DOI:
10.1039/C6RA24096H
(Paper)
RSC Adv., 2016,
6, 114374-114382
Construction of a magnetic Z-scheme photocatalyst with enhanced oxidation/reduction abilities and recyclability for the degradation of tetracycline†
Received
28th September 2016
, Accepted 14th November 2016
First published on 24th November 2016
Abstract
Here, we successfully designed and prepared the magnetic recyclable Z-scheme photocatalyst WO3/Fe3O4/g-C3N4 for the first time. The structures, morphology and photocatalytic properties of as-prepared photocatalysts were characterized by a series of modern characterization methods. According to the photoluminescence, transient fluorescence, transient photocurrent and electrochemical impedance spectra, the introduction of Fe3O4, with its high conductivity, into WO3/Fe3O4/g-C3N4 enhanced the lifetime of photogenerated holes and electrons based on the Z-scheme charge carrier transfer mechanism. More importantly, WO3/Fe3O4/g-C3N4 showed good recyclability without loss of apparent photocatalytic activity even after five cycles. Therefore, this kind of material may be a promising candidate for applications in the fields of environmental protection and sewage treatment.
1 Introduction
Over the past few decades, increasing environmental problems have become a huge challenge to the development of modern human society,1–3 with water pollution being an especially challenging issue. The antibiotic drug tetracycline shows strong sterilization abilities, displays antibacterial activity, and is widely used for the treatment of diseases in humans and animals. However, the large residual amounts of tetracycline in the aquatic environment have serious negative effects on the lives of people. For example, tetracycline has been shown to cause gastrointestinal maladies such as nausea, vomiting, abdominal distension, and diarrhea. Therefore, finding a reasonable and efficient method to treat this antibiotic pharmaceutical in wastewater is very important. Many approaches and technologies such as adsorption,4 microbial degradation,5 and electrolysis6 have been developed and widely used to address this problem, but they all appear to us to have their own drawbacks. However, photocatalysis has drawn significant attention due to the ability of this approach to remove organic pollutants from aqueous solutions by completely decomposing mineralized forms of these organic pollutants to carbon dioxide.
Recently, g-C3N4, an effective organic semiconductor photocatalyst, has attracted particular attention for treatment of organic pollutants, because of its low cost, thermal stability, and narrow band gap of 2.7 eV.7 However, the disadvantages of pure g-C3N4 limit its application. First, it undergoes a rapid recombination of photogenerated electron–hole pairs, and this process results in a low efficiency of its visible-light utilization. Thus, the visible-light activity of g-C3N4 is low in practical applications.8–10 In order to enhance the photocatalytic activity of g-C3N4, many strategies such as coupling or doping g-C3N4 with semiconductor materials, nonmetals, and metals have been used to modify g-C3N4.
Tungsten oxide (WO3), a typical n-type semiconductor, is recognized as a promising photocatalyst with an optical band gap of 2.4–2.8 eV, excellent chemical stability, a long photo-generated electron lifetime, and high electron mobility, and for these reasons as well as its low cost has been extensively studied for environmental remediation.11–13 Unfortunately, WO3 does suffer from certain drawbacks hindering the photocatalytic process, mainly (i) its conduction band (CB) level being more positive than that of other semiconductors, which results in the electrons generated in its CB having a limited reductive ability, and (ii) its relatively high electron–hole recombination rate during photocatalytic reactions.14 Therefore, the problems described above remain unsolved and require urgent attention.
Recently, the concept of all-solid-state Z-scheme photocatalysts with strong redox capacities and improved photocatalytic performance relative to the single-component photocatalysts was proposed. The sandwich-structured CdS/Au/TiO2 photocatalyst15 was reported as such a Z-scheme photocatalyst, with the Au nanoparticles (NPs) anchored between CdS and TiO2 and acting as electron-transfer mediators. It was found that the photogenerated electrons in the CB of TiO2 could transfer through the Au layer and recombine with the photogenerated holes left in the valence band (VB) of CdS, and prolong the lifetime of the carriers, and hence further enhance the photocatalytic activity.
Inspired by this concept and example, the Z-scheme WO3/Fe3O4/g-C3N4 photocatalyst was constructed to overcome the individual above-described drawbacks of WO3 and g-C3N4. Moreover, the introduced Fe3O4 not only has preferable magnetic properties, and can be hence easily separated from the mixture with a magnet and be recycled conveniently, but it can also serve as a conductor between the WO3 and g-C3N4 components and can participate in the transmit of photogenerated charge carriers. Combining magnetic Fe3O4 with a semiconductor for improving the photocatalytic activity has been reported, with such composites including TiO2/Fe3O4, WO3/Fe3O4, and graphene/Fe3O4.16–18 Therefore, Fe3O4 was introduced between g-C3N4 and WO3 and was able to significantly overcome the above drawbacks.
In the present study, magnetic Z-scheme-structured WO3/Fe3O4/g-C3N4 photocatalysts with high activity was successfully prepared for the first time. Moreover, this as-prepared photocatalyst was extensively characterized using X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), transmission electron microscopy (TEM), vibrating sample magnetometry (VSM), UV-vis diffuse reflectance spectroscopy (UV-vis), photoluminescence spectroscopy (PL), photocurrent-time measurements, transient photocurrents and electrochemical impedance spectroscopy (EIS), Brunauer–Emmett–Teller (BET) measurements, and electron spin resonance (ESR). As expected, WO3/Fe3O4/g-C3N4 displayed strong photocatalytic activity for removing tetracycline under visible light. Finally, the mechanism by which WO3/Fe3O4/g-C3N4 catalyzed the degradation of tetracycline was also systematically investigated.
2 Experimental
2.1. Chemicals
Tetracycline was purchased from the National Institutes for Food and Drug Control. Melamine (AR), iron(III) chloride hexahydrate (FeNO3·9H2O, AR), C2H3O2Na·3H2O (AR), polyethylene glycol 1500 (PEG, 1500), ethylene glycol (98.0%), polyvinyl pyrrolidone (PVP) and sodium tungstate (Na2WO4·2H2O, AR), were all supplied by Aladdin Chemistry Co., Ltd. NH4OH in solution form (25.0%), HCl in solution form (37%), p-benzoquinone (BQ, AR), isopropanol (IPA, AR), triethanolamine (OA, AR), and ethanol (C2H5OH, AR) were all purchased from Sinopharm Chemical Reagent Co., Ltd. Deionized water was used throughout this work.
2.2. Structural analysis
The as-prepared samples were characterized by using an X-ray diffractometer (XRD, Rigaku, Japan) between 5° and 80° with a scanning step of 5° min−1. Transmission electron microscope (TEM) images were acquired with a JEM-2100 transmission electron microscope (JEOL, Japan). Fourier transform infrared (FT-IR) spectra were recorded on a Nicolet Nexus 470 FT-IR (Thermo Nicolet Co., USA) at a resolution of 2.0 cm−1 between 400 cm−1 and 4000 cm−1. Magnetic measurements were taken using a vibrating sample magnetometer (VSM) (7300, Lakeshore) under a magnetic field up to 10 kOe. UV-vis diffuse reflectance spectra (UV-vis DRS) of the samples were obtained using a Shimadzu UV-3600 spectrometer with BaSO4 as a reference. Photoluminescence (PL) spectra were obtained from a F4500 PL detector (Hitachi, Japan). PL of C3N4 was investigated by using a spectrophotometer (Cary Eclipse spectrophotometer, VARIAN, USA). Transient photocurrents and electrochemical impedance spectroscopy (EIS) were investigated by using a ZENNIUM electrochemical workstation (Zahner Instruments, Germany).
2.3. Preparation of the magnetic WO3/Fe3O4/g-C3N4 photocatalyst
First, g-C3N4 was prepared by using a previously published method with some modifications. Typically, a mass of 3.0 g of melamine in an open crucible was subjected to a constant temperature of 500 °C for 2 h in a muffle, then heated with a ramping rate of 2.3 °C min−1 to 550 °C and held at this temperature for another 2 h, after which it was allowed to naturally cool to room temperature to obtain the g-C3N4 powders.
Second, a mass of 0.5 g Na2WO4·2H2O was dissolved in 30 mL deionized water with vigorous stirring for 30 min, and 0.2 mol L−1 HCl was introduced dropwise into the above aqueous solution to adjust the pH to 1. The resulting solution was then transferred into a 50 mL Teflon-lined stainless steel autoclave and solvothermally treated at 120 °C for 5 h. Afterwards, the product was then dried at 80 °C for 15 h and calcined at 500 °C for 1 h to yield the WO3 powder.
Finally, certain amounts of the as-prepared g-C3N4 and WO3 were dispersed in 30 mL ethanediol containing 0.3 g Fe(NO3)3·9H2O, 0.1 g C2H3O2Na·3H2O, 0.03 g PEG, and 0.005 g PVP. After the mixed components fully dissolved, the solution was transferred into a 50 mL Teflon-lined stainless steel autoclave and kept at 190 °C for 15 h. After the product was cooled to ambient temperature, it was washed with deionized water and ethanol several times to obtain WO3/Fe3O4/g-C3N4.
2.4. Photocatalytic and active species trapping experiments
The photocatalytic performances of the as-prepared samples were evaluated by monitoring the degradation of tetracycline under irradiation with visible light. A 300 W xenon lamp covered with a UV filter (λ > 400 nm) was used as a light source. In brief, a mass of 0.1 g of catalyst in 100 mL of an aqueous solution of tetracycline (20 mg L−1) was magnetically stirred in the dark to reach an adsorption–desorption equilibrium. Every 20 min, a volume of 3 mL of the mixture was removed and separated by using a magnet, and the absorbance of each such sample was measured by using a UV-vis spectrophotometer at λ = 357 nm. The active species trapping experiments were carried out in a manner similar to the way the photocatalytic experiments were performed, except that only 1 mmol triethanolamine (OA, a quencher of h+), 1 mmol isopropanol (IPA, a quencher of ·OH), and 1 mmol benzoquinone (BQ, a quencher of ·O2−) were, respectively, added into the aqueous tetracycline solution (20 mg L−1, 100 mL) before the start of the reaction; the next steps were the same in the degradation experiments described above.
2.5. Photoelectrochemical (PEC) measurements
To prepare the electrodes for the photoelectrochemical (PEC) experiments, a mass of 0.1 g of the as-prepared photocatalyst was dispersed in a mixture of 1.0 mL ethanol and 1.0 mL ethanol glycol, then subjected to spin-coating on a fluorine-doped tin oxide glass electrode (1 × 1 cm2), and calcined in air at 80 °C. The PEC measurements were taken on a CHI760D electrochemical analyzer (ChenHua Instruments, Shanghai, China). The working electrodes were photocatalyst thin films. A platinum wire electrode and Ag/AgCl electrode were used as the counter electrode and reference electrode, respectively. The PEC measurements were taken with 0.5 M Na2SO4 electrolyte, and a 300 W Xe lamp served as the light source for these measurements.
3 Results and discussion
3.1. XRD and FT-IR analyses
Fig. 1 shows the XRD patterns of pure g-C3N4, Fe3O4/g-C3N4, and WO3/Fe3O4/g-C3N4. Two distinct diffraction peaks, at 27.5° and 13.1°, were observed for the prepared g-C3N4 (Fig. 1a), which were indexed to the (002) and (100) peaks in JCPDS 87-1526. Fe3O4/g-C3N4 also yielded distinct diffraction peaks at 2θ = 30.2, 35.5, 43.2, 57.3 and 62.6° (Fig. 1b), consistent with diffraction peaks of Fe3O4.19,20 More importantly, WO3O/Fe3O4/g-C3N4 yielded additional strong diffraction peaks (Fig. 1c), and all of the peaks here matched well with the monoclinic phase WO3 (JCPDS card no. 72-0677).21,22 In addition, all the diffraction peaks at 27.4° and 35.5°, 43.2°, 57.3°, 62.6° are corresponding to lattice plane of (002) of g-C3N4 and (311), (400), (511), (440) of Fe3O4 could also be found in WO3/Fe3O4/g-C3N4. These observations hence suggested that the phase of g-C3N4 did not change with the introduction of WO3, and that WO3/Fe3O4/g-C3N4 was successfully synthesized.23,24
 |
| | Fig. 1 XRD patterns of g-C3N4 (a), Fe3O4/g-C3N4 (b), and WO3/Fe3O4/g-C3N4 (c). | |
FT-IR spectra of g-C3N4, Fe3O4/g-C3N4 and WO3/Fe3O4/g-C3N4 were also acquired, and are shown in ESI (Fig. S1†). A series of peaks from 1650 cm−1 to 1200 cm−1 (1251, 1325, 1419, 1571, and 1639 cm−1) and a peak near 808 cm−1 appeared (Fig. S1a–c†), which corresponded to the typical stretching modes of CN heterocycles.25 Clearly, the main peaks of g-C3N4 appeared in the spectrum of Fe3O4/g-C3N4 (Fig. S1b†) and were similar to those of pure g-C3N4, indicating that g-C3N4 in Fe3O4/g-C3N4 maintained the same chemical structure as did pure g-C3N4 after solvothermal treatment. However, Fe3O4/g-C3N4 also yielded an extra peak at 550–650 cm−1 (Fig. S1b†) which might have been due to an Fe–O bond vibration.26,27 The peaks mentioned above were all weakened in the WO3/Fe3O4/g-C3N4 spectrum (Fig. S1c†), i.e., after deposition of WO3 nanoparticles, because the surface functional groups of g-C3N4 may have been covered by the deposited WO3, and as the FT-IR results showed the introduction of Fe3O4 and WO3 to have no effect on the chemical structure of g-C3N4.
3.2. TEM analysis
The morphology of the as-prepared sample was further investigated by using TEM. The g-C3N4 nanosheets were clearly observed to be thin and to overlap each other (Fig. 2a). Meanwhile, the TEM image of Fe3O4/g-C3N4 (Fig. 2b) showed the Fe3O4 nanoparticles to have an average diameter of about 50–100 nm, and to be dispersed homogeneously on the g-C3N4 surface. And the TEM image of WO3/Fe3O4/g-C3N4 (Fig. 2c) showed the presence of small particles on the Fe3O4/g-C3N4 surface; moreover, the Fe3O4 nanoparticles and g-C3N4 nanosheet were less transparent in this composite than in the other two forms examined. In addition, analysis of the EDS spectrum of WO3/Fe3O4/g-C3N4 (Fig. 2d) clearly revealed the presence of the elements Fe, O, W, C and N. The EDS and XRD results taken together confirmed the presence of WO3, Fe3O4, and g-C3N4 in the WO3/Fe3O4/g-C3N4 Z-scheme system. In addition, the specific surface areas of g-C3N4, Fe3O4/g-C3N4 and WO3/Fe3O4/g-C3N4 were 6.9, 50.3 and 87.6 m2 g−1, respectively. Fe3O4/g-C3N4 were lower than that of WO3/Fe3O4/g-C3N4. The surface roughness levels of the as-prepared samples were also observed to differ. This difference might have contributed to the photocatalytic activity of WO3/Fe3O4/g-C3N4 being greater than those of g-C3N4 and Fe3O4/g-C3N4: the more expansive and rougher BET surface of WO3/Fe3O4/g-C3N4 can adsorb more tetracycline and further improve the photocatalytic degradation efficiency.
 |
| | Fig. 2 TEM images of g-C3N4 (a), Fe3O4/g-C3N4 (b), WO3/Fe3O4/g-C3N4 (c), and an EDS spectrum of WO3/Fe3O4/g-C3N4 (d). | |
3.3. Photocatalytic activity
We first sought to determine the amount of Fe3O4 in Fe3O4/g-C3N4 that optimized its photocatalytic performance. From degradation dynamics curves of tetracycline over Fe3O4/g-C3N4 with different contents of Fe3O4 (Fig. 3a), Fe3O4/g-C3N4 was determined to exhibit the highest photocatalytic activity when the percentage of Fe3O4/g-C3N4 made up of Fe3O4 was 5.0 wt%. Thus, Fe3O4 not only has the magnetic separation performance, but also played an important role in enhancing the photocatalytic activity. We also determined the efficiency levels of the degradation of tetracycline by WO3/Fe3O4/g-C3N4 with different amounts of WO3 (Fig. 3b). The photocatalytic activity of WO3/Fe3O4/g-C3N4 was optimal when 1.0 wt% of it was made up of WO3 nanoparticles: this composition degraded 89% of tetracycline over the course of a photocatalytic reaction of 120 min. This result suggested that too much WO3 covering the Fe3O4/g-C3N4 surface would diminish the ability of the material to harvest light. In contrast, too little WO3 would weaken the formation of heterojunctions between WO3 and g-C3N4. Therefore, the formation of heterojunctions between WO3 and g-C3N4 appeared to be a major enhancer of photocatalytic activity owing to the ability of these heterojunctions to efficiently separate the charge carriers. The degradation dynamics curves of tetracycline in Fig. 3c showed the decomposition effect of g-C3N4 to have been significantly enhanced after it was modified with Fe3O4 and WO3. We also acquired absorbance spectra of tetracycline over WO3/Fe3O4/g-C3N4 for various photodegradation reaction times (Fig. 3d). The absorbance nearly disappeared in 120 min. This result suggested that the tetracycline molecules were completely decomposed into smaller molecules.28
 |
| | Fig. 3 (a and b) Degradation of tetracycline over different samples under visible light irradiation. (a) Fe3O4/g-C3N4 with different Fe3O4 contents: 40 wt%, 20 wt%, 10 wt%, 5.0 wt%, and 3.0 wt%. (b) WO3/Fe3O4/g-C3N4 with different WO3 contents: 10 wt%, 5.0 wt%, 2.0 wt%, 1.0 wt%, and 0.5 wt%. (c) The photocatalytic effect curves of photo-degradation solutions over pure g-C3N4, Fe3O4/g-C3N4, and WO3/Fe3O4/g-C3N4. (d) Absorbance of 20 mg L−1 tetracycline solutions after various reaction times with WO3/Fe3O4/g-C3N4. | |
3.4. UV-vis analysis
UV-vis diffuse reflectance spectroscopy was used to determine the absorption levels of the as-prepared pure g-C3N4 and the Fe3O4/g-C3N4 and WO3/Fe3O4/g-C3N4 composites (Fig. 4). The pure g-C3N4 displayed an absorption edge around 450 nm, which corresponded to a band gap of 2.76 eV. The absorption edge of Fe3O4/g-C3N4 in the visible-light range was significantly increased, with a red shift of more than 700 nm relative to that of the pure g-C3N4. More importantly, the diffuse reflectance absorption of WO3/Fe3O4/g-C3N4 was stronger than that of Fe3O4/g-C3N4, and the enhanced light absorption was caused by the introduced WO3 nanoparticles. The enhanced light absorption may have generated more photo-induced electron–hole pairs under visible-light irradiation, which subsequently enhanced the photocatalytic activity. Therefore, the heterojunction character of the WO3/Fe3O4/g-C3N4 catalyst was able to effectively enhance its light absorption and photocatalytic activity. UV-vis diffuse reflectance spectra with different Fe3O4 contents are shown in Fig. S2.†
 |
| | Fig. 4 UV-vis absorbance spectra of g-C3N4, Fe3O4/g-C3N4, and WO3/Fe3O4/g-C3N4. | |
3.5. Photoluminescence emission spectra
PL emission spectra were acquired to investigate the recombination and separation of the photoexcited electron–hole pairs, and the intensity of the PL emission spectra indicated the recombination speeds of these pairs. Generally, a higher PL intensity means a higher recombination rate of electron–hole pairs.29,30 As shown in Fig. 5, the PL intensity of g-C3N4 was the highest of the three as-prepared samples, and as expected, the PL response of WO3/Fe3O4/g-C3N4 was the lowest. This difference indicated the importance of WO3 and Fe3O4 in the Z-scheme system and indirectly showed WO3/Fe3O4/g-C3N4 to have the lowest electron–hole pair recombination rate.
 |
| | Fig. 5 Photocurrent response curves of g-C3N4, Fe3O4/g-C3N4, and WO3/Fe3O4/g-C3N4 under irradiation with visible light. | |
3.6. Photocurrent tests
Photocurrent testing is considered to be an efficient method for demonstrating the generation and separation of electron–hole pairs in photocatalytic materials. Here, the higher the photocurrent, the higher the electron–hole pair separation efficiency, and the higher the photocatalytic activity.31,32 Fig. 6 shows the photocurrent responses of pure g-C3N4, Fe3O4/g-C3N4 and WO3/Fe3O4/g-C3N4. The photocurrent of pure g-C3N4 was the lowest of the three samples. And satisfyingly, the photocurrent of WO3/Fe3O4/g-C3N4 was the highest, about three times that of pure g-C3N4, and remained at a nearly constant value after the light was turned on and after it was turned off. Therefore, the results of the photocurrent test indirectly showed the WO3/Fe3O4/g-C3N4 to have the lowest electron–hole pair recombination rate and the highest photocatalytic activity. The photocurrent response curves of WO3/Fe3O4/g-C3N4 with different contents of WO3 were also acquired, and are displayed in Fig. S3.†
 |
| | Fig. 6 Photocurrent response curves of g-C3N4, Fe3O4/g-C3N4, and WO3/Fe3O4/g-C3N4 under visible light irradiation. | |
3.7. Electrochemical impedance spectroscopy
Electrochemical impedance spectroscopy (EIS) measurements were taken (Fig. 7) to investigate the charge transfer resistance and separation efficiency of the photoinduced charge carriers. Of the three samples tested, the diameter of the Nyquist semicircle was greatest and lowest for g-C3N4 and WO3/Fe3O4/g-C3N4, respectively, indicating them to have the highest and lowest levels of resistance, respectively. These EIS results indicated that the introduction of Fe3O4 and WO3 into g-C3N4 enhanced the separation and transfer efficiency of photoinduced electron–hole pairs, which is a favorable condition for improving the photocatalytic activity. These result reveals that the WO3/Fe3O4/g-C3N4 has a preferable efficiency of charge transfer after modified by WO3 nanoparticles. In addition, EIS spectra of WO3/Fe3O4/g-C3N4 with different contents of WO3 were also acquired and are shown in Fig. S4.†
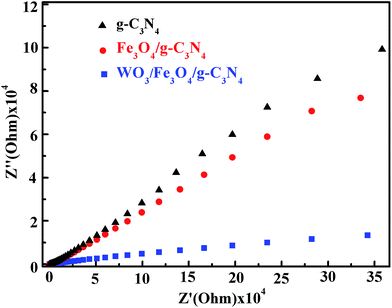 |
| | Fig. 7 EIS spectra of g-C3N4, Fe3O4/g-C3N4, and WO3/Fe3O4/g-C3N4. | |
3.8. Detection of reactive species
When photocatalysts are irradiated by visible light, active species such as holes (h+), hydroxyl radicals (·OH), and superoxide radicals (·O2−) are generated during the photocatalytic process.33,34 In the current work, we carried out radical trapping experiments (results shown in Fig. 8), using benzoquinone (BQ) to reduce the levels of ·O2−, isopropanol (IPA) to impair the formation of ·OH, and triethanolamine (TEAO) to remove h+. Only 10% of the tetracycline was degraded over WO3/Fe3O4/g-C3N4 in the presence of IPA, in contrast to the near-90% degradation without any trapping agents. This result revealed the very strong effect that ·OH had during the photo-degradation of tetracycline. Also, only about 25% of the tetracycline was degraded in the presence of TEAO, indicating that h+ also played crucial role in the degradation of tetracycline. In addition, when BQ was used as the superoxide radical scavenger agent, 30% of the tetracycline was degraded. These results taken together indicated the order of the influence of the activated species on the tetracycline photo-degradation process to be ·OH > h+ > ·O2−.
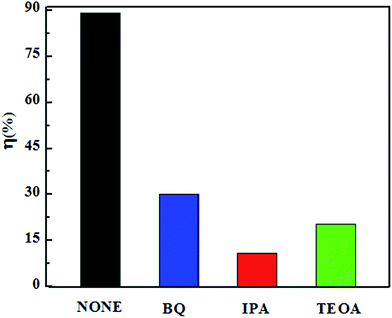 |
| | Fig. 8 Effects of reactive species of different scavengers on the photocatalytic activity of WO3/Fe3O4/g-C3N4. | |
3.9. Detection of reactive oxygen species using ESR
The ESR technique has been used to detect the presence of ·OH and ·O2− radicals in photocatalytic reaction systems. As shown in Fig. 9a, four characteristic peaks for DMPO–·OH adducts were observed (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 quartet pattern) with WO3/Fe3O4/g-C3N4 subjected to irradiation with visible light. Similarly, four characteristic peaks (Fig. 9b) corresponding to the DMPO–·O2− adduct were observed, indicating that ·O2− radicals were also produced when WO3/Fe3O4/g-C3N4 was irradiated with visible light. The ESR results confirmed that both ·O2− and ·OH were present, and that the active oxidation species played an important role in the photodegradation of tetracycline.
1 quartet pattern) with WO3/Fe3O4/g-C3N4 subjected to irradiation with visible light. Similarly, four characteristic peaks (Fig. 9b) corresponding to the DMPO–·O2− adduct were observed, indicating that ·O2− radicals were also produced when WO3/Fe3O4/g-C3N4 was irradiated with visible light. The ESR results confirmed that both ·O2− and ·OH were present, and that the active oxidation species played an important role in the photodegradation of tetracycline.
 |
| | Fig. 9 ESR spectra of WO3/Fe3O4/g-C3N4 (a) DMPO–·OH and (b) DMPO–·O2− resulting from irradiation with visible light. | |
3.10. VSW and reusability
A vibrating sample magnetometer (VSM) was employed to compare the magnetic properties of Fe3O4/g-C3N4 and WO3/Fe3O4/g-C3N4. Their symmetric hysteresis loops are shown in Fig. S5.† The values of magnetization saturation (Ms) of Fe3O4/g-C3N4 and WO3/Fe3O4/g-C3N4 were 30.06 Oe and 20.41 Oe, respectively. The relatively weakened Ms value of WO3/Fe3O4/g-C3N4 could be explained by the introduced WO3 nanoparticles. However, when an external magnetic field was applied, the WO3/Fe3O4/g-C3N4 became attracted to the wall of the beaker and the dispersion became clear and transparent, indicating that the WO3/Fe3O4/g-C3N4 still showed good magnetic properties. In addition, the photocatalytic stability of WO3/Fe3O4/g-C3N4 was also tested, because such stability is crucially important for practical applications. The recycling capability of WO3/Fe3O4/g-C3N4 was verified by carrying out a five-run test, which is shown in Fig. S6.† The results of this test revealed no obvious decrease in the degradation rate, which revealed the excellent photocatalytic stability of the Z-scheme WO3/Fe3O4/g-C3N4 photocatalyst.
3.11. Mechanism of enhanced photocatalytic performance
A schematic drawing illustrating the proposed synergistic effect in the photocatalytic degradation of tetracycline over WO3/Fe3O4/g-C3N4 is shown in Fig. 10. Under irradiation with visible light, the electrons and holes in this proposal are excited from WO3 and g-C3N4 (eqn (1) and (2)).35 And then, the weak reductive photoelectrons in the conduction band (CB) of g-C3N4 could transfer to the CB of WO3 (eqn (3)). Simultaneously, the weak oxidative holes in the VB of WO3 could transfer to the VB of g-C3N4 (eqn (4)). Then, the photogenerated electrons and holes could annihilate at Fe3O4 (eqn (5)).36–38 And the lifetime of charge carriers photogenerated from g-C3N4 and WO3 might be increased. As a result, the WO3/Fe3O4/g-C3N4 photocatalyst would show higher photocatalytic activity. Moreover, according to this proposal, the photogenerated electrons from g-C3N4 react with O2 and reduce it to ·O2− (eqn (6)). Subsequently, the ·O2− would react with H+ in the solution and generate ·OH (eqn (7)). As both the ·O2− and ·OH are the reactive species, they would be the agents causing tetracycline to decompose into smaller molecules (eqn (8) and (9)). Meanwhile, the holes generated from the valence band of g-C3N4 and WO3 can also directly oxidize the tetracycline dye (eqn (10)). Finally, the tetracycline gradually becomes consumed as the reaction progresses. This photocatalytic mechanism can be summarized as follows:| | |
WO3 + hν → WO3 (e− + h+)
| (1) |
| | |
g-C3N4 + hν → g-C3N4 (e− + h+)
| (2) |
| | |
g-C3N4 (e−) → WO3 (CB)
| (3) |
| | |
WO3 (h+) → g-C3N4 (VB)
| (4) |
| | |
WO3 (e−) + g-C3N4 (h+) → Fe3O4 (annihilate)
| (5) |
| | |
O2 + g-C3N4 (e−) → ·O2−
| (6) |
| | |
·O2− + tetracycline → small-molecule products
| (8) |
| | |
·OH + tetracycline → small-molecule products
| (9) |
| | |
h+ + tetracycline → small-molecule products
| (10) |
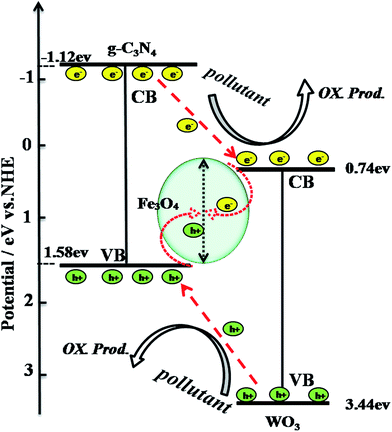 |
| | Fig. 10 Schematic illustration of the generation, separation and transport of photogenerated charge carriers at the WO3/Fe3O4/g-C3N4 interfaces. | |
4 Conclusions
In summary, a novel Z-scheme photocatalyst, WO3/Fe3O4/C3N4, was successfully prepared and showed high photocatalytic activity (90%) towards the degradation of tetracycline according to the results of PL spectroscopy, transient photocurrent, EIS spectroscopy and scavenger experiments. The increased photocatalytic activity was attributed to the high conductivity of Fe3O4, which acted as an intermediate agent for making the holes (g-C3N4) and electrons (WO3) annihilate effectively, and further enhanced the activity of the photocatalysts. On the basis of the results of this study, the present approach used to prepare the photocatalyst and the described mechanism can provide valuable information for the development of magnetic-based Z-scheme photocatalysts that remove pollutants from the environment.
Acknowledgements
This work was financially supported by the Young Teacher Funding Scheme from Pingdingshan University (No. 2014037) and the National Scientific Research Project Cultivating Foundation of Pingdingshan University (No. PXY-PYJJ2016005).
References
- F. Sastre, A. V. Puga, L. Liu, A. Corma and H. García, Complete Photocatalytic Reduction of CO2 to Methane by H2 under Solar Light Irradiation, J. Am. Chem. Soc., 2014, 136, 6798–6801 CrossRef CAS PubMed.
- C. H. Lin, J. H. Chao, C. H. Liu, J. C. Chang and F. C. Wang, Effect of Calcination Temperature on the Structure of a Pt/TiO2 Nanofiber and its Photocatalytic Activity in Generating H2, Langmuir, 2008, 24, 9907–9915 CrossRef CAS.
- Y. H. Zhang, Z. R. Tang, X. Z. Fu and Y. J. Xu, TiO2–Graphene Nanocomposites for Gas-Phase Photocatalytic Degradation of Volatile Aromatic Pollutant: Is TiO2–Graphene Truly Different from Other TiO2–Carbon Composite Materials, ACS Nano, 2010, 4, 7303–7314 CrossRef CAS PubMed.
- L. L. Ji, W. Chen, S. R. Zheng, Z. Y. Xu and D. Q. Zhu, Adsorption of sulfonamide antibiotics to multiwalled carbon nanotubes, Langmuir, 2009, 25, 11608–11613 CrossRef CAS PubMed.
- G. Buitron and I. Moreno-Andrade, Biodegradation kinetics of a mixture of phenols in a sequencing batch moving bed biofilm reactor under starvation and shock loads, J. Chem. Technol. Biotechnol., 2011, 186, 669–674 CrossRef.
- L. Sun, C. Wang, M. Ji and X. Kong, Treatment of mixed chemical wastewater and the agglomeration mechanism via an internal electrolysis filter, Chem. Eng. J., 2013, 50–56, 215–216 Search PubMed.
- Z. Zhu, X. Tang, S. Kang, P. W. Huo, M. S. Song, W. D. Shi, Z. Y. Lu and Y. Y. Yan, Constructing of the magnetic photocatalytic nanoreactor MS@FCN for cascade catalytic degrading of tetracycline, J. Phys. Chem. C, 2016 DOI:10.1021/acs.jpcc.6b05642.
- Y. S. Jun, J. Park, S. U. Lee, A. Thomas, W. H. Hong and G. D. Stucky, Three-Dimensional Macroscopic Assemblies of Low-Dimensional Carbon Nitrides for Enhanced Hydrogen Evolution, Angew. Chem., Int. Ed., 2013, 125, 11289–11293 CrossRef.
- P. Niu, L. Zhang, G. Liu and H. Cheng, Graphene-Like Carbon Nitride Nanosheets for Improved Photocatalytic Activities, Adv. Funct. Mater., 2012, 22, 4763–4770 CrossRef CAS.
- Z. Lin and X. Wang, Nanostructure engineering and doping of conjugated carbon nitride semiconductors for hydrogen photosynthesis, Angew. Chem., 2013, 125, 1779–1782 CrossRef.
- A. Srinivasan and M. Miyauchi, Chemically Stable WO3 Based Thin-Film for Visible-Light Induced Oxidation and Superhydrophilicity, J. Phys. Chem. C, 2012, 116, 15421–15426 CAS.
- T. Nakajima, T. Kitamura and T. Tsuchiya, Visible light photocatalytic activity enhancement for water purification in Cu(II)-grafted WO3 thin films grown by photoreaction of nanoparticles, Appl. Catal., B, 2011, 47–53, 108–109 Search PubMed.
- F. G. Wang, C. D. Valentin and G. Pacchioni, Electronic and Structural Properties of WO3: A Systematic Hybrid DFT Study, J. Phys. Chem. C, 2011, 115, 8345–8353 CAS.
- S. Chen, Y. Hu, S. Meng and X. Fu, Study on the separation mechanisms of photogenerated electrons and holes for composite photocatalysts g-C3N4/WO3, Appl. Catal., B, 2014, 150–151, 564–573 CrossRef CAS.
- H. Tada, T. Mitsui, T. Kiyonaga, T. Akita and K. Tanaka, All-solid-state Z-scheme in CdS–Au–TiO2 three-component nanojunction system, Nat. Mater., 2006, 5, 782–786 CrossRef CAS PubMed.
- W. J. Chen, P. J. Tsai and Y. C. Chen, Functional Fe3O4/TiO2 Core/Shell Magnetic Nanoparticles as Photo killing Agents for Pathogenic Bacteria, Small, 2008, 4, 485–491 CrossRef CAS PubMed.
- J. Xie, K. Chen, H. Y. Lee, C. H. Xu, A. R. Su, S. Peng, X. Chen and S. Sun, Ultra small c(RGDyK)-Coated Fe3O4 Nanoparticles and Their Specific Targeting to Integrin rv3-Rich Tumor Cells, J. Am. Chem. Soc., 2008, 130, 7542–7543 CrossRef CAS PubMed.
- L. Ren, S. Huang, W. Fan and T. Liu, One-Step Preparation of Hierarchical Superpara magnetic Iron Oxide/Graphene Composites Via Hydrothermal Method, Appl. Surf. Sci., 2011, 258, 1132–1138 CrossRef CAS.
- Z. Zhu, Z. Y. Lu and X. X. Zhao, Surface imprinting of a g-C3N4 photocatalyst for enhanced photocatalytic activity and selectivity towards photodegradation of 2-mercaptobenzothiazole, RSC Adv., 2015, 5, 40726–40736 RSC.
- G. Quan, Y. Liao, L. Yin, J. Long, X. Wang and C. Xue, Template-free synthesis of porous graphitic carbon nitride microspheres for enhanced photocatalytic hydrogen generation with high stability, Appl. Catal., B, 2015, 165, 503–510 CrossRef.
- T. Wang, W. Quan, D. Jiang, L. Chen, D. Li and S. Meng, Synthesis of redox-mediator-free direct Z-scheme AgI/WO3 nanocomposite photocatalysts for the degradation of tetracycline with enhanced photocatalytic activity, Chem. Eng. J., 2016, 300, 280–290 CrossRef CAS.
- A. K. Chakraborty and M. A. Kebede, Preparation and characterization of WO3/Bi3O4Cl nanocomposite and its photocatalytic behavior under visible light irradiation, React. Kinet., Mech. Catal., 2012, 106, 83–98 CrossRef CAS.
- Y. Q. Yang, Facile synthesis of ZnO/Ag nanocomposites with enhanced photocatalytic properties under visible light, Mater. Lett., 2016, 180, 97–100 CrossRef CAS.
- Y. Guo, S. Lin, X. Li and Y. Liu, Amino acids assisted hydrothermal synthesis of hierarchically structured ZnO with enhanced photocatalytic activities, Appl. Surf. Sci., 2016, 384, 83–91 CrossRef CAS.
- S. Cao, W. Yuan, Y. P. Fang, J. Shahjamali and M. M. Barber, Growth of CdS quantum dots on g-C3N4 nanosheets for highly efficient photocatalytic hydrogen generation under visible light irradiation, Int. J. Hydrogen Energy, 2013, 38, 1258–1266 CrossRef CAS.
- J. Xie, K. Chen, H. Y. Lee, C. H. Xu, A. R. Su, S. Peng, X. Chen and S. Sun, Ultrasmall c(RGDyK)-Coated Fe3O4 Nanoparticles and Their Specific Targeting to Integrin rv3-Rich Tumor Cells, J. Am. Chem. Soc., 2008, 130, 7542–7543 CrossRef CAS PubMed.
- J. Park, K. J. An and Y. S. Wang, Ultra-large-scale syntheses of monodisperse nanocrystals, Nat. Mater., 2004, 3, 891–895 CrossRef CAS PubMed.
- Z. Zhu, X. Tang, C. Ma, M. Song, N. Gao and Y. Wang, Fabrication of conductive and high-dispersed PPy@Ag/g-C3N4, composite photocatalysts for removing various pollutants in water, Appl. Surf. Sci., 2016, 387, 366–374 CrossRef CAS.
- J. Cao, B. D. Luo, H. L. Lin, B. Y. Xu and S. F. Chen, Thermodecomposition synthesis of WO3/H2WO4 heterostructures with enhanced visible light photocatalytic properties, Appl. Catal., B, 2012, 111–112, 288–296 CrossRef CAS.
- C. Li, C. Gang, J. Sun, Y. Feng, J. Liu and H. Dong, Ultrathin nanoflakes constructed erythrocyte-like Bi2WO6 hierarchical architecture via, anionic self-regulation strategy for improving photocatalytic activity and gas-sensing property, Appl. Catal., B, 2015, 163, 415–423 CrossRef CAS.
- Z. Y. Lu, F. Chen, M. He, M. S. Song, Z. F. Ma, W. D. Shi, Y. S. Yan and J. Z. Lan, Microwave synthesis of a novel magnetic imprinted TiO2 photocatalyst with excellent transparency for selective photodegradation of enrofloxacin hydrochloride residues solution, Chem. Eng. J., 2014, 249, 15–26 CrossRef CAS.
- F. Tian, Y. Zhang, J. Zhang and C. Pan, Raman Spectroscopy: A New Approach to Measure the Percentage of Anatase TiO2 Exposed (001) Facets, J. Phys. Chem. C, 2012, 11, 7515–7519 Search PubMed.
- Z. A. Huang, Q. Sun, K. L. Lv, Z. H. Zhang, M. Li and B. Li, Effect of contact interface between TiO2 and g-C3N4 on the photoreactivity of g-C3N4/TiO2 photocatalyst (001) vs. (101) facets of TiO2, Appl. Catal., B, 2015, 164, 420–427 CrossRef CAS.
- K. Dai, D. Li, L. Lu, Q. Liu, C. Liang and J. Lv, Plasmonic TiO2/AgBr/Ag ternary composite nanosphere with heterojunction structure for advanced visible light photocatalyst, Appl. Surf. Sci., 2014, 314, 864–871 CrossRef CAS.
- S. Chen, Y. Hu, X. Jiang, S. Meng and X. Fu, Fabrication and characterization of novel Z-scheme photocatalyst WO3/g-C3N4 with high efficient visible light photocatalytic activity, Mater. Chem. Phys., 2015, 149–150, 512–521 CrossRef CAS.
- Z. Zhu, Z. Y. Lu, D. D. Wang, X. Tang, Y. S. Yan, W. D. Shi and Y. S. Wang, Construction of high-dispersed Ag/Fe3O4/g-C3N4 photocatalyst by selective photo-deposition and improved photocatalytic activity, Appl. Catal., B, 2016, 182, 115–122 CrossRef CAS.
- W. Li, F. Chang and S. Dai, Fabrication of sulfur-doped g-C3N4/Au/CdS Z-scheme photocatalyst to improve the photocatalytic performance under visible light, Appl. Catal., B, 2015, 168–169, 465–471 CrossRef CAS.
- Z. Zhu, Z. Y. Lu, D. D. Wang, X. Tang, Y. S. Yan, W. D. Shi and Y. S. Wang, Construction of high-dispersed Ag/Fe3O4/g-C3N4 photocatalyst by selective photo-deposition and improved photocatalytic activity, Appl. Catal., B, 2016, 182, 115–122 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra24096h |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 quartet pattern) with WO3/Fe3O4/g-C3N4 subjected to irradiation with visible light. Similarly, four characteristic peaks (Fig. 9b) corresponding to the DMPO–·O2− adduct were observed, indicating that ·O2− radicals were also produced when WO3/Fe3O4/g-C3N4 was irradiated with visible light. The ESR results confirmed that both ·O2− and ·OH were present, and that the active oxidation species played an important role in the photodegradation of tetracycline.
1 quartet pattern) with WO3/Fe3O4/g-C3N4 subjected to irradiation with visible light. Similarly, four characteristic peaks (Fig. 9b) corresponding to the DMPO–·O2− adduct were observed, indicating that ·O2− radicals were also produced when WO3/Fe3O4/g-C3N4 was irradiated with visible light. The ESR results confirmed that both ·O2− and ·OH were present, and that the active oxidation species played an important role in the photodegradation of tetracycline.






