Rapid and efficient biphasic liquid extraction of metals with bio-derived lipophilic β-diketone†
Kaana Asemave,
Fergal Byrne,
Thomas J. Farmer ,
James H. Clark and
Andrew J. Hunt*
,
James H. Clark and
Andrew J. Hunt*
Green Chemistry Centre of Excellence, Department of Chemistry, University of York, YO10 5DD, UK. E-mail: Andrew.hunt@york.ac.uk
First published on 30th September 2016
Abstract
A bio-derived lipophilic β-diketone (14,16-hentriacontanedione) was isolated from wheat straw wax and tested in the liquid biphasic removal of Ni2+, Co2+, Cu2+ and Cr3+ in comparison to dibenzoylmethane and acetylacetone. The bio-derived β-diketone exhibited excellent capacity for metal ions extraction from aqueous solutions.
The sustainability of elements has become a hot topic for both policy makers and researchers alike. The recovery of metals from wastes/spent products can be considered as a method for enhancing an elements sustainability.1–3 Additionally, metal ion extraction can also reduce the release of potentially toxic elements entering into the environment.4–6
Use of chelating agents is one such method of recovering metal from aqueous solutions used in mining. Many common chelating agents are not readily biodegradable and persist in the environment, thus leading to significant interest in finding environmentally acceptable bio-derived alternatives.7 β-Diketones are well known for their metal chelation8,9 and importantly, long-chain β-diketones have been reported as major components in waxes from wheat, barley, oats, rye, eucalyptus, festuca, agropyron and vanilla bean.10–12 Hunt et al. reported the extraction of wax from wheat straw using supercritical carbon dioxide, with yields of 1.8% from biomass.12 This work identified the presence of significant quantities of 14,16-hentriacontanedione 1, however, limited research has focussed on the purification and application of these β-diketone as a metal chelating agents.
Herein, the first reported purification of 1 from wheat straw wax and testing as bio-derived chelators is presented. The bio-derived lipophilic β-diketone, 1 was tested in a liquid biphasic extraction of Ni2+, Co2+, Cu2+ and Cr3+ in comparison with acetylacetone, 2, and dibenzoylmethane, 3 (Fig. 1). Nickel, cobalt, copper and chromium were selected due to their use in critical energy applications including nuclear energy, wind energy, carbon capture & storage, biofuels and solar energy.4
The lipophilic β-diketone, 1, was isolated using an adapted method described by Horn et al. previously reporting the extraction from eucalyptus.13 Through the chelation of 1 with copper acetate (Cu(OAc)2) it was possible to recover a yield of 18 ± 0.5 wt% from wheat straw wax. The 1H-NMR, 13C-NMR, MS and FTIR spectra of the purified product confirmed this compound as 1.†
Enolization in β-diketones is a prerequisite for their metal chelation ability.14 Therefore the keto–enol tautomerism of 1 was investigated in a range of organic solvents including cyclohexane, toluene, acetone and THF. These studies were monitored by NMR spectroscopy in comparison to 2 and 3 and the results of which are presented in Table 1.
| Chelator | Solvent | π*17 | % enol | % keto |
|---|---|---|---|---|
| a 1 = 14,16-hentriacontanedione, 2 = acetylacetone and 3 = dibenzoylmethane; π* = Kamlet–Taft polarizability.b In these entries, acetone sparingly dissolves 1.c In these entries, THF overlaps with methylene proton peaks in 1 and 2. | ||||
| 1 | Cyclohexane-d12 | 0.00 | 97.15 ± 0.12 | 2.85 ± 0.12 |
| Toluene-d8 | 0.54 | 90.45 ± 1.91 | 9.55 ± 1.91 | |
| Acetone-d6 | 0.71 | 71.99b | 28.01b | |
| THF-d8 | 0.58 | 87.76c | 12.24c | |
| 2 | Cyclohexane-d12 | 0.00 | 97.92 ± 0.03 | 2.08 ± 0.03 |
| Toluene-d8 | 0.54 | 92.42 ± 0.04 | 7.58 ± 0.04 | |
| Acetone-d6 | 0.71 | 77.89 ± 1.13 | 22.11 ± 1.13 | |
| THF-d8 | 0.58 | 76.44c | 23.56c | |
| 3 | Cyclohexane-d12 | 0.00 | 99.50 ± 0.04 | 0.50 ± 0.04 |
| Toluene-d8 | 0.54 | 98.88 ± 0.17 | 1.12 ± 0.17 | |
| Acetone-d6 | 0.71 | 97.16 ± 0.23 | 2.85 ± 0.23 | |
| THF-d8 | 0.58 | 98.67 ± 0.21 | 1.34 ± 0.21 | |
The percentage of enol relative to keto for the bio-derived β-diketone 1 at constant concentration in these solvents is in the order cyclohexane > toluene > THF > acetone (i.e. 1 gives highest% enol in cyclohexane). The enolization of the long chain lipophilic β-diketone in these solvents is consistent with the results of 2. The extension of the conjugated system in the enol form of 3 makes this particular diketone more enolizable than 1 and 2 in the solvents selected, as shown in Table 1. Generally for β-diketones, the keto tautomers are more favoured in protic solvents and solvents with higher dipole moment (a measure of degree of polarizability), while enol tautomer predominates in aprotic and non-polar solvents.15,16 The bio-derived lipophilic β-diketone, 1 has also exhibited this behaviour where polar solvents with higher π* (polarizability) such as acetone and THF provided greater stabilisation of the keto tautomer while aprotic and non-polar solvents (cyclohexane and toluene) had an enhanced stabilising effect on the enol tautomer. More so, cyclohexane and toluene do not donate H-bonds to the β-diketone and hence 1 forms a higher percentage of enol in cyclohexane and toluene.
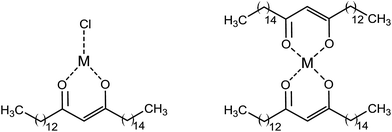
As the polarizability increases from cyclohexane to acetone, there is a gradual decrease in the percentage of enol in the β-diketones. The capacity for 1 to form the enol in cyclohexane is thus highlighted in its significant ability for metal ion chelation (ESI Fig. S4†). Therefore, liquid biphasic (water/cyclohexane) extraction of metal ions with the bio-derived lipophilic β-diketone, 1, was tested in comparison to 2 and 3. A detailed procedure for the metal extraction is described in the electronic ESI.† Due to the relatively low lipophilicity of 2 there was little metal removal observed with this β-diketone under the described experimental conditions. Shigematsu et al. reported that it is often problematic to compare 2 to lipophilic β-diketones in metals extraction.18 This is partly attributed to the fact that the partition coefficient of metal acetylacetonates in most organic solvents in an aqueous/organic biphasic system is significantly less than metal chelates of highly lipophilic β-diketones. The bio-derived β-diketone and 3 are both highly lipophilic and can therefore be readily used for the extraction of metal ions to the organic phase in a biphasic system. Results of metal removal with the bio-derived β-diketone, 1, showed that 86 mg L−1 and 108 mg L−1 of Cu2+ from Cu(OAc)2 and CuCl2 solutions respectively could be extracted into the organic phase for recovery. These results were comparable to 3 which removed 118 mg L−1 Cu2+ from aqueous Cu(OAc)2 solution and 120 mg L−1 Cu2+ from aqueous CuCl2 solution at a similar pH of 4–6. The extraction of Cu2+ from Cu(NO3)2 and CuSO4·5H2O solutions by both 1 and 3 were less favoured under these conditions. This shows that the Cl− counter ions in the Cu2+ extraction medium have enhanced the amount of copper removed compared to SO42− and NO3− ions with 1. In addition, it has been reported that the extraction of Cu2+ from SO42− medium using hydrophobic pyridyl ketoximes was found to be inefficient, but the extraction was enhanced with added chloride ions.19 Therefore, Cl− is a suitable ligand to generate extractable and charge-neutral complexes, [MClx(L)n] as it can act as ligand or counter ions pair unlike SO42− which is a weak ligand and does not readily form [MSO4x2−(L)n] (Fig. 2).20 This explains why extraction of some critical metals are preferred from chloride media.20 In the same vein, the removal of Co2+ at pH 7 was effective from an aqueous CoCl2 solution in the case of 1 and 3 but was poor from a Co(NO3)2 solution due to the counter ion effect as earlier explained. Meanwhile, the observed amount of cobalt removed from CoCl2 solution with 2 was significantly lower than that of 1 and 3 as shown in Fig. 3. Gerald et al.21 also reported excellent extraction of Co2+ from aqueous CoSO4·7H2O using a β-diketone (1-phenyl-3-methyl-4-(p-nitrobenzoyl)pyrazolone) in 30 min contact time with chloroform, benzene, ethyl acetate and toluene diluents at pH between 5.5–7.0.21
For the extraction of Ni2+ at pH 7.3 from NiCl2 solution the bio-derived β-diketone, 1, removed 112 mg L−1 Ni2+. A kinetic study was used to investigate the extraction of Ni2+ with chelators 1 and 3. This demonstrated no removal of nickel with chelator 3 over two hours. It has been reported that longer contact times of up to 3 days are required to extract Ni2+ with 3.8,22 In contrast, with the aliphatic β-diketone 1 the recovery of Ni2+ was rapid and reached equilibrium within 60 minutes (ESI Fig. S7†). This demonstrates that 1 can chelate to Ni2+ at a faster rate than observed for 3 because of the increased rate of nickel transfer into the organic phase; thereby offering significant advantages in processing efficiency. This adheres to the principle put forward by Wilson et al. who reported that an ideal chelator should readily facilitate metal transfer into the organic phase.20
6 mg L−1 of Cr3+ at pH of 2.6 was also extracted from an aqueous CrCl3·6H2O solution using 1. 3 proved less effective for Cr3+ removal, once again due to the long chelation times and low pH of the aqueous solution. Previous studies report that a pH higher than 2.58 are required for a better extraction of Cr3+ and that the contact time for removal of most metals with 3 is 4–5 hours.20,22 It has been previously reported that higher concentrations of 2 (and likely other β-diketones) is required for the extraction of metals from acidic medium.22
It was found that the concentration (mg L−1) of Co2+ removed from CoCl2 solutions was higher than all of the other metals for 1 and 3. This could be attributed to the higher solubility of the Co2+ chelated complex in cyclohexane compared to the other metals. Cu2+ chelates from CuCl2 and Cu(OAc)2 solutions and Ni2+ chelate from NiCl2 were less soluble and precipitated readily after 30 minutes of contact time. Due to their solvating properties, traditional solvents such as toluene, carbon tetrachloride, dichloromethane,9 chloroform and benzene,22 are often used in the extraction of metals and are typically regarded as better solvents than cyclohexane in this application. However, these traditional solvents have highly negative environmental and safety considerations.23 Cyclohexane was selected because of its ability to enolize the bio-derived β-diketone 1, and its improved safety credentials as compared to traditional organic solvents.
The metal extraction was tested at varying concentrations of 0.015 M and 0.03 M of Cu2+. For 0.015 M initial Cu2+ ion, 19 and 25 mg L−1 were extracted with 1 and 3 respectively. It was found that increasing the initial Cu2+ ion concentration to 0.03 M the amount of Cu2+ extracted was also increased to 108 and 120 mg L−1 for 1 and 3. This result is consistent with literature sources for other β-diketones and metals which observe enhancement with increasing the initial metal concentration.9 Similarly, the extraction of Cu2+ found to increase by increasing the concentrations of 1 (ESI Fig. S9†). This is linked to the fact that the total number of active chelating sites increased with great concentration of chelator. The extraction of 0.015 M Cu2+ was investigated at 20–50 °C (ESI Fig. S10†), the results showed that, 26 ± 4 mg L−1, 15 ± 5 mg L−1, 10 ± 3 mg L−1 and 15 ± 3 mg L−1 of Cu2+ were extracted at 20 °C, 30 °C, 40 °C and 50 °C respectively.
The competitive extraction of Co2+ in the presence of Cu2+ at pH 5 indicated comparable extractions of the two metal ions with 1 and 3. 64 mg L−1 of Cu was extracted with 1 and 3, while 88 mg L−1 and 59 mg L−1 Co was extracted by 1 and 3 respectively.
Is noteworthy that the final stage of the isolation of the 14,16-hentriacontanedione 1 from the wheat straw wax involves stripping off the Cu with HCl. An ideal chelant should readily release the metal ion under a changed conditions ready for it to be used again.20 The acid stripping process was used to recover and reuse of 1 from the cyclohexane phase after the extraction, the recovered β-diketone was tested a further 2 times with no observed degradation or loss in activity.
The capacity of the bio-derived β-diketone, 1, to extract copper and chromium was comparable to petroleum-derived dibenzoylmethane. Importantly, due to the increased lipophilicity of 1, shorter contact times and in the cases of Ni2+ and Co2+, enhanced recovery was observed compared to 2 and 3. Fast facilitation of metal transfer into the organic phase using 1, highlight its suitability as an efficient and sustainable metal chelator. Future studies on the extraction of metals in real-life waste with 1, will promote the commercial exploitation of this molecule. It is hoped that the development of bio-derived chelators from agricultural wastes such as wheat straw may aid in creating holistic methods for metal recovery that can be utilised as part of a circular economy.24
Acknowledgements
KA would like to thank the Tertiary Education Trust Fund (TET Fund) – Nigeria and Benue State University, Makurdi, Nigeria for their kind financial support.References
- J. R. Dodson, A. J. Hunt, H. L. Parker, Y. Yang and J. H. Clark, Chem. Eng. Process., 2012, 51, 69–78 CrossRef CAS.
- A. J. Hunt, T. J. Farmer and J. H. Clark, Element Recovery and Sustainability, Royal Society of Chemistry, London, 2013 Search PubMed.
- J. R. Dodson, H. L. Parker, A. M. García, A. Hicken, K. Asemave, T. J. Farmer, H. He, J. H. Clark and A. J. Hunt, Green Chem., 2015, 1–15 CAS.
- R. L. Moss, E. Tzimas, H. Kara, P. Willis and J. Kooroshy, Critical Metals in Strategic Energy Technologies: Assessing Rare Metals as Supply-Chain Bottlenecks, in Low-Carbon Energy Technologies, 2011, pp. 1–164 Search PubMed.
- I. K. Wernick and N. J. N. J. Themelis, Annu. Rev. Energy, 1998, 23, 465 CrossRef.
- E. MacIngova and A. Luptakova, Chemical Engineering Transactions, 2012, 28, 109–114 Search PubMed.
- H. Hyvönen, Studies on metal complex formation of environmentally friendly aminopolycarboxylate chelating agents, University of Helsinki, Finland, 2008 Search PubMed.
- S. S. Przeszlakowski and H. Wydra, Hydrometallurgy, 1982, 8, 49–64 CrossRef CAS.
- D. Fanou, B. Yao, S. Siaka and G. Ado, J. Appl. Sci., 2007, 7, 310–313 CrossRef CAS.
- J. A. Kenar, J. Am. Oil Chem. Soc., 2003, 80, 1027–1032 CrossRef CAS.
- A. P. P. Tulloch, in Chemistry and Biochemistry of Natural Waxes, ed. P. E. Kolattukkudy, Elsevier, Amsterdam, 1976, pp. 235–287 Search PubMed.
- E. H. K. Sin, R. Marriott, A. J. Hunt and J. H. Clark, C. R. Chim., 2014, 17, 293–300 CrossRef CAS.
- D. H. S. Horn, Z. H. Kranz and J. A. Lamberton, Aust. J. Chem., 1964, 17, 464–476 CrossRef CAS.
- W. Urbaniak, K. Jurek, K. Witt and A. Gorączko, Chemik, 2011, 65, 273–282 CAS.
- P. O. Sandusky, J. Chem. Educ., 2014, 91, 739–742 CrossRef CAS.
- J. H. Clark, D. J. Macquarrie and J. Sherwood, Chem.–Eur. J., 2013, 19, 5174–5182 CrossRef CAS PubMed.
- K. Hofmann, K. Schreiter, A. Seifert, T. Rüffer, H. Lang and S. Spange, New J. Chem., 2008, 1–8 Search PubMed.
- T. Shigematsu, M. Tabushi and T. Tarumoto, Bull. Inst. Chem. Res., Kyoto Univ., 1963, 40, 388–399 Search PubMed.
- K. Wieszczyckaa, M. Krupaa and A. Olszanowskia, Sep. Sci. Technol., 2012, 47, 1278–1284 CrossRef.
- A. M. Wilson, P. J. Bailey, P. A. Tasker, J. R. Turkington, R. A. Grant and J. B. Love, Chem. Soc. Rev., 2014, 43, 123–134 RSC.
- O. Gerald, A. Judith and O. Martin, J. Miner. Mater. Charact. Eng., 2013, 1, 90–94 Search PubMed.
- J. Stary and E. Hladky, Anal. Chim. Acta, 1963, 28, 227–235 CrossRef CAS.
- B. Gupta and I. Singh, Hydrometallurgy, 2013, 134–135, 11–18 CrossRef CAS.
- J. H. Clark, T. J. Farmer, L. Herrero-Davila and J. Sherwood, Green Chem., 2016, 18, 3914–3934 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental and characterisation data and figures of extraction. See DOI: 10.1039/c6ra24104b |
| This journal is © The Royal Society of Chemistry 2016 |



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: