Synthesis and application of PNP pincer ligands in rhodium-catalyzed hydroformylation of cycloolefins†
Abstract
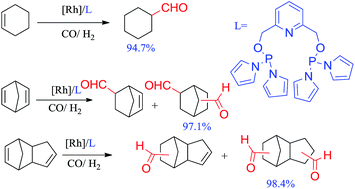
Two new phosphorus ligands (L1 and L2) were developed for rhodium-catalyzed hydroformylation of cycloolefins. L1 produced high conversion for cyclohexene (94.7%) and high dialdehyde selectivity for NBD (97.1%) and DCPD (98.4%). Analogue pincer ligands L3–L6 with different steric and electronic character were also investigated in these hydroformylations.

 Please wait while we load your content...
Please wait while we load your content...