and temperature-dependent crosslinking velocity that can promote the complex hydrogel gelation quickly at 15 °C during printing. After printing, the alginate component forms final cross-linking by incubation with calcium ions to retain the designed geometries of hydrogel for in vitro culture and in vivo implantation.43 The carboxymethyl chitosan component improves the mechanical stability and antibacterial activity of the hydrogel.High viscosity of the hydrogels could ensure good printing fidelity, but may lead to the low cell viability. In our work, BMSCs-laden Gel/SA/CMCS hydrogel, showed a slightly higher viscosity than that of Gel/SA hydrogel, but still remained well-defined 3D structures with interconnected porosity (Fig. 7A) for necessary nutrition and oxygen delivery. Meanwhile, live/dead cell staining demonstrates that the cell viability is above 85% at all time points, showing good biocompatibility of the 3D bioprinted Gel/SA/CMCS hydrogel. And few cells indicated by the arrows display spread and extended morphology (Fig. 7B), which is similar to the phenomenon observed by other researchers.44 Clearly, investigation of the long-term cellular viability and functions in the 3D printed scaffolds is highly desired for their practical applications. Moreover, transmission optical imaging technology was used to observe the location of the BMSCs in the hydrogel scaffold (Fig. S1†). Because the BMSCs are not in the same plane, leading to some BMSCs are not very clear. However, the transmission optical image still showed that the BMSCs (white circles in Fig. S1†) located in the hydrogel pattern (blue dashed line in Fig. S1†).
Conclusions
We have developed Gel/SA/CMCS hydrogel and explored its application as BMSCs-laden scaffold for 3D bioprinting. Using a customized 3D printer, the microstructure of the printed scaffolds was able to be tuned, and the physical properties of Gel/SA/CMCS hydrogels can be controlled by manipulating the crosslinking time of CaCl2. Compared to the commonly used 3D bioprinting material, Gel/SA hydrogel, Gel/SA/CMCS hydrogel showed excellent equilibrium water content, mechanical properties, antibacterial effect, and degradation performance. Moreover, the printed BMSCs showed high cell viability (>85%), which is very important for their practical applications in tissue engineering. Given the improved physicochemical and biological properties that the complex Gel/SA/CMCS hydrogel demonstrated, we conclude that it may have potential applications in tissue engineering scaffolds and 3D bioprinting.Acknowledgements
The authors acknowledge financial support from the Key Research Program of the Chinese Academy of Sciences (No. ZDRW-ZS-2016-2-3), the Ministry of Science and Technology of China (No. 2014CB965003, 2016YFC1000809).References
- T. Boland, T. Xu, B. Damon and X. F. Cui, Biotechnol. J., 2006, 1, 910–917 CrossRef CAS PubMed.
- J. L. Drury and D. J. Mooney, Biomaterials, 2003, 24, 4337–4351 CrossRef CAS PubMed.
- B. Guillotin and F. Guillemot, Trends Biotechnol., 2011, 29, 183–190 CrossRef CAS PubMed.
- V. Mironov, N. Reis and B. Derby, Tissue Eng., 2006, 12, 631–634 CrossRef PubMed.
- C. C. Chang, E. D. Boland, S. K. Williams and J. B. Hoying, J. Biomed. Mater. Res., Part B, 2011, 98, 160–170 CrossRef PubMed.
- J. Malda, J. Visser, F. P. Melchels, T. Jüngst, W. E. Hennink, W. J. A. Dhert, J. Groll and D. W. Hutmacher, Adv. Mater., 2013, 25, 5011–5028 CrossRef CAS PubMed.
- X. Q. Jia and K. L. Kiick, Macromol. Biosci., 2009, 9, 140–156 CrossRef CAS PubMed.
- A. M. Kloxin, C. J. Kloxin, C. N. Bowman and K. S. Anseth, Adv. Mater., 2010, 22, 3484–3494 CrossRef CAS PubMed.
- T. C. Flanagan, B. Wilkins, A. Black, S. Jockenhoevel, T. J. Smith and A. S. Pandit, Biomaterials, 2006, 27, 2233–2246 CrossRef CAS PubMed.
- T. C. Flanagan, C. Cornelissen, S. Koch, B. Tschoeke, J. S. Sachweh, T. Schmitz-Rode and S. Jockenhoevel, Biomaterials, 2007, 28, 3388–3397 CrossRef CAS PubMed.
- W. Schuurman, P. A. Levett, M. W. Pot, P. R. van Weeren, W. J. A. Dhert, D. W. Hutmacher, F. P. W. Melchels, T. J. Klein and J. Malda, Macromol. Biosci., 2013, 13, 551–561 CrossRef CAS PubMed.
- B. Duan, E. Kapetanovic, L. A. Hockaday and J. T. Butcher, Acta Biomater., 2014, 10, 1836–1846 CrossRef CAS PubMed.
- M. C. Du, B. Chen, Q. Y. Meng, S. M. Liu, X. F. Zheng, C. Zhang, H. R. Wang, H. Y. Li, N. Wang and J. W. Dai, Biofabrication, 2015, 7, 044104 CrossRef PubMed.
- S. Das, F. Pati, Y. J. Choi, G. Rijal, J. H. Shim, S. W. Kim, A. R. Ray, D. W. Cho and S. Ghosh, Acta Biomater., 2015, 11, 233–246 CrossRef CAS PubMed.
- J. H. Y. Chung, S. Naficy, Z. L. Yue, R. Kapsa, A. Quigley, S. E. Moulton and G. G. Wallace, Biomater. Sci., 2013, 1, 763–773 RSC.
- T. C. Tseng, L. Tao, F. Y. Hsieh, Y. Wei, I. M. Chiu and S. H. Hsu, Adv. Mater., 2015, 27, 3518–3524 CrossRef CAS PubMed.
- R. Gaetani, P. A. Doevendans, C. H. G. Metz, J. Alblas, E. Messina, A. Giacomello and J. P. G. Sluijter, Biomaterials, 2012, 33, 1782–1790 CrossRef CAS PubMed.
- B. Duan, L. A. Hockaday, K. H. Kang and J. T. Butcher, J. Biomed. Mater. Res., Part A, 2013, 101, 1255–1264 CrossRef PubMed.
- Z. S. Li, M. Leung, R. Hopper, R. Ellenbogen and M. Q. Zhang, Biomaterials, 2010, 31, 404–412 CrossRef CAS PubMed.
- Z. S. Li, H. R. Ramay, K. D. Hauch, D. M. Xiao and M. Q. Zhang, Biomaterials, 2005, 26, 3919–3928 CrossRef CAS PubMed.
- Z. S. Li and M. Q. Zhang, J. Biomed. Mater. Res., Part A, 2005, 75, 485–493 CrossRef PubMed.
- S. V. Murphy and A. Atala, Nat. Biotechnol., 2014, 32, 773–785 CrossRef CAS PubMed.
- X. F. Liu, Y. L. Guan, D. Z. Yang, Z. Li and K. D. Yao, J. Appl. Polym. Sci., 2001, 79, 1324–1335 CrossRef CAS.
- K. Chawla, T. B. Yu, S. W. Liao and Z. B. Guan, Biomacromolecules, 2011, 12, 560–567 CrossRef CAS PubMed.
- S. G. Wang, R. Castro, X. An, C. L. Song, Y. Luo, M. W. Shen, H. Tomás, M. F. Zhu and X. Y. Shi, J. Mater. Chem., 2012, 22, 23357–23367 RSC.
- Y. Luo, H. Shen, Y. X. Fang, Y. H. Cao, J. Huang, M. X. Zhang, J. W. Dai, X. Y. Shi and Z. J. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 6331–6339 CAS.
- P. S. K. Murthy, Y. Murali Mohan, K. Varaprasad, B. Sreedhar and K. Mohana Raju, J. Colloid Interface Sci., 2008, 318, 217–224 CrossRef CAS PubMed.
- S. F. Han, Y. N. Zhao, Z. F. Xiao, J. Han, B. Chen, L. Chen and J. W. Dai, J. Genet. Genomics, 2012, 39, 633–641 CrossRef CAS PubMed.
- X. G. Chen and H. J. Park, Carbohydr. Polym., 2003, 53, 355–359 CrossRef CAS.
- Y. H. Chen, J. Li, Y. B. Hao, J. X. Qi, N. G. Dong, C. L. Wu and Q. Wang, J. Appl. Polym. Sci., 2015, 132, 41898 Search PubMed.
- J. M. Dang, D. D. N. Sun, Y. Shin-Ya, A. N. Sieber, J. P. Kostuik and K. W. Leong, Biomaterials, 2006, 27, 406–418 CrossRef CAS PubMed.
- L. Zhang, J. Hu and K. A. Athanasiou, Crit. Rev. Bioeng., 2009, 37, 1–57 Search PubMed.
- M. W. Tibbitt and K. S. Anseth, Biotechnol. Bioeng., 2009, 103, 655–663 CrossRef CAS PubMed.
- G. J. Tsai and W. H. Su, J. Food Prot., 1999, 62, 239–243 CrossRef CAS PubMed.
- K. Iwami, T. Noda, K. Ishida, K. Morishima, M. Nakamura and N. Umeda, Biofabrication, 2010, 2, 014108 CrossRef CAS PubMed.
- L. Shor, S. Güçeri, R. Chang, J. Gordon, Q. Kang, L. Hartsock, Y. An and W. Sun, Biofabrication, 2009, 1, 015003 CrossRef PubMed.
- S. M. Peltola, F. P. W. Melchels, D. W. Grijpma and M. Kellomäki, Ann. Med., 2008, 40, 268–280 CrossRef CAS PubMed.
- S. D. Thorpe, T. Nagel, S. F. Carroll and D. J. Kelly, PLoS One, 2013, 8, e60764 CAS.
- T. B. F. Woodfield, C. A. Van Blitterswijk, J. De Wijn, T. J. Sims, A. P. Hollander and J. Riesle, Tissue Eng., 2005, 11, 1297–1311 CrossRef CAS PubMed.
- W. Zhu, B. Holmes, R. I. Glazer and L. G. Zhang, Nanomedicine: Nanotechnology, Biology and Medicine, 2016, 12, 69–79 CrossRef CAS PubMed.
- J. Knychala, N. Bouropoulos, C. J. Catt, O. L. Katsamenis, C. P. Please and B. G. Sengers, Ann. Biomed. Eng., 2013, 41, 917–930 CrossRef CAS PubMed.
- F. Pati, J. Jang, J. W. Lee and D. W. Cho, in Essentials of 3D Biofabrication and Translation, ed. A. Atala and J. J. Yoo, Academic Press, San Diego, 1st edn, 2015, vol. 7, pp. 123–152 Search PubMed.
- J. K. Carrow, P. Kerativitayanan, M. K. Jaiswal, G. Lokhande and A. K. Gaharwar, in Essentials of 3D Biofabrication and Translation, ed. A. Atala and J. J. Yoo, Academic Press, San Diego, 1st edn, 2015, vol. 13, pp. 229–248 Search PubMed.
- W. B. Wan, Q. T. Li, H. Y. Gao, L. P. Ge, Y. Q. Liu, W. Zhong, O. Y. Jun and M. Xing, J. Mater. Chem. B, 2015, 3, 1990–2005 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra24231f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |