DOI:
10.1039/C6RA23631F
(Paper)
RSC Adv., 2016,
6, 114361-114373
Synthesis, characterization of nitrogen-doped mesoporous carbon spheres and adsorption performance
Received
23rd September 2016
, Accepted 22nd November 2016
First published on 30th November 2016
Abstract
Nitrogen-doped mesoporous carbon spheres (NMCS) were prepared by a nanocasting route using benzoxazine resins as the precursor of nitrogen and carbon, and ordered mesoporous silica spheres as the hard template to remove methyl orange (MO) dye in aqueous solution. The prepared NMCS were respectively characterized by transmission electron microscopy, scanning electron microscopy, nitrogen sorption, X-ray diffraction, energy dispersive spectrometry, elemental analysis, thermal gravimetric analysis and X-ray photoelectron spectroscopy technologies. The characterization results show that NMCS are amorphous spherical nanoparticles with worm-like mesoporous channels, the specific surface area, pore volume and nitrogen contents were 634 m2 g−1, 0.91 cm3 g−1 and 3.50 (wt%), respectively. MO adsorption on NMCS was well fitted with the Langmuir adsorption isotherm and monolayer maximum adsorption capacity was up to 352.1 mg g−1 at 318 K, it was suggested that NMCS have excellent adsorption capacity for methyl orange. MO adsorption kinetics was found to follow the pseudo-second-order kinetic model, and the intra-particle diffusion model indicates that the rate-controlling step of adsorption was determined simultaneously by external mass transfer and intra-particle diffusion. The thermodynamic parameters indicated MO adsorption was a spontaneous, endothermic and feasible process. The free energy change was −27.79 kJ mol−1, −29.07 kJ mol−1 and −30.32 kJ mol−1 at 298 K, 308 K and 318 K, and the enthalpy and entropy change was 9.835 kJ mol−1 and 126.3 J (mol−1 K−1). Moreover, good regeneration properties and long life were observed for NMCS through the desorption experiments. MO adsorption should be mainly controlled by electrostatic attraction mechanism.
1. Introduction
The synthetic organic dyes, known as a specific group of pollutants, have attracted widespread attention.1–3 The different dyes with complex molecular structures are non-biodegradable, even a small amount of dye wastewater is toxic to some organisms and even carcinogenic.4–8 To remove dyes in dye wastewater, many conventional methods are adopted such as coagulation,9–13 electrochemical,14–17 oxidation,18–21 photodecomposition,22–33 ultrafiltration34–39 and adsorption40–48 methods. Among these techniques, the adsorption process as one of the reliable and efficient techniques has been successfully used for dyes removal from wastewater.49–51 A number of porous materials, including activated carbon, inorganic oxides, carbon nanotubes/nanofibers, and mesoporous carbon have been reported to remove dyes from aqueous solutions.52–57 Recently, due to outstanding properties such as high mechanical strength, large specific surface area, opened mesoporous structures, high chemical and thermal stabilities, mesoporous carbons (pore size ranged from 2 to 50 nm) have been widely employed as adsorbents to remove dyes.58–61 The mesoporous carbons are difficult to disperse homogeneously in water, which greatly limit their application. Therefore, many efforts have been made in enhancement of the dispersion property and adsorption capacities.62 In general, adsorption functional groups (–NH2, –COOH, –CN, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) are usually introduced into mesoporous carbons by chemical post-modification processes. However, post-modification processes are uncontrollable in terms of both the amount and distribution of dopants. In addition, the preparation steps require extra chemical reagents such as sulfuric acid, nitric acid, aniline, which increases operation difficulty and adsorption costs.
O) are usually introduced into mesoporous carbons by chemical post-modification processes. However, post-modification processes are uncontrollable in terms of both the amount and distribution of dopants. In addition, the preparation steps require extra chemical reagents such as sulfuric acid, nitric acid, aniline, which increases operation difficulty and adsorption costs.
The nitrogen-doped mesoporous carbon not only maintains excellent mesoporous characteristics, but also presents better hydrophilicity and stronger electronegativity due to doping nitrogen atoms.63,64 Therefore, compared with the conventional mesoporous carbon, nitrogen-doped mesoporous carbon (NMC) have better adsorption performance, and nitrogen atom can be introduced into carbon skeleton by in situ doping without post-modification processes.65,66 However, to our knowledge, there are very few reports about nitrogen-doped mesoporous carbon used for adsorbing methyl orange from aqueous solution in the publicized literatures.67
In this work, we successfully fabricate a new efficient adsorbent of nitrogen-doped mesoporous carbon spheres (NMCS) used for adsorbing methyl orange. The adsorption performance of nitrogen-doped mesoporous carbon for methyl orange was systematically investigated, and two models Langmuir and Freundlich isotherms were applied to analyze the adsorption isotherm. Furthermore, kinetic and thermodynamic studies are also carried out to determine the adsorption process. Desorption were conducted to investigate the regeneration and reusability of NMCS. The adsorption mechanism and thermal stability of NMCS was also investigated.
2. Experimental section
2.1 Chemicals and materials
Diethylenetriamine (DETA, 99%) was purchased from Aladdin Reagent Co., Ltd. Tetraethyl orthosilicate (TEOS, 99 wt%), 1-methylimidazole, 1-bromohexadecane, phenol, toluene, formaldehyde (35–40% aqueous solution), anhydrous ether, anhydrous ethanol, ammonium hydroxide (25 wt% NH3 in water), hydrofluoric acid, methyl orange were purchased from Sinopharm Chemical Reagent Co., Ltd. Mesoporous silica spheres (self-made, it was synthesized according to the Stöber method68).
2.2 Synthesis
2.2.1 Synthesis of DETA-based benzoxazine (BOZ-DETA). 0.05 M of DETA, 0.2 M of paraformaldehyde and 15 mL of toluene were placed in a 250 mL round-bottomed flask with 300 rpm for 1.0 h. Then the solution was gradually heated to 80 °C. 0.1 M phenol with 15 mL toluene were added drop-wise and reacted under reflux for 5.0 h. Solvent of toluene was removed via rotary evaporator, the mixture was dissolved in anhydrous ether and washed three times with 1.0 M NaOH solution, and then washed with deionized water to pH = 7.0. The product was dried at 50 °C in vacuum for 24 h.
2.2.2 Synthesis of NMCS. The nitrogen-doped mesoporous spheres were synthesized by using mesoporous silica spheres as hard template, BOZ-DETA as carbon precursor. In a typical synthesis of BOZ (DETA)–silica composites: 0.4 g BOZ (DETA) was added into 2.5 mL THF, and stirred to clear and homogeneous solution obtained. Then, 1 g MSS was added into above solution stirring at room temperature, and the obtained-mixtures were sonicated for 5 h to obtain the BOZ (DETA)–silica composites. These composites were collected by centrifugation, and dried at 60 °C in vacuum for 24 h. The as-prepared BOZ (DETA)–silica composites were cured as follows: composites were carbonized at 800 °C for 2 h under a high purity nitrogen atmosphere to obtain the carbon/silica composites. Subsequently, 20 wt% HF solution was employed to etch the silica in the resulting carbon/silica composites for 24 h at room temperature. Finally, the sample was washed several times with deionized water and absolute ethanol before drying in an oven at 60 °C.
2.3 Apparatus
Structural and morphological characterizations were performed by transmission electron microscopy (TEM, FEI TecnaiG2 instrument operated at 300 kV) and scanning electron microscopy (SEM, Hitachi S-4800 instrument). Nitrogen adsorption isotherms were collected at 77 K on ASAP 2010 volumetric adsorption analyzers using nitrogen of 99.998% purity. The specific surface area was calculated according to the BET (Brunauer–Emmet and Teller) model, while the pore size and pore volume were calculated using the Barrett–Joyner–Halen-da (BJH) formula based on the desorption branch of the isotherm. The X-ray diffraction (XRD) measurements of carbonized samples were recorded using a D8 Advance diffractometric with Cu Kα radiation at an operating voltage of 40 kV. Elemental analysis was performed on FLASH EA 1112 Elemental Analyzer. The component of materials was investigated by Fourier transform-infrared spectroscopy (Thermo Nicolet FT-IR2000), and the samples were dried at 323 K in vacuum for 24 h before the measurement. The surface electronic states were determined by X-ray photoelectron spectroscopy (XPS) on a PHI quantera SXM spectrometer with an Al Kα = 280.00 eV excitation source, where binding energies were calibrated by referencing the C1s peak (284.8 eV) to reduce the sample charge effect. Thermal gravimetric analysis (TGA) curves were obtained using the NETZSCH STA 449F3 instrument.
2.4 Adsorption studies
The MO concentrations were prepared by dissolving different amounts of methyl orange in distilled water. The concentration of MO was determined by a UV spectrophotometer (Shimadzu UV spectrophotometer, UV-1800), and the maximum absorbance wavelength of methyl orange is 464 nm. The adsorption studies were conducted by 10 mg carbon adsorbents in 50 mL of dye concentrations from 20 to 200 mg L−1 (pH ∼ 5.6). The mixture was kept in a thermostatic water bath shaker with 120 rpm at a certain temperature until the adsorption equilibrium. To determine the effect of temperature and thermodynamic parameters, adsorption experiments were carried out at (298, 308, and 318) K, respectively. The residual concentrations were measured by UV spectrophotometer, and the equilibrium adsorption capacities (qe) were calculated by the following formula:| |
 | (1) |
where C0 and Ce are the initial and equilibrium dye concentrations (mg L−1), respectively. V is the volume of solution (L) and W is the mass of adsorbent (g).
2.5 Regeneration of NMCS
After equilibration, the used NMCS was washed with 100 mL distilled water to remove the unadsorbed traces of the dye. The saturated adsorbents were then treated with 50 mL ethanol and kept in a thermostatic water bath shaker with 120 rpm. After desorption, the adsorbent of NMCS was dried and used to investigate the regeneration properties and reusability.
3. Results and discussion
3.1 Characterization of adsorbent
3.1.1 N2 adsorption/desorption isotherms of NMCS. Nitrogen sorption experiments were performed to examine the porous nature of NMCS. The results showed that the mesopores are concentrated at a size of 2.3 nm. The specific surface area and pore volume are 634 m2 g−1 and 0.91 cm3 g−1, respectively. As shown in Fig. 1a that nitrogen adsorption isotherms of NMCS are a typical IV curves, indicating a mesoporous material, which is fairly similar with reported literature.69
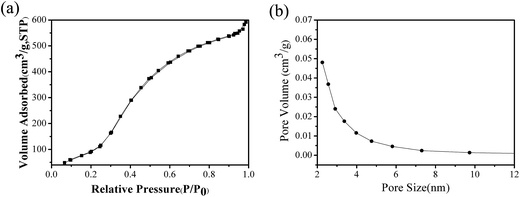 |
| | Fig. 1 (a) Nitrogen adsorption isotherms and (b) pore size distribution curves for NMCS. | |
3.1.2 SEM and TEM. The morphology of as-prepared NMCS was investigated via scanning electron microscopy (SEM) and transmission electron microscopy (TEM). As shown in Fig. 2a and b, mono-dispersed spherical nanoparticles with the average diameter about 244 nm are observed. The intrinsic structure of as-prepared NMCS was further detected with HRTEM measurements. NMCS are spherical nanoparticles with worm-like mesoporous channels, which is basically same with literature.70
 |
| | Fig. 2 SEM image of NMCS (a). TEM images of NMCS (b and c). | |
3.1.3 XRD. The NMCS were further characterized by X-ray diffraction (shown in Fig. 3). The broad and low intensity peaks centered at 23.1° and 43° reveal that the NMCS materials are amorphous with low-graphitization degree,71 and complete crystal shape at (100), (110) crystal peak.
 |
| | Fig. 3 XRD pattern of NMCS. | |
3.1.4 Energy dispersive spectrometry (EDS). From Fig. 4, energy-dispersive spectroscopy (EDS) analysis reveals the presence of C, O and N and uniform dispersion of the N, O and C elements in NMCS. The in situ carbonization could provide a facile method to get N-doped carbon materials with homogeneous distribution of N.72 Together with the corresponding carbon, nitrogen and oxygen elemental maps, reveal that these elements are uniformly distributed for throughout sample. More importantly, nitrogen image is same as that of carbon with a uniform distribution, signifying that nitrogen is homogeneously doped into the carbon framework.
 |
| | Fig. 4 EDS spectrum and mapping images of NMCS. | |
3.1.5 Elemental analysis. Table 1 collects the weight percentages of carbon (77.50 wt%), nitrogen (3.50 wt%), oxygen (17.58 wt%) and hydrogen (1.42 wt%). The presence of high O content can greatly improve the hydrophilicity of NMCS, and N content of 3.50 wt% provide more active sites on the surface of synthetic material.
Table 1 Surface composition of NMCS
| Element |
C |
H |
O |
N |
| Content (wt%) |
77.50 |
1.42 |
17.58 |
3.50 |
3.1.6 TG-DTG-DSC analysis. The thermal stability of NMCS was investigated by TG-DTG curves, which was presented in Fig. 5. The NMCS materials has slightly weightlessness caused by the evaporation of surface in 50–105 °C, and heating weightlessness was contributed to the decomposition of molecule chain dehydrogenation, amino, carboxyl and phenol hydroxyl in 400 °C. The total weight loss rate can reach 76.61%, thermal decomposition phenomenon not appearing before 400 °C showed that it has good thermal stability.
 |
| | Fig. 5 The TG-DTG curve of the NMCS material. | |
3.2 Adsorption isotherms and thermodynamic
3.2.1 Adsorption isotherms. For solid–liquid system, adsorption isotherm is an important physico-chemical aspect to describe adsorption behavior and obtain the information on the surface properties of the adsorbents.60,73 In the paper, two well-known models Langmuir and Freundlich isotherms were applied to analyze the isothermal adsorption experiment data.74The Langmuir isotherm assumes that the adsorption is a monolayer adsorption within a homogenous adsorbent surface. The adsorption takes place at specific homogeneous site over the coverage surface, and the adsorbed molecules don't interact over the entire adsorption.64,68 The equation is given as follows:
| |
 | (2) |
where
Ce,
qe,
KL and
Qm stand for the equilibrium concentration of the adsorbate (mg L
−1), the amount of adsorbate adsorbed per unit mass of adsorbent (mg g
−1), the Langmuir adsorption constant (L mg
−1), and the maximum adsorption capacity (mg g
−1), respectively.
The dimensionless separation factor RL is usually used to describe essential characteristic of Langmuir isotherm equation, and is noted as:59
| |
 | (3) |
The
RL value indicates that (a) 0 <
RL < 1 for favorable; (b)
RL > 1 for unfavorable; (c)
RL = 1 for linear adsorption; and (d)
RL = 0 for irreversible adsorption.
As an empirical equation, the Freundlich isotherm assumes that the adsorption surface becomes heterogeneous and adsorption process is not restricted to the formation of the monolayer.74 The Freundlich isotherm equation is represented as follows:
| |
 | (4) |
where
qe is the amount of adsorbate adsorbed at equilibrium (mg g
−1),
Ce is the equilibrium concentration of the adsorbate (mg L
−1),
KF is the Freundlich adsorption constant (mg g
−1) indicating the adsorption capacity and
n is the heterogeneity factor related to the adsorption intensity of the adsorbent.
The adsorption isotherms of methyl orange on NMCS at 298 K, 308 K and 318 K are shown in Fig. 6a. The equilibrium adsorption capacity increases along with the increase of the equilibrium concentration of methyl orange, while this trend slows down gradually. The adsorption capacity of MO increases with increasing temperature, which demonstrates the adsorption processes are endothermic in nature. Langmuir and Freundlich isotherms for methyl orange on NMCS are respectively shown in Fig. 6b and c. As shown in Table 2, at (298, 308 and 318) K, the Langmuir isotherms (with correlation coefficient range from 0.995 to 0.997 and the F values range from 1659.33 to 2075.78) exhibit more fitting results for isotherm adsorption experimental data than Freundlich model (with correlation coefficient range from 0.987 to 0.991 and the F value range from 483.23 to 1411.05). It suggests that monolayer adsorption takes place on the surface of NMCS adsorbent, and the active sites are homogenously distributed.75 Moreover, the calculated maximum adsorption capacity (Qm) at (298, 308 and 318) K were 284.1 mg g−1, 313.5 mg g−1, and 352.1 mg g−1 by Langmuir model, respectively, which is closed to the experimental values of equilibrium adsorption amount (qe). It is indicated that the adsorption process is favorable and rather irreversible due to values of the dimensionless constant (RL) range from 0.016961 to 0.18. Furthermore, as seen from Table 2, the values of n are found to be more than 1, indicating the high adsorption intensity.
 |
| | Fig. 6 (a) Adsorption isotherms for MO adsorption (b) Langmuir plots of the isotherms and (c) Freundlich plots of the isotherms. | |
Table 2 Isotherm parameters for the adsorption of MO
| Dye |
T (K) |
Langmuir model |
Freundlich model |
| Qm (mg g−1) |
KL (L mg−1) |
RL |
R2 |
F value |
KF |
n |
R2 |
F value |
| MO |
298 |
284.1 |
0.2272 |
0.0215–0.18 |
0.995 |
1681.08 |
130.0 |
6.386 |
0.987 |
483.23 |
| 308 |
313.5 |
0.2596 |
0.0189–0.16 |
0.995 |
1659.33 |
143.2 |
6.272 |
0.989 |
598.34 |
| 318 |
352.1 |
0.2916 |
0.0169–0.14 |
0.997 |
2075.78 |
160.7 |
6.130 |
0.991 |
1411.05 |
A comparison of the maximum adsorption capacities (Qm) of MO on NMCS and reported adsorbents are shown in Table 3. All of the others adsorbents in the previous works can be obtained via a post-modification process. The NMCS show a far better performance for the removal of MO than those reported adsorbents, and the adsorption capacity of NMCS was 352 mg g−1, indicating that the in situ doped materials NMCS shows great potential on adsorption of MO from aqueous solution.
Table 3 Comparison of the reported maximum adsorption capacities for MO on various adsorbents
| Adsorbent |
qe (mg g−1) |
Conditions (adsorbent dose, temperature, initial concentrations) |
Reference |
| Alkali-activated multiwalled carbon nanotubes |
149 |
0.75 g L−1, 25 °C, 80–150 mg L−1 |
56 |
| Chitosan/Fe2O3/CNTs |
66.09 |
0.6 g L−1, 24 °C, 5–50 mg L−1 |
76 |
| MWCNT–starch–iron oxide |
135.6 |
0.5 g L−1, 25 °C, 327.33 mg L−1 |
77 |
| Activated carbon/Fe3O4–HNO3 |
244.32 |
2 g L−1, 30 °C, 250–1000 mg L−1 |
78 |
| Acid modified carbon coated monolith |
147.06 |
10 g L−1, 30 °C, 50–600 mg L−1 |
79 |
| N-Doped mesoporous carbons |
150.85 |
1 g L−1, 25 °C, 2 g L−1 |
67 |
| Surfactant modified silkworm exuviae |
87.03 |
2 g L−1, 30 °C, 50–350 mg L−1 |
2 |
| Protonated ethylenediamine-grafted MIL-101 |
241 |
0.2 g L−1, 45 °C, 20–200 mg L−1 |
54 |
| NMCS |
352 |
0.2 g L−1, 45 °C, 20–200 mg L−1 |
This work |
3.2.2 Adsorption thermodynamic. Thermodynamic properties of MO adsorption process on NMCS were further studied to estimate the effect of temperature. Thermodynamic parameters, the free energy change (ΔG0), enthalpy change (ΔH0), and entropy change (ΔS0) were calculated by eqn (5) and (6):| |
ΔG0 = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KL KL
| (5) |
where ΔG0 is free energy change (kJ mol−1), R is the gas constant (8.314 J (mol−1 K−1)), T is the absolute temperature (K), KL is the Langmuir adsorption constant (L mol−1).Based on the Van't Hoff equation,55 the enthalpy change ΔH0 (kJ mol−1) and entropy change ΔS0 (J (mol−1 K−1)) could be obtained by eqn (6):
| |
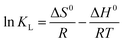 | (6) |
The enthalpy change Δ
H0 and entropy change (Δ
S0) were calculated from the slope and the intercept of the liner plot of ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KL
KL against 1/
T, respectively. The calculated values of Δ
G0, Δ
H0, and Δ
S0 are listed in
Table 4. From
Table 4, the negative free energy change (Δ
G0) suggests that the adsorption NMCS is spontaneous, and the decrease in negative values suggests an increase in the feasibility and spontaneity of the MO adsorption with increasing temperature. The positive enthalpy change indicates that the adsorption reaction is endothermic, which is in accord with the increasing adsorption capacity with increasing adsorption temperature. The positive values of Δ
S0 suggest that the randomness at the solid-solution interface increases during adsorption, which may be ascribed that the number of desorbed water molecule is far larger than that of the adsorbed MO molecule.
54
Table 4 Thermodynamic parameters for the adsorption of MO at different temperatures
| Dye |
T (K) |
ΔG0 (kJ mol−1) |
ΔH0 (kJ mol−1) |
ΔS0 (J (mol−1 K−1)) |
% ξH |
% ξS |
| MO |
298 |
−27.79 |
9.835 |
126.3 |
20.75 |
79.25 |
| 308 |
−29.07 |
20.18 |
79.82 |
| 318 |
−30.32 |
19.67 |
80.33 |
In order to estimate the major driving force in the adsorption process, the relative contributions of the molar enthalpy and molar entropy to the molar Gibbs free energy are compared by eqn (7) and (8) (ref. 80 and 81) and are listed in Table 4.
| |
 | (7) |
| |
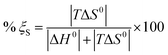 | (8) |
As seen from Table 4, the values of % ξS are far greater than those of % ξH. Therefore, the major contributing factor to the molar Gibbs free energy for MO adsorption on NMCS is entropy effect (% ξS > 79.25%) rather than enthalpy change.
3.3 Adsorption kinetics
The adsorption kinetics is essential to obtain the information about efficiency and mechanism of adsorption process. In order to identify potential rate-controlling steps and provide guidance for designing an adsorption treatment process, the pseudo-first-order and pseudo-second-order models are used to analyze adsorption kinetics data and investigate the mechanism of adsorption processes.
The pseudo-first-order model is based on the hypothesis that the adsorption rate with time is directly proportional to the difference in the amount of adsorbate adsorbed at equilibrium and the adsorbed amount.52 The pseudo-first-order kinetic model can be expressed as:
| |
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t
| (9) |
where
k1 (min
−1) is the adsorption rate constant of pseudo-first-order adsorption,
qe and
qt (mg g
−1) are adsorption amount at equilibrium and time
t, respectively. The values of
qe and
k1 (presented in
Table 5) are acquired from the intercepts and the slopes of the plots of ln(
qe −
qt)
versus t, respectively.
Table 5 Kinetic parameters for the adsorption of MO at different temperatures and different initial concentrations
| C0 (mg L−1) |
T (K) |
qe (exp) (mg g−1) |
Pseudo-first-order model |
Pseudo-second-order model |
| qe (cal) (mg g−1) |
k1 × 103 (min−1) |
R2 |
F value |
qe (cal) (mg g−1) |
k2 × 105 (g (mg−1 min−1)) |
h0 (mg (g−1 min−1)) |
R2 |
F value |
| 40 |
298 |
169.72 |
95.17 |
7.43 |
0.983 |
352.22 |
175.75 |
18.6 |
5.75 |
0.998 |
2969.09 |
| 40 |
308 |
181.29 |
82.30 |
7.98 |
0.977 |
295.11 |
186.91 |
24.2 |
8.44 |
0.999 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 274.97 274.97 |
| 40 |
318 |
196.10 |
69.31 |
7.88 |
0.975 |
279.28 |
199.20 |
33.2 |
13.18 |
0.999 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 464.98 464.98 |
| 120 |
298 |
265.13 |
168.61 |
9.48 |
0.987 |
459.81 |
270.27 |
10.5 |
7.70 |
0.997 |
4475.89 |
| 120 |
308 |
293.26 |
190.73 |
7.89 |
0.993 |
995.91 |
298.50 |
10.8 |
9.26 |
0.999 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 098.51 098.51 |
| 120 |
318 |
306.72 |
206.11 |
6.29 |
0.954 |
146.12 |
310.56 |
14.6 |
14.10 |
0.996 |
1758.15 |
The pseudo-second-order kinetic model can be represented by the following equation:
| |
 | (10) |
The initial adsorption rate
h0 (mg (g
−1 min
−1)) at
t = 0 is defined as follows:
46where
k2 (g (mg
−1 min
−1)) is the adsorption rate constant of pseudo-second-order adsorption,
qe and
qt (mg g
−1) are adsorption amount at equilibrium and time
t, respectively. It is noticed that
k2 and
qe in
eqn (10) can be calculated from the intercept and slope of the plot of
t/
qt versus t. The straight line plots of
t/
qt versus t with initial concentrations 40 mg L
−1 and 120 mg L
−1 at (298, 308 and 318 K) are shown in
Fig. 7. The kinetic parameters are given in
Table 5.
 |
| | Fig. 7 (a) pseudo-first-order kinetics plots with C0 (40 mg L−1) (b) pseudo-second-order kinetics plots with C0 (40 mg L−1) (c) pseudo-first-order kinetics plots with C0 (120 mg L−1) (d) pseudo-second-order kinetics plots with C0 (120 mg L−1). | |
As shown in Table 5, the correlation coefficients of pseudo-second-order model are found to be higher than 0.996, which is far greater than those of pseudo-first-order model range from 0.954 to 0.993. The F values of pseudo-second-order model (1758 to 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 274) are much higher than those of pseudo-first-order model (146 to 995). Moreover, the calculated values qe (cal) obtained from the pseudo-second-order model agree well with experimental values qe (exp) at initial concentrations 40 mg L−1 and 120 mg L−1. It can be concluded that the pseudo-second-order model is more suitable to describe the kinetics of MO adsorption on NMCS than the pseudo-first-order model. In addition, in Table 5, the initial adsorption rates (h0) of the pseudo-second-order model increase with the initial concentration increase. This may be attributed to the driving force of diffusion between the liquid and solid phase.82,83 Moreover, the initial adsorption rate (h0) and k2 increase with the increasing temperature, indicating that the adsorption reaction would be faster to achieve adsorption equilibrium with the increasing temperature, which is well consistent with results of thermodynamic study. From Fig. 8, the adsorption plots shows a rapid rate at the initial period, and the adsorption rate decreases with the increased contact time until adsorption equilibrium. This phenomenon can be explained by two stages.84 The first stage, film diffusion, is that dye molecules will overcome the boundary layer effect and across the film to the exterior surface of adsorbent, and this process is relatively quick due to the lager available surface area of adsorbent.56 Then, the adsorption on exterior surface get saturation, the adsorbate will diffuse into the internal pore and adhere to the interior surface of the particles, which is called inner diffusion. This stage will spend a long time because the intraparticle diffusion rate will decrease due to the increased diffusion resistance.85
274) are much higher than those of pseudo-first-order model (146 to 995). Moreover, the calculated values qe (cal) obtained from the pseudo-second-order model agree well with experimental values qe (exp) at initial concentrations 40 mg L−1 and 120 mg L−1. It can be concluded that the pseudo-second-order model is more suitable to describe the kinetics of MO adsorption on NMCS than the pseudo-first-order model. In addition, in Table 5, the initial adsorption rates (h0) of the pseudo-second-order model increase with the initial concentration increase. This may be attributed to the driving force of diffusion between the liquid and solid phase.82,83 Moreover, the initial adsorption rate (h0) and k2 increase with the increasing temperature, indicating that the adsorption reaction would be faster to achieve adsorption equilibrium with the increasing temperature, which is well consistent with results of thermodynamic study. From Fig. 8, the adsorption plots shows a rapid rate at the initial period, and the adsorption rate decreases with the increased contact time until adsorption equilibrium. This phenomenon can be explained by two stages.84 The first stage, film diffusion, is that dye molecules will overcome the boundary layer effect and across the film to the exterior surface of adsorbent, and this process is relatively quick due to the lager available surface area of adsorbent.56 Then, the adsorption on exterior surface get saturation, the adsorbate will diffuse into the internal pore and adhere to the interior surface of the particles, which is called inner diffusion. This stage will spend a long time because the intraparticle diffusion rate will decrease due to the increased diffusion resistance.85
 |
| | Fig. 8 Kinetic curves of MO adsorption on NMCS: (a) C0 (40 mg L−1), (b) C0 (120 mg L−1). | |
In this study, the intra-particle diffusion model would be employed to estimate the actual rate-controlling step of the MO adsorption process, and it is expressed as:
| |
 | (12) |
where
qt is the amount of adsorbate adsorbed at equilibrium (mg g
−1),
kid is the adsorption rate constant of intra-particle diffusion (mg (g min
1/2)
−1). The
kid and
I can be calculated from the slope and the intercept in the plot of
qt versus t0.5. The intraparticle diffusion plots for MO adsorption are shown in
Fig. 9 and the corresponding parameters are listed in
Table 6. The plots show an initial steep-sloped portion and an intermediate linear portion of the intraparticle diffusion. The initial portion is related to boundary layer diffusion and the linear portion is attributed to intraparticle diffusion. In the first portion, the large value of
I represents the thickness of the boundary and the liner plots do not pass through the origin, suggesting that the intraparticle diffusion is not the sole rate-controlling step. It also indicates that the adsorption process is a multi-step process involving in the external mass transfer and intra-particle diffusion.
86
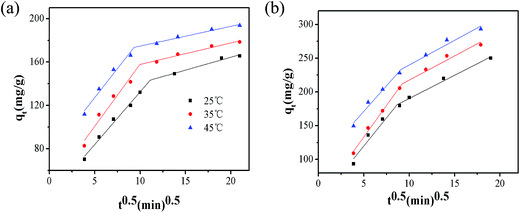 |
| | Fig. 9 intraparticle diffusion kinetics plots with, (a) C0 (40 mg L−1) and (b) C0 (120 mg L−1). | |
Table 6 Intraparticle diffusion model parameters for adsorption of MO
| C0 (mg L−1) |
T (K) |
Intraparticle diffusion model |
| kid (mg (g min1/2)−1) |
I (mg g−1) |
R2 |
| 40 |
298 |
2.38 |
35.45 |
0.948 |
| 40 |
308 |
1.99 |
43.34 |
0.959 |
| 40 |
318 |
1.82 |
73.66 |
0.963 |
| 120 |
298 |
6.87 |
36.42 |
0.985 |
| 120 |
308 |
7.19 |
39.78 |
0.947 |
| 120 |
318 |
7.32 |
95.65 |
0.947 |
3.4 Effect of initial pH on MO adsorption
MO has two chemical structures of basic form and acidic structure. Whether chromophore is anthraquinone or azo bond depends on the pH of solution, as is represented in Fig. 10.87 The MO adsorption on NMCS at the initial solution pH from 2 to 10 is shown in Fig. 11. From the Fig. 11, the adsorption capacity decreases with increased pH of the solution, which is quite similar to the reported results.2,88 This can be attributed to the surface charge of the adsorbent and the ionic charge of anionic dyes. NMCS present strong electronegativity due to doping nitrogen atoms. The amount of nitrogen doping can supply more many active sites and improve the adsorption capacity of NMCS. As pH value is less than 7 (acidic solution), protonation of the dye increase, the negative charge on the surface of the adsorbent can serve as active sites and give rise to strong electrostatic attraction with the protonation of dye in solution. Hence, it is more available to adsorb MO. While pH increases to 8.0, protonation of dye is gradually weakened. The negative charged bond site on NMCS do not favor to the adsorption of anionic dye due to electrostatic repulsion, which causes evidently decrease in MO adsorption.
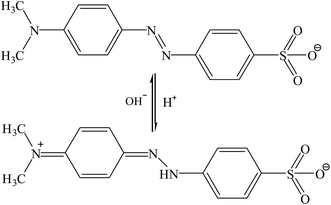 |
| | Fig. 10 The basic and acidic structures of MO. | |
 |
| | Fig. 11 Effect of pH on the adsorption of MO (experimental conditions: initial MO concentration: 40 mg L−1, adsorbent dose: 10 mg/50 mL, temperature: 298 K, contact time: 10 h). | |
3.5 Adsorbent regeneration
The regeneration of adsorbent is of great importance for commercial feasibility. The re-usability of as-prepared NMCS is shown in Fig. 12. After the first desorption, the adsorbed amounts of MO is 159.8 mg g−1, which is almost 98.9% of the adsorption capacity by the fresh material. For the fourth cycles, the adsorption capacity is 144.9 mg g−1 (89.04% of the fresh material), which suggests NMCS show well regeneration properties and long life.
 |
| | Fig. 12 Adsorption capacity on MO for four consecutive adsorption–desorption cycles (experimental conditions: initial MO concentration: 40 mg L−1, adsorbent dose: 10 mg/50 mL, temperature: 298 K, contact time: 10 h, ethanol dose: 50 mL per times). | |
3.6 Adsorption mechanism
The adsorption mechanisms are expressed by FITR spectra analysis to identify the surface functional groups of adsorbents. FITR spectra analyses of NMCS, MO/NMCS and MO are shown in Fig. 13.
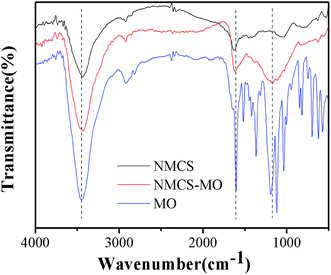 |
| | Fig. 13 FTIR spectra of MO and NMCS before and after adsorption of MO. | |
FITR of MO shows the characteristic functional groups of –S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1035 cm−1), –CH3 (1190 cm−1), C–N (1366 cm−1), and N
O (1035 cm−1), –CH3 (1190 cm−1), C–N (1366 cm−1), and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (1519 cm−1). Compared with NMCS and MO/NMCS, peaks at 2925 cm−1 and 2857 cm−1, ascribed to asymmetric and symmetric stretching of –CH2 groups, keep the same intensity and wavenumbers after adsorption, which suggest that these functional groups do not participate in the sorption process.89 After the adsorption process, the intense absorption band at 3437 cm−1, ascribed to N–H bond stretching, shows a significant increase in intensity and presents a bathochromic-shift to 3432 cm−1, indicating that the NMCS donate electrons to MO. The exhibition of band at 1629 cm−1 is the characteristic stretching vibration of C
N (1519 cm−1). Compared with NMCS and MO/NMCS, peaks at 2925 cm−1 and 2857 cm−1, ascribed to asymmetric and symmetric stretching of –CH2 groups, keep the same intensity and wavenumbers after adsorption, which suggest that these functional groups do not participate in the sorption process.89 After the adsorption process, the intense absorption band at 3437 cm−1, ascribed to N–H bond stretching, shows a significant increase in intensity and presents a bathochromic-shift to 3432 cm−1, indicating that the NMCS donate electrons to MO. The exhibition of band at 1629 cm−1 is the characteristic stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O from the group of O
O from the group of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH2, and the intensity of peak increased after adsorption of MO, it may be due to the functional groups attached to the aromatic rings of MO molecules, which suggest the MO dye adhere to the surface of NMCS. The peak at 1037 cm−1, associated with the stretching vibration of C–O,90 presents a shift to 1175 cm−1, and the broad bond shows an evident increase in intensity. This indicates that electrostatic attraction between NMCS and MO occurs in the adsorption process.
C–NH2, and the intensity of peak increased after adsorption of MO, it may be due to the functional groups attached to the aromatic rings of MO molecules, which suggest the MO dye adhere to the surface of NMCS. The peak at 1037 cm−1, associated with the stretching vibration of C–O,90 presents a shift to 1175 cm−1, and the broad bond shows an evident increase in intensity. This indicates that electrostatic attraction between NMCS and MO occurs in the adsorption process.
To further confirm the chemical composition of the NMCS materials and infrared spectroscopy results, the XPS of sample was conducted in corresponding conditions. From Fig. 14, the binding energy at 532.32 eV and 533.02 eV were specified as O–H and O–C, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in O1s spectra, respectively. 400 eV, 284.6 eV, 285.5 eV, 286.6 eV was separately assigned to N–H, C–C/C–H, C–OH, C–O–C. It is suggested that the result of XPS was consistent with infrared spectroscopy, and the functional groups such as amino, carboxyl and phenol hydroxyl exist in the material surface.
C in O1s spectra, respectively. 400 eV, 284.6 eV, 285.5 eV, 286.6 eV was separately assigned to N–H, C–C/C–H, C–OH, C–O–C. It is suggested that the result of XPS was consistent with infrared spectroscopy, and the functional groups such as amino, carboxyl and phenol hydroxyl exist in the material surface.
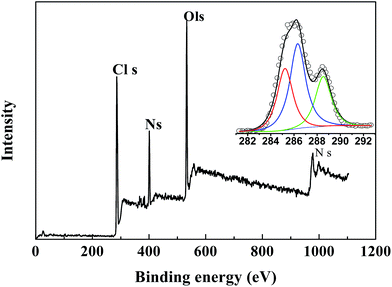 |
| | Fig. 14 The XPS of NMCS materials. | |
4. Conclusion
In present study, nitrogen-doped mesoporous carbon spheres (NMCS) were prepared successfully and applied to remove methyl orange dye. The prepared NMCS were characterized by TEM, SEM, N2 sorption, XRD, EDS and elemental analysis, TG-DTG and XPS techniques. NMCS are amorphous spherical nanoparticles (average diameter about 244 nm) with worm-like mesoporous channels and have a high surface area (634 m2 g−1), large pore volume (0.91 cm3 g−1), moderate nitrogen (3.50 wt%) contents and good thermal stability. The adsorption isotherm experiments were conducted at 298 K, 308 K, and 318 K. The experimental data are well fit with Langmuir models, and the adsorbent has high monolayer adsorption capacity of 284.1 mg g−1, 313.5 mg g−1 and 352.1 mg g−1 under 298 K, 308 K and 318 K, respectively. In addition, thermodynamic parameters, including free energy change (ΔG0 < 0), enthalpy change (ΔH0 > 0), and entropy change, (ΔS0 > 0) indicate that adsorption is a spontaneous, endothermic and feasible process. The pseudo-second-order kinetic model agrees well with the dynamic behavior for the adsorption of MO at three different temperatures, and the intra-particle diffusion kinetic study reveals that the adsorption process is controlled by external mass transfer together with intra-particle diffusion. Moreover, NMCS show well regeneration properties and long life, the adsorption capacity is 144.9 mg g−1 after five cycles, and it may be used in re-generable adsorptive removal of anionic dye materials. MO adsorption on NMCS should be mainly controlled by electrostatic attraction.
References
- X. Peng, D. Huang, T. Odoom-Wubah, D. Fu, J. Huang and Q. Qin, Adsorption of anionic and cationic dyes on ferromagnetic ordered mesoporous carbon from aqueous solution: equilibrium, thermodynamic and kinetics, J. Colloid Interface Sci., 2014, 430, 272–282 CrossRef CAS PubMed.
- H. Chen, J. Zhao, J. Wu and G. Dai, Isotherm, thermodynamic, kinetics and adsorption mechanism studies of methyl orange by surfactant modified silkworm exuviae, J. Hazard. Mater., 2011, 192, 246–254 CAS.
- C. Kannan, K. Muthuraja and M. R. Devi, Hazardous dyes removal from aqueous solution over mesoporous aluminophosphate with textural porosity by adsorption, J. Hazard. Mater., 2013, 244–255, 10–20 CrossRef PubMed.
- S. Wang, D. Li, C. Sun, S. Yang, Y. Guan and H. He, Synthesis and characterization of g-C3N4/Ag3VO4 composites with significantly enhanced visible-light photocatalytic activity for triphenylmethane dye degradation, Appl. Catal., B, 2014, 144, 885–892 CrossRef CAS.
- M. A. Adebayo, L. D. T. Prola, E. C. Lima, M. J. Puchana-Rosero, R. Cataluña, C. Saucier, C. S. Umpierres, J. C. P. Vaghetti, L. G. da Silva and R. Ruggiero, Adsorption of Procion Blue MX-R dye from aqueous solutions by lignin chemically modified with aluminium and manganese, J. Hazard. Mater., 2014, 268, 43–50 CrossRef CAS PubMed.
- E. Forgacs, T. Cserhati and G. Oros, Removal of synthetic dyes from wastewaters, Environ. Int., 2004, 30, 953–971 CrossRef CAS PubMed.
- V. K. Gupta, Suhas, Application of low-cost adsorbents for dye removal, J. Environ. Manage., 2009, 90, 2313–2342 CrossRef CAS PubMed.
- H. S. Rai, M. S. Bhattacharyya, J. Singh, T. K. Bansal, P. Vats and U. C. Banerjee, Removal of dyes from the effluent of textile and dyestuff manufacturing industry: a review of emerging techniques with reference to biological treatment, Crit. Rev. Environ. Sci. Technol., 2005, 35, 219–238 CrossRef CAS.
- V. K. Gupta, S. Agarwal and T. A. Saleh, Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal, J. Hazard. Mater., 2011, 185, 17–23 CrossRef CAS PubMed.
- A. K. Jain, V. K. Gupta and A. Bhatnagar, A comparative study of adsorbents prepared form industrial waste for removal of dyes, Sep. Sci. Technol., 2003, 38, 463–481 CrossRef CAS.
- V. K. Gupta, A. Nayak and S. Agarwal, Bioadsorbents for remediation of heavy metals: current status and their future prospects, Environ. Eng. Res., 2015, 20, 1–18 CrossRef.
- V. K. Gupta and T. A. Saleh, Sorption of pollutants by porous carbon, carbon nanotubes and fullerene-an overview, Environ. Sci. Pollut. Res., 2013, 20, 2828–2843 CrossRef CAS PubMed.
- V. K. Gupta, A. Mittal, D. Jhare and J. Mittal, Batch and bulk removal of hazardous colouring agent rose bengal by adsorption techniques using bottom ash as adsorbent, RSC Adv., 2012, 2, 8381–8389 RSC.
- H. Khani, M. K. Rofouei, P. Arab and V. K. Gupta, Multi-walled carbon nanotubes-ionic liquid-carbon paste electrode as a super selectivity sensor: application to potentiometric monitoring of mercury ion(II), J. Hazard. Mater., 2010, 183, 402–409 CrossRef CAS PubMed.
- M. Devaraj, R. Aaravanan, R. K. Deivasigamani, V. K. Gupta, F. Gracia and S. Jayadevan, Fabrication of novel shape Cu and Cu/Cu2O nano-particles modified electrode for the determination of dopamine and paracetamol, J. Mol. Liq., 2016, 221, 930–941 CrossRef CAS.
- R. Saravanan, F. Gracia, M. M. Khan, V. Poomima, V. K. Gupta, V. Narayanan and A. Stephen, ZnO/CdO nano-particles for textile effluent degradation and electro-chemical detection, J. Mol. Liq., 2015, 209, 374–380 CrossRef CAS.
- R. Saravanan, T. Prakash, V. K. Gupta and A. Stephen, Tailoring the electrical and dielectric of ZnO nano-rods by substitution, J. Mol. Liq., 2014, 193, 160–165 CrossRef CAS.
- A. Mittal, J. Mittal, A. Malviya and V. K. Gupta, Adsorption removal of hazardous anionic dye Congo red from wastewater using waste materials and recovery by desorption, J. Colloid Interface Sci., 2009, 340, 16–26 CrossRef CAS PubMed.
- V. K. Gupta, I. Ali, T. A. Saleh, A. Nayak and S. Agarwal, Chemical treatment technologies for waste-water recycling: an overview, RSC Adv., 2012, 2, 6380–6388 RSC.
- S. Karthikeyan, V. K. Gupta, R. Boopathy, A. Titus and G. Sekaran, A new approach for the degradation of high concentration of aromatic amine by heterocatalytic Fenton oxidation: kinetic and spectroscopic studies, J. Mol. Liq., 2012, 173, 153–163 CrossRef CAS.
- R. Saravanan, V. K. Gupta, T. Prakash, V. Narayanan and A. Stephen, Synthesis and characterization and photocatalytic activity of novel Hg doped ZnO nanorods prepared by thermal decomposition method, J. Mol. Liq., 2013, 178, 88–93 CrossRef CAS.
- V. K. Gupta, R. Jain, A. Mittal, T. A. Saleh, A. Nayak, S. Agarwal and S. Sikarwar, Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions, Mater. Sci. Eng., C, 2012, 32, 12–17 CrossRef CAS PubMed.
- V. K. Gupta, R. Jain, A. Nayak, S. Agarwal and M. Shrivastava, Removal of the hazardous dye-tartrazine by photodegradation on titanium dioxide surface, Mater. Sci. Eng., C, 2011, 31, 1062–1067 CrossRef CAS.
- T. A. Saleh and V. K. Gupta, Photo-catalyzed degradation of hazardous dye methyl orange by use of a composite catalyst consisting of multi-walled carbon nanotubes and titanium dioxide, J. Colloid Interface Sci., 2012, 371, 101–106 CrossRef CAS PubMed.
- R. Saravanan, E. Sacari, F. Gracia, M. M. Khan, E. Mosquera and V. K. Gupta, Conducting PANI stimulated ZnO system for visible light photocatalytic degradation of coloured dyes, J. Mol. Liq., 2016, 221, 1029–1033 CrossRef CAS.
- R. Saravanan, V. K. Gupta, E. Mosquera, F. Gracia, V. narayanan and A. Stephen, Visible light induced degradation of methyle orange using β-Ag0.333V2O5 nanorod catalysts by facile thermal decomposition method, J. Saudi Chem. Soc., 2015, 19, 521–527 CrossRef.
- R. Saravanan, M. M. Khan, V. K. Gupta, E. Mosquera, F. Gracia, V. Narayanan and A. Stephen, ZnO/Ag/Mn2O3 nanocomposite for visible light-induced industrial textile effluent degradation, uric acid and a scorbic acid sensing and antimicrobial activity, RSC Adv., 2015, 5, 34645–34651 RSC.
- R. Saravanan, M. M. Khan, V. K. Gupta, E. Mosquera, F. Gracia, V. Narayanan and A. Stephen, ZnO/Ag/Mn2O3 nanocomposite for visible light-induced photocatalytic degradation of industrial textile effluents, J. Colloid Interface Sci., 2015, 452, 126–133 CrossRef CAS PubMed.
- R. Saravanan, V. K. Gupta, E. Mosquera and F. Gracia, Preparation and characterization of V2O5/ZnO nanocomposite system for photocatalytic application, J. Mol. Liq., 2014, 198, 409–412 CrossRef CAS.
- R. Saravanan, N. Karthikeyan, V. K. Gupta, E. Thirumal, P. Thangadurai, V. Narayanan and A. Stephen, ZnO/Ag nanocomposite: an efficient catalyst for degradation studies of textile effluents under visible light, Mater. Sci. Eng., C, 2013, 33, 2235–2244 CrossRef CAS PubMed.
- R. Saravanan, V. K. Gupta, V. Narayanan and A. Stephen, Comparative study on photocatalytic activity of ZnO prepared by different methods, J. Mol. Liq., 2013, 181, 133–141 CrossRef CAS.
- R. Saravanan, S. Karthikeyan, V. K. Gupta, G. Sekaran, V. Narayanan and A. Stephen, Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination, Mater. Sci. Eng., C, 2013, 33, 91–98 CrossRef CAS PubMed.
- R. Saravanan, E. Thirumal, V. K. Gupta, V. Narayanan and A. Stiphen, The photocatalytic activity of ZnO prepared by simple thermal decomposition method at various temperatures, J. Mol. Liq., 2013, 177, 394–401 CrossRef CAS.
- T. A. Saleh and V. K. Gupta, Processing methods and characteristics of porous carbons derived form waste tires: a review, Adv. Colloid Interface Sci., 2014, 211, 92–100 CrossRef PubMed.
- V. Kumar, R. Kumar, A. Nayak, T. A. Saleh and M. A. Barakat, Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: a review, Adv. Colloid Interface Sci., 2013, 193–194, 24–34 Search PubMed.
- S. Rajendran, M. M. Khan, F. Gracia, J. Qin, V. K. Gupta and A. Stephen, Ce3+-ion-induced visible-light photocatalytic degradation and electrochemical activity of ZnO/CeO2 nanocomposite, Sci. Rep., 2016, 6, 31641 CrossRef CAS PubMed.
- R. Saravanan, V. K. Gupta, V. Narayanan and A. Stephen, Visible light degradation of textile effluent using novel catalyst ZnO/γ-Mn2O3, J. Taiwan Inst. Chem. Eng., 2014, 45, 1910–1917 CrossRef CAS.
- R. Saravanan, S. Joicy, V. K. Gupta, V. Narayanan and A. Stephen, Visible light induced degradation of methylene blue using CeO2/V2O5 and CeO2/CuO catalysts, Mater. Sci. Eng., C, 2013, 33, 4725–4731 CrossRef CAS PubMed.
- T. A. Saleh and V. K. Gupta, Functionalization of tungsten oxide into MWCNT and its application for sunlight-induced degradation of rhodamine B, J. Colloid Interface Sci., 2011, 362, 337–344 CrossRef CAS PubMed.
- V. K. Gupta, S. K. Srivastava, D. Mohan and S. Sharma, Design parameters for fixed bed reactors of activated carbon developed from fertilizer waste for the removal of some heavy metal ions, Waste Manag., 1998, 19, 517–522 CrossRef.
- A. Mittal, J. Mittal, A. Malviya, D. Kaur and V. K. Gupta, Decoloration treatment of a hazardous triarymethane dye, light green SF (Yellowish) by waste material adsorbents, J. Colloid Interface Sci., 2010, 342, 518–527 CrossRef CAS PubMed.
- A. Mittal, D. Kaur, A. Malviya, J. Mittal and V. K. Gupta, Adsorption studies on the removal of coloring agent phenol red from wastewater using waste materials as adsorbents, J. Colloid Interface Sci., 2009, 337, 345–354 CrossRef CAS PubMed.
- A. Mittal, J. Mittal, A. Malviya and V. K. Gupta, Removal and recovery of chrysoidine Y from aqueous solutions by waste materials, J. Colloid Interface Sci., 2010, 344, 497–507 CrossRef CAS PubMed.
- V. K. Gupta and A. Nayak, Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles, Chem. Eng. J., 2012, 180, 81–90 CrossRef CAS.
- T. Yao, S. Guo, C. F. Zeng, C. Q. Wang and L. X. Zhang, Investigation on efficient adsorption of cationic dyes on porous magnetic polyacrylamide microspheres, J. Hazard. Mater., 2015, 292, 90–97 CrossRef CAS PubMed.
- C. Galindo, P. Jacques and A. Kalt, Photodegradation of the aminoazobenzene acid orange 52 by three advanced oxidation processes: UV/H2O2, UV/TiO2 and VIS/TiO2: comparative mechanistic and kinetic investigations, J. Photochem. Photobiol., A, 2000, 130, 35 CrossRef CAS.
- T. Robinson, G. McMullan, R. Marchant and P. Nigam, Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative, Bioresour. Technol., 2001, 77, 247–255 CrossRef CAS PubMed.
- Y. Fu and T. Viraraghavan, Fungal decolorization of dye wastewaters: a review, Bioresour. Technol., 2001, 79, 251–262 CrossRef CAS PubMed.
- A. Dabrowski, Adsorption-from theory to practice, Adv. Colloid Interface Sci., 2001, 93, 135–224 CrossRef CAS PubMed.
- H. Gao, T. Kan, S. Zhao, Y. Qian, X. Cheng, W. Wu, X. Wang and L. Zheng, Removal of anionic azo dyes from aqueous solution by functional ionic liquid cross-linked polymer, J. Hazard. Mater., 2013, 261, 83–90 CrossRef CAS PubMed.
- Y. Yu, N. B. Murthy, J. G. Shapter, K. T. Constantopoulos, N. H. Voelcker and A. V. Ellis, Benzene carboxylic acid derivatized graphene oxide nanosheets on natural zeolites as effective adsorbents for cationic dye removal, J. Hazard. Mater., 2013, 260, 330–338 CrossRef CAS PubMed.
- X. Han, W. Wang and X. Ma, Adsorption characteristics of methylene blue onto low cost biomass material lotus leaf, Chem. Eng. J., 2011, 171, 1–8 CrossRef CAS.
- E. Haque, J. W. Jun and S. H. Jhung, Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal–organic framework material, iron terephthalate (MOF-235), J. Hazard. Mater., 2011, 185, 507–511 CrossRef CAS PubMed.
- E. Haque, J. E. Lee, I. T. Jang, Y. K. Hwang, J. S. Chang, J. Jegal and S. H. Jhung, Adsorptive removal of methyl orange from aqueous solution with metal–organic frameworks, porous chromium–benzenedicarboxylates, J. Hazard. Mater., 2010, 181, 535–542 CrossRef CAS PubMed.
- A. Özcan, E. M. Öncü and S. Özcan, Kinetics, isotherm and thermodynamic studies of adsorption of Acid Blue 193 from aqueous solutions onto natural sepiolite, Colloids Surf., A, 2006, 277, 90–97 CrossRef.
- J. Ma, F. Yu, L. Zhou, L. Jin, M. Yang, J. Luan, Y. Tang, H. Fan, Z. Yuan and J. Chen, Enhanced adsorptive removal of methyl orange and methylene blue from aqueous solution by alkali-activated multiwalled carbon nanotubes, ACS Appl. Mater. Interfaces, 2012, 4, 5749–5760 CAS.
- M. Teng, J. Qiao, F. Li and P. K. Bera, Electrospun mesoporous carbon nanofibers produced from phenolic resin and their use in the adsorption of large dye molecules, Carbon, 2012, 50, 2877–2886 CrossRef CAS.
- X. Yuan, S. P. Zhou, W. Xing, H. D. Cui, X. D. Dai, X. M. Liu and Z. F. Yan, Aqueous dye adsorption on ordered mesoporous carbons, J. Colloid Interface Sci., 2007, 310, 83–89 CrossRef CAS PubMed.
- J. Goscianska, M. Marciniak and R. Pietrzak, Mesoporous carbons modified with lanthanum(III) chloride for methyl orange adsorption, Chem. Eng. J., 2014, 247, 258–264 CrossRef CAS.
- N. Mohammadi, H. Khani, V. K. Gupta, E. Amereh and S. Agarwal, Adsorption process of methyl orange dye onto mesoporous carbon material-kinetic and thermodynamic studies, J. Colloid Interface Sci., 2011, 362, 457–462 CrossRef CAS PubMed.
- J. Galan, A. Rodríguez, J. M. Gómez, S. J. Allen and G. M. Walker, Reactive dye adsorption onto a novel mesoporous carbon, Chem. Eng. J., 2013, 219, 62–68 CrossRef CAS.
- P. Ma, N. A. Siddiqui, G. Marom and J. Kim, Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: a review, Composites, Part A, 2010, 41, 1345–1367 CrossRef.
- Y. D. Xia and R. Mokaya, Ordered mesoporous carbon monoliths: CVD nanocasting and hydrogen storage properties, J. Phys. Chem. C, 2007, 111, 10035–10039 CAS.
- Y. Huang, F. Yang, Z. Xu and J. Shen, Nitrogen-containing mesoporous carbons prepared from melamine formaldehyde resins with CaCl2 as a template, J. Colloid Interface Sci., 2011, 363, 193–198 CrossRef CAS PubMed.
- J. Wei, D. Zhou, Z. Sun, Y. Deng, Y. Xia and D. Zhao, A controllable synthesis of rich nitrogen-doped ordered mesoporous carbon for CO2 Capture and supercapacitors, Adv. Funct. Mater., 2013, 23, 2322–2328 CrossRef CAS.
- J. Yu, M. Guo, F. Muhammad, A. Wang, G. Yu, H. Ma and G. Zhu, Simple fabrication of an ordered nitrogen-doped mesoporous carbon with resorcinol–melamine–formaldehyde resin, Microporous Mesoporous Mater., 2014, 190, 117–127 CrossRef CAS.
- Á. Sánchez-Sánchez, F. Suárez-García, A. Martínez-Alonso and J. M. D. Tascón, Synthesis, characterization and dye removal capacities of N-doped mesoporous carbons, J. Colloid Interface Sci., 2015, 450, 91–100 CrossRef.
- K. Yano and Y. Fukushima, Synthesis of mono-dispersed mesoporous silica spheres with highly ordered hexagonal regularity using conventional alkyltrimethylammonium halide as a surfactant, J. Mater. Chem., 2004, 14, 1579–1584 RSC.
- R. Wang, P. Wang, X. Yan, J. Lang, C. Peng and Q. Xue, Promising porous carbon derived from celtuce leaves with outstanding supercapacitance and CO2 capture performance, ACS Appl. Mater. Interfaces, 2012, 4, 5800–5806 CAS.
- Z. Teng, Y. Han, J. Li, F. Yan and W. Yang, Preparation of hollow mesoporous silica spheres by a sol–gel/emulsion approach, Microporous Mesoporous Mater., 2010, 127, 67–72 CrossRef CAS.
- J. Cai, J. Qi, C. Yang and X. Zhao, Poly(vinylidene chloride)-Based Carbon with Ultrahigh Microporosity and Outstanding Performance for CH4 and H2 Storage and CO2 Capture, ACS Appl. Mater. Interfaces, 2014, 6, 3703–3711 CAS.
- S. Zhang, A. K. Ueno, Z. Chen, K. Dokko and M. Watanabe, Protic-Salt-Derived Nitrogen/Sulfur-Codoped Mesoporous Carbon for the Oxygen Reduction Reaction and Supercapacitors, ChemSusChem, 2015, 8, 1608–1617 CrossRef CAS PubMed.
- Y. Bulut, N. Gözübenli and H. Aydın, Equilibrium and kinetics studies for adsorption of direct blue 71 from aqueous solution by wheat shells, J. Hazard. Mater., 2007, 144, 300–306 CrossRef CAS PubMed.
- A. A. Jalil, S. Triwahyono, S. H. Adam, N. D. Rahim, M. A. A. Aziz, N. H. H. Hairom, N. A. M. Razali, M. A. Z. Abidin and M. K. A. Mohamadiah, Adsorption of methyl orange from aqueous solution onto calcined Lapindo volcanic mud, J. Hazard. Mater., 2010, 181, 755–762 CrossRef CAS PubMed.
- O. G. Apul, T. Shao, S. Zhang and T. Karanfil, Impact of carbon nanotube morphology on phenanthrene adsorption, Environ. Toxicol. Chem., 2012, 31, 73–78 CrossRef CAS PubMed.
- H. Y. Zhu, R. Jiang, L. Xiao and G. M. Zeng, Preparation, characterization, adsorption kinetics and thermodynamics of novel magnetic chitosan enwrapping nanosized γ-Fe2O3 and multi-walled carbon nanotubes with enhanced adsorption properties for methyl orange, Bioresour. Technol., 2010, 101, 5063–5069 CrossRef CAS PubMed.
- P. R. Chang, P. Zheng, B. Liu, D. P. Anderson, J. Yu and X. Ma, Characterization of magnetic soluble starch-functionalized carbon nanotubes and its application for the adsorption of the dyes, J. Hazard. Mater., 2011, 186, 2144–2150 CrossRef CAS PubMed.
- M. H. Do, N. H. Phan, T. D. Nguyen, T. T. S. Pham, V. K. Nguyen, T. T. T. Vu and T. K. P. Nguyen, Activated carbon/Fe3O4 nanoparticle composite: fabrication, methyl orange removal and regeneration by hydrogen peroxide, Chemosphere, 2011, 85, 1269–1276 CrossRef CAS PubMed.
- W. Cheah, S. Hosseini, M. A. Khan, T. G. Chuah and T. S. Y. Choong, Acid modified carbon coated monolith for methyl orange adsorption, Chem. Eng. J., 2013, 215–216, 747–754 CrossRef CAS.
- G. L. Perlovich, S. V. Kurkov and A. Bauer-Brandl, Thermodynamics of solutions II flurbiprofen and diflunisal as models for studying solvation of drug substances, Eur. J. Pharm. Sci., 2003, 19, 423–432 CrossRef CAS PubMed.
- Q. Zhang, Y. Yang, C. Cao, L. Cheng, Y. Shi, W. Yang and Y. Hu, Thermodynamic models for determination of the solubility of dibenzothiophene in (methanol + acetonitrile) binary solvent mixtures, J. Chem. Thermodyn., 2015, 80, 7–12 CrossRef CAS.
- A. Khaled, A. E. Nemr, A. El-Sikaily and O. Abdelwahab, Removal of direct N Blue-106 from artificial textile dye effluent using activated carbon from orange peel: adsorption isotherm and kinetic studies, J. Hazard. Mater., 2009, 165, 100–110 CrossRef CAS PubMed.
- M. A. Al-Ghouti, M. A. M. Khraisheh, M. N. M. Ahmad and S. Allen, Adsorption behaviour of methylene blue onto Jordanian diatomite: a kinetic study, J. Hazard. Mater., 2009, 165, 589–598 CrossRef CAS PubMed.
- M. Ghaedi, A. Hassanzadeh and S. N. Kokhdan, Multi-walled carbon nanotubes as a adsorbents for the kinetic and equilibrium study of the removal of alizarin Red S and Morin, J. Chem. Eng. Data, 2011, 56, 2511–2520 CrossRef CAS.
- S. Rengaraj, Y. Kim, C. K. Joo and J. Yi, Removal of copper from aqueous solution by aminated and protonated mesoporous aluminas: kinetics and equilibrium, J. Colloid Interface Sci., 2004, 273, 14–21 CrossRef CAS PubMed.
- M. Dogan, H. Abak and M. Alkan, Adsorption of methylene blue onto hazelnut shell: kinetics, mechanism and activation parameters, J. Hazard. Mater., 2009, 164, 172–181 CrossRef CAS PubMed.
- Y. Yao, B. He, F. Xu and X. Chen, Equilibrium and kinetic studies of methyl orange adsorption on multi-walled carbon nanotubes, Chem. Eng. J., 2011, 170, 82–89 CrossRef CAS.
- V. K. Gupta, B. Gupta, A. Rastogi, S. Agarwal and A. Nayak, A comparative investigation on adsorption performances of mesoporous activated carbon prepared from waste rubber tire and activated carbon for a hazardous azo dye-Acid Blue 113, J. Hazard. Mater., 2011, 186, 891–901 CrossRef CAS PubMed.
- F. M. Machado, C. P. Bergmann, T. H. M. Fernandes, E. C. Lima, B. Royer, T. Calvete and S. B. Fagan, Adsorption of reactive Red M-2BE dye from water solutions by multi-walled carbon nanotubes and activated carbon, J. Hazard. Mater., 2011, 192, 1122–1131 CrossRef CAS PubMed.
- N. F. Cardoso, R. B. Pinto, E. C. Lima, T. Calvete, C. V. Amavisca, B. Royer, M. L. Cunha, T. H. M. Fernandes and I. S. Pinto, Removal of remazol black B textile dye from aqueous solution by adsorption, Desalination, 2011, 269, 92–103 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) are usually introduced into mesoporous carbons by chemical post-modification processes. However, post-modification processes are uncontrollable in terms of both the amount and distribution of dopants. In addition, the preparation steps require extra chemical reagents such as sulfuric acid, nitric acid, aniline, which increases operation difficulty and adsorption costs.
O) are usually introduced into mesoporous carbons by chemical post-modification processes. However, post-modification processes are uncontrollable in terms of both the amount and distribution of dopants. In addition, the preparation steps require extra chemical reagents such as sulfuric acid, nitric acid, aniline, which increases operation difficulty and adsorption costs.





![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KL
KL
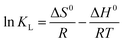
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KL against 1/T, respectively. The calculated values of ΔG0, ΔH0, and ΔS0 are listed in Table 4. From Table 4, the negative free energy change (ΔG0) suggests that the adsorption NMCS is spontaneous, and the decrease in negative values suggests an increase in the feasibility and spontaneity of the MO adsorption with increasing temperature. The positive enthalpy change indicates that the adsorption reaction is endothermic, which is in accord with the increasing adsorption capacity with increasing adsorption temperature. The positive values of ΔS0 suggest that the randomness at the solid-solution interface increases during adsorption, which may be ascribed that the number of desorbed water molecule is far larger than that of the adsorbed MO molecule.54
KL against 1/T, respectively. The calculated values of ΔG0, ΔH0, and ΔS0 are listed in Table 4. From Table 4, the negative free energy change (ΔG0) suggests that the adsorption NMCS is spontaneous, and the decrease in negative values suggests an increase in the feasibility and spontaneity of the MO adsorption with increasing temperature. The positive enthalpy change indicates that the adsorption reaction is endothermic, which is in accord with the increasing adsorption capacity with increasing adsorption temperature. The positive values of ΔS0 suggest that the randomness at the solid-solution interface increases during adsorption, which may be ascribed that the number of desorbed water molecule is far larger than that of the adsorbed MO molecule.54

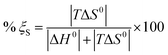
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t
qe − k1t
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 274.97
274.97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 464.98
464.98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 098.51
098.51
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 274) are much higher than those of pseudo-first-order model (146 to 995). Moreover, the calculated values qe (cal) obtained from the pseudo-second-order model agree well with experimental values qe (exp) at initial concentrations 40 mg L−1 and 120 mg L−1. It can be concluded that the pseudo-second-order model is more suitable to describe the kinetics of MO adsorption on NMCS than the pseudo-first-order model. In addition, in Table 5, the initial adsorption rates (h0) of the pseudo-second-order model increase with the initial concentration increase. This may be attributed to the driving force of diffusion between the liquid and solid phase.82,83 Moreover, the initial adsorption rate (h0) and k2 increase with the increasing temperature, indicating that the adsorption reaction would be faster to achieve adsorption equilibrium with the increasing temperature, which is well consistent with results of thermodynamic study. From Fig. 8, the adsorption plots shows a rapid rate at the initial period, and the adsorption rate decreases with the increased contact time until adsorption equilibrium. This phenomenon can be explained by two stages.84 The first stage, film diffusion, is that dye molecules will overcome the boundary layer effect and across the film to the exterior surface of adsorbent, and this process is relatively quick due to the lager available surface area of adsorbent.56 Then, the adsorption on exterior surface get saturation, the adsorbate will diffuse into the internal pore and adhere to the interior surface of the particles, which is called inner diffusion. This stage will spend a long time because the intraparticle diffusion rate will decrease due to the increased diffusion resistance.85
274) are much higher than those of pseudo-first-order model (146 to 995). Moreover, the calculated values qe (cal) obtained from the pseudo-second-order model agree well with experimental values qe (exp) at initial concentrations 40 mg L−1 and 120 mg L−1. It can be concluded that the pseudo-second-order model is more suitable to describe the kinetics of MO adsorption on NMCS than the pseudo-first-order model. In addition, in Table 5, the initial adsorption rates (h0) of the pseudo-second-order model increase with the initial concentration increase. This may be attributed to the driving force of diffusion between the liquid and solid phase.82,83 Moreover, the initial adsorption rate (h0) and k2 increase with the increasing temperature, indicating that the adsorption reaction would be faster to achieve adsorption equilibrium with the increasing temperature, which is well consistent with results of thermodynamic study. From Fig. 8, the adsorption plots shows a rapid rate at the initial period, and the adsorption rate decreases with the increased contact time until adsorption equilibrium. This phenomenon can be explained by two stages.84 The first stage, film diffusion, is that dye molecules will overcome the boundary layer effect and across the film to the exterior surface of adsorbent, and this process is relatively quick due to the lager available surface area of adsorbent.56 Then, the adsorption on exterior surface get saturation, the adsorbate will diffuse into the internal pore and adhere to the interior surface of the particles, which is called inner diffusion. This stage will spend a long time because the intraparticle diffusion rate will decrease due to the increased diffusion resistance.85

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1035 cm−1), –CH3 (1190 cm−1), C–N (1366 cm−1), and N
O (1035 cm−1), –CH3 (1190 cm−1), C–N (1366 cm−1), and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (1519 cm−1). Compared with NMCS and MO/NMCS, peaks at 2925 cm−1 and 2857 cm−1, ascribed to asymmetric and symmetric stretching of –CH2 groups, keep the same intensity and wavenumbers after adsorption, which suggest that these functional groups do not participate in the sorption process.89 After the adsorption process, the intense absorption band at 3437 cm−1, ascribed to N–H bond stretching, shows a significant increase in intensity and presents a bathochromic-shift to 3432 cm−1, indicating that the NMCS donate electrons to MO. The exhibition of band at 1629 cm−1 is the characteristic stretching vibration of C
N (1519 cm−1). Compared with NMCS and MO/NMCS, peaks at 2925 cm−1 and 2857 cm−1, ascribed to asymmetric and symmetric stretching of –CH2 groups, keep the same intensity and wavenumbers after adsorption, which suggest that these functional groups do not participate in the sorption process.89 After the adsorption process, the intense absorption band at 3437 cm−1, ascribed to N–H bond stretching, shows a significant increase in intensity and presents a bathochromic-shift to 3432 cm−1, indicating that the NMCS donate electrons to MO. The exhibition of band at 1629 cm−1 is the characteristic stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O from the group of O
O from the group of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH2, and the intensity of peak increased after adsorption of MO, it may be due to the functional groups attached to the aromatic rings of MO molecules, which suggest the MO dye adhere to the surface of NMCS. The peak at 1037 cm−1, associated with the stretching vibration of C–O,90 presents a shift to 1175 cm−1, and the broad bond shows an evident increase in intensity. This indicates that electrostatic attraction between NMCS and MO occurs in the adsorption process.
C–NH2, and the intensity of peak increased after adsorption of MO, it may be due to the functional groups attached to the aromatic rings of MO molecules, which suggest the MO dye adhere to the surface of NMCS. The peak at 1037 cm−1, associated with the stretching vibration of C–O,90 presents a shift to 1175 cm−1, and the broad bond shows an evident increase in intensity. This indicates that electrostatic attraction between NMCS and MO occurs in the adsorption process.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in O1s spectra, respectively. 400 eV, 284.6 eV, 285.5 eV, 286.6 eV was separately assigned to N–H, C–C/C–H, C–OH, C–O–C. It is suggested that the result of XPS was consistent with infrared spectroscopy, and the functional groups such as amino, carboxyl and phenol hydroxyl exist in the material surface.
C in O1s spectra, respectively. 400 eV, 284.6 eV, 285.5 eV, 286.6 eV was separately assigned to N–H, C–C/C–H, C–OH, C–O–C. It is suggested that the result of XPS was consistent with infrared spectroscopy, and the functional groups such as amino, carboxyl and phenol hydroxyl exist in the material surface.











