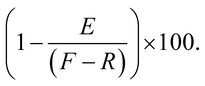Synthesis and characterization of lipophilic cationic Ga(III) complexes based on the H2CHXdedpa and H2dedpa ligands and their 67/68Ga radiolabeling studies†
C. F. Ramogidaab,
D. Schindlera,
C. Schneidera,
Y. L. K. Tana,
S. Huha,
C. L. Ferreirac,
M. J. Adamb and
C. Orvig *a
*a
aMedicinal Inorganic Chemistry Group, Department of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, British Columbia, Canada V6T 1Z1. E-mail: orvig@chem.ubc.ca
bLife Sciences Division, TRIUMF, 4004 Wesbrook Mall, Vancouver, British Columbia, Canada V6T 2A3
cNordion, 4004 Wesbrook Mall, Vancouver, British Columbia, Canada V6T 2A3
First published on 19th October 2016
Abstract
68Ga is an attractive isotope for incorporation into a positron-emission tomography (PET) imaging agent, and is finding use as an alternative generator-produced isotope to 99mTc particularly in imaging of myocardial perfusion. We have synthesized six new chelating ligands based on our previously reported H2dedpa and H2CHXdedpa scaffolds (CHX = cyclohexyl, H2dedpa = 1,2-[[carboxypyridin-2-yl]methylamino]ethane). These ligands are designed to incorporate several lipophilic appendages at the secondary nitrogens, and upon coordination to 68Ga(III) will form lipophilic, cationic complexes designed to mimic the properties of other clinically relevant myocardial perfusion imaging agents. The non-radioactive Ga(III) complexes were prepared and characterized by NMR spectroscopy; each ligand retained its predicted hexadentate N4O2 binding to Ga(III). The radiolabeling properties of the six ligands were assessed using the longer-lived 68Ga surrogate, 67Ga. The absence of ‘free’ uncomplexed 67Ga in the HPLC radio-chromatograms indicated >99% radiochemical yields (10 minutes at ambient temperature, ligand concentrations of 10−4 M). However, the N,N′-benzyl functionalized derivatives displayed multiple peaks corresponding to the presence of additional 67Ga-complexes which complicated further study. Selected 67Ga-CHXdedpa complexes were tested for in vitro stability against the metal-binding protein apo-transferrin, and were found to be sufficiently stable (>80%) in a 2 h challenge assay, suggesting that alternative N,N′-alkylated derivatives which introduce more lipophilic character will be of interest in future studies.
Introduction
Myocardial perfusion imaging (MPI) is a common and powerful technique used to illustrate the function of the heart muscle. Its main application is in diagnosis of coronary artery disease (CAD) and it is of great importance for early detection of heart disease, one of the leading causes of death in the western world.1 The aim of MPI is to visualise the perfusion of the heart muscle; this blood flow analysis relies on the ensuing accumulation of a radiopharmaceutical in the heart. Areas with good blood circulation will show increased radionuclide uptake compared to areas with poor flow or damaged tissue, thus defining the extent and severity of disease.1–4MPI is dominated by the use of 99mTc labelled agents for single-photon emission computed tomography (SPECT) imaging. The FDA approved radiopharmaceuticals 99mTc-sestamibi (Cardiolite), 99mTc-tetrofosmin (Myoview), and 99mTc-teboroxime (Fig. 1) are among the most popular agents used clinically. It is believed that the elevated myocardial accumulation of these 99mTc tracers is rooted in the physical properties of the complexes: these are highly lipophilic and monocationic or neutral charged complexes which contain at least two ether-like linkages.4,5 Limitations such as low first-pass extraction and high liver uptake of such agents have sustained interest in developing new radiopharmaceuticals for MPI. The ideal perfusion radiotracer should have high heart uptake and retention, with minimal liver and lung uptake to create diagnostically useful images.4
 | ||
| Fig. 1 Commercially available 99mTc SPECT agents (top) and recently investigated 68Ga PET agents (bottom) for myocardial perfusion imaging. | ||
The advantageous properties of the β+ emitter 68Ga (t1/2 = 68 min) make it an ideal candidate for incorporation into an MPI agent for positron-emission tomography (PET). A 68Ga-based perfusion imaging agent would possess the specific advantages of spatial resolution obtainable by PET compared to SPECT, whilst relying on the convenience of a generator-produced isotope.6–8 Consequently, much effort has been made towards development of new lipophilic, cationic Ga-complexes with myocardial retention and low liver uptake.9–12 Specifically, bis- and tris(salicylaldimine) chelators such as tris(4,6-dimethoxysalicylaldimin-N,N′-bis(3-aminopropyl)-N,N′-ethylenediamine) (BAPEN)9,13 and its derivatives10,11,14 (Fig. 1) showed some promising properties for myocardial perfusion imaging with 68Ga, but non-trivial labelling procedures have limited the progression of these ligands towards the clinic.
Our recent reports of the promising acyclic hexadentate 67/68Ga chelator H2dedpa which forms a monocationic complex with Ga(III) sparked interest in developing derivatives which could potentially lead to compounds of increased and prolonged heart uptake.12,15 A small library of H2dedpa analogues of varying lipophilicity which possess benzyl residues and a variety of methoxy or ethoxy substituents was evaluated in vivo.12 Despite modest biodistribution results showing initial high uptake and slow clearance from the liver, two complexes showed persistent heart uptake over the course of 2 h.12 These conclusions have motivated us to develop a second class of dedpa2− analogues which possess varying degrees of lipophilicity.
Herein, we report five new derivatives of the chiral acyclic chelator H2CHXdedpa,16 and one new derivative of H2dedpa, that have been functionalized to include varying lipophilic appendages. Much like H2dedpa, the chiral chelator H2CHXdedpa forms 68/67Ga complexes of high thermodynamic stability (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KML = 27.61(8)), shows promising kinetic inertness in vitro, and exhibits fast and mild 67/68Ga complexation kinetics (radiochemical yields >99% in 10 minutes at RT at ligand concentrations of 10−5 M), making H2CHXdedpa an ideal candidate for further elaboration into a Ga(III) radiopharmaceutical.16,17 The six novel lipophilic analogues prepared here contain either diethyl ether-like or benzyl linkages with varying number of methoxy groups added onto the benzyl or 4-position of the picolinate moieties (Fig. 2). Moreover, the cyclohexanediamine backbone of CHXdedpa2− inherently introduces a degree of added lipophilicity. The addition of ether groups was found to reduce radiotracer liver uptake of cationic 99mTc complexes resulting in improved target/background ratios.4 We hope to take advantage of this theory with some of our derivatives by incorporating such ether linkages in our ligands. Indeed, many of the structural components that have been added to this new class of compounds (1–6) have been strategically chosen to mimic other 99mTc and 68Ga MPI agents under investigation. The gallium coordination potential, labelling properties, in vitro stability, and log
KML = 27.61(8)), shows promising kinetic inertness in vitro, and exhibits fast and mild 67/68Ga complexation kinetics (radiochemical yields >99% in 10 minutes at RT at ligand concentrations of 10−5 M), making H2CHXdedpa an ideal candidate for further elaboration into a Ga(III) radiopharmaceutical.16,17 The six novel lipophilic analogues prepared here contain either diethyl ether-like or benzyl linkages with varying number of methoxy groups added onto the benzyl or 4-position of the picolinate moieties (Fig. 2). Moreover, the cyclohexanediamine backbone of CHXdedpa2− inherently introduces a degree of added lipophilicity. The addition of ether groups was found to reduce radiotracer liver uptake of cationic 99mTc complexes resulting in improved target/background ratios.4 We hope to take advantage of this theory with some of our derivatives by incorporating such ether linkages in our ligands. Indeed, many of the structural components that have been added to this new class of compounds (1–6) have been strategically chosen to mimic other 99mTc and 68Ga MPI agents under investigation. The gallium coordination potential, labelling properties, in vitro stability, and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P of the six chelating ligands will be explored to assess their potential as myocardial perfusion imaging agents.
P of the six chelating ligands will be explored to assess their potential as myocardial perfusion imaging agents.
Results and discussion
Ligand synthesis and characterization
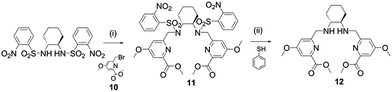
The synthesis of pro-ligands 3–6 required the use of methyl 6-(bromomethyl)-4-methoxypicolinate (10) which was prepared using a previously reported synthesis.12 Using the methoxy-modified bromopicolinate (10), the precursor 12 was prepared using nosyl protection/deprotection chemistry (Scheme 1) analogous to that used in the preparation of previous H2dedpa and H2CHXdedpa analogues.16,18 The precursor 12 was nicknamed ‘Me2CHXdedpaOMe’ because it is similar to the previously synthesized analogue Me2CHXdedpa16,19 in which there are no methoxy (OMe) groups appended to the 4-position of the picolinate moieties.A similar synthetic pathway was used to prepare five ligands (1–5) (Scheme 2). Preparation began with N,N′-alkylation of Me2dedpa,15,18 Me2CHXdedpa,16 or Me2CHXdedpaOMe (12) with a slight excess of the appropriate bromo-alkylating agent under basic condition (K2CO3) to yield the methyl ester protected precursors 13–17. For alkylation reactions with 1-bromo-2-ethoxyethane (Br-ee), 2-ethoxyethane (ee) also replaced the methyl protecting group of one or both of the carboxylic acid(s) which resulted in a mixture of products. For precursors 14 and 15, separation and isolation of the over-alkylated products via column chromatography was not possible, consequently 1H and 13C NMR spectra of pure compound were not collected. Nonetheless, hydrolysis of all ester protected ligands was accomplished in the final deprotection step using lithium hydroxide in order to yield all five ligands 1–5 as pure compounds after RP-HPLC purification.
The commercial availability of an aldehyde starting material (2,4,6-trimethoxybenzaldehyde) allowed ligand 6 to be prepared in three synthetic steps, and avoided the need for nosyl protection and deprotection chemistry which was utilized for the preparation of ligands 1–5 through intermediate 12. Pro-ligand 6 was synthesized in three steps (Scheme 3) through imine formation followed by reductive amination of 2,4,6-trimethoxybenzaldehyde with (1R,2R)-(−)-cyclohexanediamine to yield 18 which was subsequently used in an N,N′-alkylation reaction with methyl 6-(bromomethyl)-4-methoxypicolinate (10). Finally, the methyl-ester protected ligand 19 was deprotected using lithium hydroxide as was done for the previous ligands to give H2CHXdedpaOMe-Bn3OMe (6).
1H NMR spectra of several pro-ligands at 25 °C revealed very broad and unresolvable peaks which precluded 13C NMR data collection. It was hypothesized that intramolecular hydrogen-bonding of the acidified ligand was the cause of such peak broadening; however, NMR samples in deuterated water adjusted to pD of >7.5 using NaOD resulted in only a slight sharpening of peaks. Consequently, variable-temperature (VT) NMR was used to collect 1H NMR spectra of the ligands at temperatures 25 to 75 °C, and resulted in an apparent sharpening of peaks with increasing temperatures (Fig. 3 and S1†); 13C NMR spectra were also obtained at the highest temperature.
Ga(III) complexation
After successful characterization of all six pro-ligands, preparation of metal complexes was attempted with non-radioactive Ga(III). The dedpa2− and CHXdedpa2− based ligands 1–6 reported herein were all able to complex Ga(III). In most cases, upon addition of appropriate equivalency of Ga(ClO4)3 salt solution to a solution of pro-ligand, precipitate formation was observed; the precipitate was isolated and its identity as the Ga-complex was confirmed by HR ESI-MS, and NMR spectroscopy. Stacked 1H NMR spectra of two ligands and their corresponding Ga-complexes are represented in Fig. 4 and S2.†The diagnostic diastereotopic splitting of hydrogens upon metal-complexation is seen in the NMR spectra of the dedpa2− based ligand 1 (Fig. 4). The two singlets (labelled A and B), assigned to the methylene hydrogens alpha to the pyridine ring and the hydrogens on the ethylenediamine bridge, respectively, of the ligand 1, become diastereotopic upon gallium complexation and each singlet splits into two doublets (labelled α,α′ and β,β′).
Diastereotopic splitting is visible in pro-ligands 2–6 due to the inherently chiral nature of the CHXdedpa2− ligands. Nonetheless, shifts in the 1H resonances of the solution NMR data (Fig. S2†) can be used to confirm successful Ga(III) complexation.
67/68Ga radiochemistry and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) P determination
P determination
With a half-life of 3.26 days, 67Ga is used as a longer-lived surrogate for 68Ga complexes, and can be used in studies requiring longer time points by monitoring its γ-emission. 68Ga was also used in the radiolabeling studies herein, when 67Ga was not available. In all cases, the concentration of chelator used for radiolabeling is in great excess relative to the radioisotope. Initial radiolabeling reactions were performed with ligand concentrations of 10−4 M and 1 mCi of 67Ga in sodium acetate buffer at pH 4.5, the reaction mixture was left for 10 min at room temperature prior to injection into the HPLC.
The dedpa2− and both CHXdedpa2− ligands that possess 2-ethoxyethane functionalities off the secondary amines (1, and 2–3, respectively) displayed one major sharp peak in the radio-chromatogram (99, 95, 99% RCY, respectively) assigned to the 67Ga-labelled complex. The remaining three CHXdedpaOMe2− ligands which possess benzyl functionalities attached to the secondary amines with none, one, or three methoxy groups per benzene ring (4, 5, and 6, respectively) display atypical 67Ga labelling radio-traces. Despite the lack of free radioisotope present in the HPLC radio-chromatogram (evinced by absence of a peak at tR = 2–3 min), three sharp peaks of varying intensity are present over a span of about 3 min (Table 1 and Fig. 5). It is possible that small impurities in the ligand solution are able to complex with 67Ga more efficiently that the desired ligand, resulting in multiple peaks in the radio-chromatogram. Ligand impurities, even in small concentrations relative to the pure ligand, can greatly affect labelling efficiency/yield since radiolabeling conditions dictate that the concentration of the chelator is in great excess compared to that of the radioisotope. The peak with the latest retention time in the RP-HPLC radio-trace is believed to be the desired 67Ga-complex, since the chelating ligand will likely be the most lipophilic molecule in the reaction mixture which will subsequently form the most lipophilic 67Ga-complex. Further confirmation to correctly identify the desired 67Ga-complex was performed by injecting an aliquot of the pre-formed non-radioactive Ga-complex ([Ga(6)]+) into the HPLC under the same conditions as the 67Ga-complex and revealed one major peak in the UV-Vis absorbance trace (tR = 14.38 min, Fig. S28†) which matches the latest peak in the radio-trace (tR = 14.57 min, Fig. 5). Given the shorter retention times of the two additional radio-peaks, the impurities are believed to be the mono-alkylated and non-alkylated by-products. The chelator purity was deemed sufficiently pure post-purification by RP-HPLC by re-injection of a small aliquot of chelator which resulted in one sharp peak and by NMR spectroscopy, so it seemed surprising that the 67Ga radio-traces of the above chelating ligands exhibited three peaks of similar intensity. A second hypothesis is also plausible, one in which multiple radiolabeling peaks arise from ligand radiolysis. If ligand radiolysis is indeed occurring during the course of the radiolabeling reaction, these ligands would be deemed sufficiently unstable and unfit for further testing in vitro or in vivo. The steric bulk imposed by the benzyl groups on pro-ligands 4, 5, and 6 may also contribute to the instability of the chelators, resulting in ease of radiolysis or decomposition overtime. In an effort to distinguish whether or not the extra 67Ga-labelled products arise from ligand impurities or radiolysis products, ligand 5 was radiolabelled with 1 mCi of 67Ga and analysed by radio-HPLC at 10 minutes and again at 1 hour. It was hypothesized that if the extra 67Ga-products at 10.91 and 11.76 min in the radio-trace were formed via ligand radiolysis, the areas of these peaks would increase over time while the assumed product peak at 13.97 min would decrease as the radiative emissions of 67Ga induce more damage to the radio-complex. However, the relative peak height and areas did not change overtime (% RCYs were within ±2% of each other, Fig. S29†). Furthermore, ligand 6 was radiolabelled with 0.1 mCi of 68Ga at constant ligand concentration (10−4 M) and analysed via RP-HPLC after 10 minutes at ambient temperature. Again, the ratio (via % area) of the three 68Ga-labelled peaks in the radio-trace did not change significantly (23, 60, 16% compared to 23, 54, and 21% for 67/68Ga-products from shortest to longest retention time, Fig. S30†). We predicted that if indeed the extra peaks at shorter retention were due to ligand radiolysis the relative areas of impurity products would change compared to the desired product peak between the [67Ga(6)]+ and [68Ga(6)]+ radiolabeling reaction, since radiolysis is dependent on factors such as the time, amount, and type of radiation exposure; 67Ga, a gamma and Auger electron emitter, would inflict a different destructive dose to the ligand compared to the primarily positron emitter 68Ga. The observation of little to no change in the radio-traces of both the 67Ga- and 68Ga-labelled products of ligand 6 further supports the hypothesis that ligand impurities in the benzyl-alkylated ligands 4–6 have contributed to the presence of multiple peaks in the radiolabeling reactions. The steric bulk imposed by the benzene rings' close proximity to the cyclohexane ring may be contributing to the instability of pro-ligands 4–6, causing ligand decomposition via de-alkylation of one or both of the secondary amines.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values for 67Ga-labelled dedpa2− and CHXdedpa2− chelators 1–6
P values for 67Ga-labelled dedpa2− and CHXdedpa2− chelators 1–6
| Complex | tR/min (RCY/%) | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
|---|---|---|
| a Retention time of 67Ga-labelled by-products produced from ligand impurities or ligand radiolysis.b Not determined. | ||
| [67Ga(1)]+ | 8.85 (99) | −2.67 |
| [67Ga(2)]+ | 11.50 (99) | −1.76 |
| [67Ga(3)]+ | 12.68 (93) | −1.53 |
| [67Ga(4)]+ | 11.15a (20), 11.40a (27), 13.42 (32) | NDb |
| [67Ga(5)]+ | 10.91a (23), 11.76a (53), 13.97 (24) | NDb |
| [67Ga(6)]+ | 11.76a (23), 12.34a (54), 14.57 (21) | NDb |
 | ||
| Fig. 5 HPLC radio-chromatograms of 67Ga labelling reactions with lipophilic dedpa2− or CHXdedpa2− chelators 1–6. * = desired radiolabelled product. | ||
Determination of partition coefficients (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) for myocardial perfusion imaging agents is of particular importance because it is believed that differences in heart uptake and liver clearance are caused by the compound lipophilicity which affects the ability to cross the plasma and mitochondrial membranes (the accepted mechanism of heart uptake).4 It has been hypothesized that cationic 99mTc radiotracers should exhibit log
P) for myocardial perfusion imaging agents is of particular importance because it is believed that differences in heart uptake and liver clearance are caused by the compound lipophilicity which affects the ability to cross the plasma and mitochondrial membranes (the accepted mechanism of heart uptake).4 It has been hypothesized that cationic 99mTc radiotracers should exhibit log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values ranging from 0.5–1.2 in order to achieve optimal myocardium retention and fast liver clearance concurrently;4 therefore, we sought to find a 68Ga-CHXdedpa complex which exhibited a partition coefficient within this range.
P values ranging from 0.5–1.2 in order to achieve optimal myocardium retention and fast liver clearance concurrently;4 therefore, we sought to find a 68Ga-CHXdedpa complex which exhibited a partition coefficient within this range.
Due to the presence of multiple species in the 67Ga-labeling reactions of benzyl functionalized ligands 4–6, (vide supra) log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values were not determined, since the value obtained through experimental procedures would not accurately depict the lipophilicity of the desired radiometal–ligand complex but instead would be influenced by the mixture of the relatively hydrophilic 67Ga by-products present in the reaction mixture. log
P values were not determined, since the value obtained through experimental procedures would not accurately depict the lipophilicity of the desired radiometal–ligand complex but instead would be influenced by the mixture of the relatively hydrophilic 67Ga by-products present in the reaction mixture. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P of the three 67Ga-ligands functionalized with ee groups (1–3) were determined to be −2.67, −1.76, and −1.53, respectively. The structure of ligands 1 and 2 differ only by the presence on the cyclohexane ring on 2, consequently by comparing directly the log
P of the three 67Ga-ligands functionalized with ee groups (1–3) were determined to be −2.67, −1.76, and −1.53, respectively. The structure of ligands 1 and 2 differ only by the presence on the cyclohexane ring on 2, consequently by comparing directly the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values of [67Ga(1)]+ and [67Ga(2)]+ one can discern the lipophilic character caused by the ‘CHX’ backbone. The addition of the cyclohexane ring to the ethylenediamine backbone resulted in a shift of 0.91
P values of [67Ga(1)]+ and [67Ga(2)]+ one can discern the lipophilic character caused by the ‘CHX’ backbone. The addition of the cyclohexane ring to the ethylenediamine backbone resulted in a shift of 0.91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P units between [67Ga(1)]+ and [67Ga(2)]+. Moreover, the added lipophilic character of [67Ga(3)]+ due to the addition of two extra methoxy groups off the 4-position of the picolinate moiety are represented by a shift of 0.23
P units between [67Ga(1)]+ and [67Ga(2)]+. Moreover, the added lipophilic character of [67Ga(3)]+ due to the addition of two extra methoxy groups off the 4-position of the picolinate moiety are represented by a shift of 0.23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P units compared to [67Ga(2)]+ (in which there is no additional methoxy groups). The comparatively hydrophilic nature of the three evaluated complexes renders their log
P units compared to [67Ga(2)]+ (in which there is no additional methoxy groups). The comparatively hydrophilic nature of the three evaluated complexes renders their log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values well out of the optimal range predicted by Liu.4 Nonetheless, valuable lessons on ligand design of lipophilic H2dedpa and H2CHXdedpa analogues have been drawn from the findings of the structures (1–6) studied herein.
P values well out of the optimal range predicted by Liu.4 Nonetheless, valuable lessons on ligand design of lipophilic H2dedpa and H2CHXdedpa analogues have been drawn from the findings of the structures (1–6) studied herein.
Human apo-transferrin stability studies
Selected 67Ga-complexes were investigated for their stability in a human apo-transferrin challenge. Compared to unaltered [67Ga(dedpa)]+ which remained 99% intact after 2 hours,15 the new CHXdedpa2− derivatives with N,N-functionalization displayed moderately reduced stabilities of 81, 86, and 80% for [67Ga(2)]+, [67Ga(3)]+, and [67Ga(5)]+ after 2 hours, respectively (Table 2). It should be noted that the [67Ga(5)]+ labelling reaction used for the stability assay contained a mixture of labelled products; as such the results of the transferrin challenge are representative of the bulk and not the desired single 67Ga-chelate complex. The in vitro stability of the pure 67Ga-complex ([67Ga(CHXdedpaOMe-BnOMe)]+) may differ from the stability of the other side products in the mixture, such as the mono-alkylated ligand.Experimental
Materials and methods
All solvents and reagents were purchased from commercial suppliers (Sigma Aldrich, TCI America, Fisher Scientific) and were used as received. Human apo-transferrin was purchased from Sigma Aldrich. The analytical thin-layer chromatography (TLC) plates were aluminum-backed ultrapure silica gel 60 Å, 250 μm thickness; the flash column silica gel (standard grade, 60 Å, 40–63 μm) was provided by Silicycle. 1H and 13C NMR spectra were recorded at 25 °C unless otherwise noted on Bruker AV300, AV400, or AV600 instruments; NMR spectra are expressed on the δ scale and referenced to residual solvent peaks. Low-resolution mass spectrometry was performed using a Waters ZG spectrometer with an ESCI electrospray/chemical-ionization source, and high-resolution electrospray-ionization mass spectrometry (HR-ESI-MS) was performed on a Micromass LCT time-of-flight instrument at the Department of Chemistry, University of British Columbia. 67Ga-(chelate) apo-transferrin stability experiments were analyzed using GE Healthcare Life Sciences PD-10 desalting columns (size exclusion for MW < 5000 Da) and counted with a Capintec CRC 15R well counter. The HPLC system used for analysis and purification of nonradioactive compounds consisted of a Waters 600 controller, Waters 2487 dual wavelength absorbance detector, and a Waters delta 600 pump. Phenomenex Synergi Hydro-RP 80 Å columns (250 mm × 4.6 mm analytical or 250 mm × 21.2 mm semipreparative) were used for purification of several of the deprotected ligands. Analysis of radiolabelled complexes was carried out using a Phenomenex Synergi 4 μ Hydro-RP 80A analytical column (250 × 4.60 mm 4 μm) using either a Waters Alliance HT 2795 separation module equipped with a Raytest Gabi Star NaI (Tl) detector and a Waters 996 photodiode array (PDA) or an Agilent HPLC system equipped with a model 1200 quaternary pump, a model 1200 UV absorbance detector (set at 250 nm), and a Raytest Gabi Star NaI(Tl) detector. 67GaCl3 was cyclotron-produced and provided by Nordion as ∼0.1 M HCl solution. 68Ga was obtained from an Eckert & Ziegler (Berlin, Germany) IGG100 68Ge/68Ga generator and was purified according to previously published procedures20 using a DGA resin column.Precursors 7–10 (Scheme 4) were prepared using a modified synthesis according to literature protocol of a similar analogue.21
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 90 mL) and cooled to 0 °C. Sodium borohydride (0.233 g, 6.15 mmol) was added in small portions, and the reaction mixture was stirred at 0 °C for 2.5 hours. The reaction mixture was quenched with water (50 mL), and the organics were decanted and collected. The aqueous phase was extracted with dichloromethane (8 × 50 mL), organics were collected, dried over MgSO4 and all the organics were concentrated in vacuo. The crude product was purified by column chromatography (CombiFlash Rf automated column system; 40 g HP silica; A: hexanes, B: ethyl acetate, 100% A to 100% B gradient) to yield the product as a white solid (0.871 g, 72%). 1H NMR (400 MHz, CDCl3) δ 7.40 (d, J = 2.3 Hz, 1H), 7.03 (d, J = 2.2 Hz, 1H), 4.70 (s, 2H), 3.84 (s, 3H), 3.79 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 167.2, 165.5, 163.0, 148.3, 110.4, 109.0, 64.7, 55.5, 52.8.
1, 90 mL) and cooled to 0 °C. Sodium borohydride (0.233 g, 6.15 mmol) was added in small portions, and the reaction mixture was stirred at 0 °C for 2.5 hours. The reaction mixture was quenched with water (50 mL), and the organics were decanted and collected. The aqueous phase was extracted with dichloromethane (8 × 50 mL), organics were collected, dried over MgSO4 and all the organics were concentrated in vacuo. The crude product was purified by column chromatography (CombiFlash Rf automated column system; 40 g HP silica; A: hexanes, B: ethyl acetate, 100% A to 100% B gradient) to yield the product as a white solid (0.871 g, 72%). 1H NMR (400 MHz, CDCl3) δ 7.40 (d, J = 2.3 Hz, 1H), 7.03 (d, J = 2.2 Hz, 1H), 4.70 (s, 2H), 3.84 (s, 3H), 3.79 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 167.2, 165.5, 163.0, 148.3, 110.4, 109.0, 64.7, 55.5, 52.8.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20 mL) was added. The reaction mixture was stirred overnight and allowed to warm to RT. Bis-imine formation was confirmed by ES-MS (m/z = 471.4 [M + H]+), and the solution was again cooled to 0 °C on ice. Sodium borohydride (0.17 g, approx. 3 equiv.) was added to the reaction mixture in small portions, and the mixture was stirred for 1.5 hours. The mixture was then quenched with saturated NH4Cl (20 mL), and extracted with dichloromethane (3 × 40 mL). The organics were collected, dried over MgSO4 and concentrated in vacuo to yield the product as a faint yellow solid (0.675 g, >99%) which was used in the subsequent step without further purification. 1H NMR (400 MHz, MeOD) δ 6.22 (s, 4H), 4.10 (d, J = 12.5 Hz, 2H), 3.82 (s, 6H), 3.79 (d, J = 12.5 Hz, 2H), 3.71 (s, 12H), 2.48 (s, 2H), 2.24 (d, J = 7.3 Hz, 2H), 1.81 (s, 2H), 1.30 (d, J = 6.3 Hz, 4H). 13C NMR (75 MHz, MeOD) δ 163.4, 160.7, 105.0, 91.6, 61.1, 56.2, 56.0, 39.2, 29.9, 25.5. MS (ES+) m/z = 475.4 [M + H]+.
1, 20 mL) was added. The reaction mixture was stirred overnight and allowed to warm to RT. Bis-imine formation was confirmed by ES-MS (m/z = 471.4 [M + H]+), and the solution was again cooled to 0 °C on ice. Sodium borohydride (0.17 g, approx. 3 equiv.) was added to the reaction mixture in small portions, and the mixture was stirred for 1.5 hours. The mixture was then quenched with saturated NH4Cl (20 mL), and extracted with dichloromethane (3 × 40 mL). The organics were collected, dried over MgSO4 and concentrated in vacuo to yield the product as a faint yellow solid (0.675 g, >99%) which was used in the subsequent step without further purification. 1H NMR (400 MHz, MeOD) δ 6.22 (s, 4H), 4.10 (d, J = 12.5 Hz, 2H), 3.82 (s, 6H), 3.79 (d, J = 12.5 Hz, 2H), 3.71 (s, 12H), 2.48 (s, 2H), 2.24 (d, J = 7.3 Hz, 2H), 1.81 (s, 2H), 1.30 (d, J = 6.3 Hz, 4H). 13C NMR (75 MHz, MeOD) δ 163.4, 160.7, 105.0, 91.6, 61.1, 56.2, 56.0, 39.2, 29.9, 25.5. MS (ES+) m/z = 475.4 [M + H]+.General procedure for methyl ester deprotection
To a stirred solution of methyl ester-protected ligand (13–17, 19) (approx. 60 mg) in THF/water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4 mL), LiOH (12 mg, approx. 5 equiv.) was added. The reaction mixture was stirred at ambient temperature for 1.5 h or until reaction progress was complete (confirmed by absence of starting material peak in MS, and disappearance of starting material spot on TLC (Rf (product) < Rf (starting materials)) in 10% MeOH/dichloromethane). The reaction mixture was concentrated in vacuo and used without further purification, or purified by semi-preparative RP-HPLC (A: CH3CN, B: 0.1% trifluoroacetic acid (TFA) in water, 5–100% A linear gradient, 25 min, 10 mL min−1). Product fractions were lyophilized to yield the ligand as a white solid (80–99% yield).
1, 4 mL), LiOH (12 mg, approx. 5 equiv.) was added. The reaction mixture was stirred at ambient temperature for 1.5 h or until reaction progress was complete (confirmed by absence of starting material peak in MS, and disappearance of starting material spot on TLC (Rf (product) < Rf (starting materials)) in 10% MeOH/dichloromethane). The reaction mixture was concentrated in vacuo and used without further purification, or purified by semi-preparative RP-HPLC (A: CH3CN, B: 0.1% trifluoroacetic acid (TFA) in water, 5–100% A linear gradient, 25 min, 10 mL min−1). Product fractions were lyophilized to yield the ligand as a white solid (80–99% yield).
General procedure for Ga(III) complexation of pro-ligands (1–6)
A solution of pro-ligand (1–6) (0.02 mmol) in water/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 mL) was adjusted to pH 2–3 using 0.1 M HCl (aq). A solution of Ga(ClO4)3·6H2O (0.03 mmol, 1.5 equiv.) in water (500 μL) was added to this solution, and the pH was then adjusted to 4–4.5 with 0.1 M NaOH (aq). The reaction mixture was stirred at room temperature overnight, over which time a white precipitate formed. The solid was subsequently isolated by centrifugation (4000 rpm, 10 min) and dried further under vacuum to yield the Ga-complex as a white solid (55–89% yield).
1, 2 mL) was adjusted to pH 2–3 using 0.1 M HCl (aq). A solution of Ga(ClO4)3·6H2O (0.03 mmol, 1.5 equiv.) in water (500 μL) was added to this solution, and the pH was then adjusted to 4–4.5 with 0.1 M NaOH (aq). The reaction mixture was stirred at room temperature overnight, over which time a white precipitate formed. The solid was subsequently isolated by centrifugation (4000 rpm, 10 min) and dried further under vacuum to yield the Ga-complex as a white solid (55–89% yield).
67/68Ga radiolabeling studies
The 67Ga radiolabeling protocol of all pro-ligands followed those previously described.16,17 Briefly, for initial labeling studies and human apo-transferrin challenge assays, all chelators were made up as stock solutions (1 mg mL−1, ∼10−3 M) in deionized water. A 100 μL aliquot of each chelator stock solution was transferred to screw-cap mass spectrometry vials and diluted with pH 4 NaOAc (10 mM) buffer such that the final volume was 1 mL after the addition of 67GaCl3, to a final chelator concentration of ∼10−4 M for each sample. An aliquot of 67GaCl3 (∼1 mCi for labeling studies and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P determination, and ∼3–6 mCi for apo-transferrin competitions) was added to the vials containing the chelator and allowed to radiolabel at ambient temperature for 10 min, then analyzed by RP-HPLC to confirm radiolabeling and calculate yields. Areas under the peaks observed in the HPLC radiotrace were integrated to determine radiolabeling yields. Elution conditions used for RP-HPLC analysis were gradient: A: 10 mM NaOAc buffer, pH 4.5, B: CH3CN; 0 to 100% B linear gradient 20 min.
P determination, and ∼3–6 mCi for apo-transferrin competitions) was added to the vials containing the chelator and allowed to radiolabel at ambient temperature for 10 min, then analyzed by RP-HPLC to confirm radiolabeling and calculate yields. Areas under the peaks observed in the HPLC radiotrace were integrated to determine radiolabeling yields. Elution conditions used for RP-HPLC analysis were gradient: A: 10 mM NaOAc buffer, pH 4.5, B: CH3CN; 0 to 100% B linear gradient 20 min.
For 68Ga radiolabeling studies, selected pro-ligands were prepared as above (1 mg mL−1, ∼10−3 M) in deionized water. A 100 μL aliquot of each chelator stock solution was transferred to screw-cap mass spectrometry vials and diluted with pH 5.5 NaOAc (100 mM) buffer such that the final volume was 1 mL after the addition of 68GaCl3 (50–100 μL), to a final chelator concentration of 10−4 M and reaction pH of 4.5. Reaction vials were left to sit at ambient temperature for 10 minutes, and analysed by RP-HPLC as above for 67Ga reactions. Elution conditions used for RP-HPLC analysis of 68Ga reactions were gradient: A: 0.1% TFA (trifluoroacetic acid), B: CH3CN; 0 to 100% B linear gradient 20 min.
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) P determination
P determination
The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P of 67Ga-complexes were measured experimentally following procedures outlined previously.17 Briefly, 67Ga-labelled complex (30 μCi/6 μL) was added to 1.5 mL centrifuge tubes containing phosphate buffered saline (PBS) (494 μL) and 1-octanol (500 μL), mixed vigorously for 1 minute, and subsequently centrifuged to separate phases (3000 rpm, 5 min). Equal aliquots (20–100 μL) of the organic and aqueous phases were diluted in a standard volume (20 mL) of water for measurement in an N-type Co-axial HPGe gamma spectrometer from Canberra fitted with a 0.5 mm beryllium window and calibrated (energy and efficiency) with a 20 mL 152Eu source. The samples were counted for 10 minutes, with a dead time of less than 5%. Measurements were carried out in triplicate.
P of 67Ga-complexes were measured experimentally following procedures outlined previously.17 Briefly, 67Ga-labelled complex (30 μCi/6 μL) was added to 1.5 mL centrifuge tubes containing phosphate buffered saline (PBS) (494 μL) and 1-octanol (500 μL), mixed vigorously for 1 minute, and subsequently centrifuged to separate phases (3000 rpm, 5 min). Equal aliquots (20–100 μL) of the organic and aqueous phases were diluted in a standard volume (20 mL) of water for measurement in an N-type Co-axial HPGe gamma spectrometer from Canberra fitted with a 0.5 mm beryllium window and calibrated (energy and efficiency) with a 20 mL 152Eu source. The samples were counted for 10 minutes, with a dead time of less than 5%. Measurements were carried out in triplicate.
Human apo-transferrin stability studies
The in vitro kinetic inertness assays of 67Ga-complexes against human apo-transferrin were performed according to a previously published method.15,17,18 The radiolabelled complexes [67Ga(2, 3, or 5)]+ were prepared with the radiolabeling protocol as described above. In duplicate for each 67Ga-complex above, solutions were prepared in vials with 500 μL of freshly prepared 1 mg mL−1 apo-transferrin in NaHCO3 (10 mM, pH 7) solution, 300 μL of 67Ga-complex, and 200 μL of phosphate buffered saline (PBS, pH 7.4), and incubated at 37 °C in a water bath. At time points 15 min, 1 and 2 hours, 300 μL of the apo-transferrin competition mixture was removed from each vial, diluted to a total volume of 2.5 mL with PBS, and then counted in a Capintec CRC 15R well counter to obtain a value for the total activity to be loaded on the PD-10 column (F). The 2.5 mL of diluted apo-transferrin mixture was loaded onto a PD-10 column that had previously been conditioned via elution with 20 mL of PBS, and the empty vial was counted in a well counter to determine the residual activity left in the vial (R). The 2.5 mL of loading volume was allowed to elute into a waste container, and then the PD-10 column was eluted with 3.5 mL of PBS and collected into a separate vial. The eluent which contained 67Ga bound/associated with apo-transferrin (size exclusion for MW < 5000 Da) was counted in a well counter (E) and then compared to the total amount of activity that was loaded on the PD-10 column to obtain the percentage of 67Ga that was bound to apo-transferrin and therefore no longer chelate-bound using the equation:Conclusions
The synthesis and characterization of six new lipophilic derivatives of H2dedpa or H2CHXdedpa for the intended purpose of myocardial perfusion imaging were described. All six derivatives were screened for their ability to bind Ga(III) and retained hexadentate N4O2 binding. Initial radiolabeling experiments with 67Ga resulted in abnormal radiolabeling HPLC chromatograms in which three 67Ga-labelled products were formed for the N,N′-benzyl functionalized ligand derivatives (4–6), while ligands (1–3), with N,N′-ethyl ether moieties, exhibited single peaks in the radio-chromatogram as expected. In an attempt to further identify the additional by-product peaks in the radiolabeling reactions of [67/68Ga(4–6)]+ as either ligand impurities or ligand radiolysis products, reaction mixture [67Ga(5)]+ was analysed over the course of one hour, and pro-ligand 6 was radiolabelled with 68Ga as well as 67Ga. The relative peak intensities of the three radiolabelled products remained constant despite the changing reaction parameters, suggesting ligand impurities present in even low concentrations are able to complex Ga(III) more efficiently than the desired pro-ligand. Even though the ligands were deemed sufficiently pure post purification via HPLC and NMR characterization, the relative steric-bulk imposed by benzyl groups next to the cyclohexane backbone may be conceivably inducing ligand decomposition overtime. Therefore, ligands 4–6 are not suitable candidates as radiopharmaceuticals, as a major requirement of such pharmaceuticals is that they be chemically stable and have a suitable shelf-life.Partition coefficients of CHXdedpa2− N,N-ethyl ether functionalized ligands (2 and 3) were determined to be in the range of −1.5 to −1.8, and are not in the predicted suitable log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P range for myocardial perfusion imaging agents. The 67Ga-dedpa complex ([Ga(1)]+, [Ga(dedpa-ee)]+) exhibited a log
P range for myocardial perfusion imaging agents. The 67Ga-dedpa complex ([Ga(1)]+, [Ga(dedpa-ee)]+) exhibited a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P nearly one full log unit lower than the analogous 67Ga-CHXdedpa complex ([Ga(2)]+, [Ga(CHXdedpa-ee)]+), −2.7 and −1.8, respectively. This finding suggests that H2CHXdedpa presents a potentially useful framework to design lipophilic ligands in future studies. Moreover, selected 67Ga-CHXdedpa complexes were sufficiently stable (>80%) in a 2 h apo-transferrin challenge assay.
P nearly one full log unit lower than the analogous 67Ga-CHXdedpa complex ([Ga(2)]+, [Ga(CHXdedpa-ee)]+), −2.7 and −1.8, respectively. This finding suggests that H2CHXdedpa presents a potentially useful framework to design lipophilic ligands in future studies. Moreover, selected 67Ga-CHXdedpa complexes were sufficiently stable (>80%) in a 2 h apo-transferrin challenge assay.
Though N,N′-alkylation of the ligands with benzyl-like attachments was an unfortunate choice of appendage which led to non-trivial radiolabeling and potentially unstable ligands, important lessons on ligand design and alteration of the H2CHXdedpa scaffold have been learned. The results obtained from this set of ligands may be used to design a new library of H2CHXdedpa ligands which offer more lipophilic features, but do not incorporate benzyl groups off the 2° nitrogens in order to prepare Ga(III) complexes within the optimal log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P range (0.5–1.2). The findings in this study are an important first step towards defining the design requirements for the next generation of H2CHXdedpa lipophilic tracers that may have potential use as myocardial perfusion imaging agents.
P range (0.5–1.2). The findings in this study are an important first step towards defining the design requirements for the next generation of H2CHXdedpa lipophilic tracers that may have potential use as myocardial perfusion imaging agents.
Acknowledgements
We acknowledge Nordion (Canada) and the Natural Sciences and Engineering Research Council (NSERC) of Canada for grant support (CR&D, Discovery), NSERC for a CGS-M/CGS-D fellowships (C. F. R.), and the University of British Columbia for 4YF fellowships (C. F. R.). C. O. acknowledges the Canada Council for the Arts for a Killam Research Fellowship (2011–2013). The authors also wish to thank Nordion for supplying 67GaCl3 and Gemma Dias of the Dr Bénard group (BC Cancer Agency) for supplying 68GaCl3.Notes and references
- American Health Association, http://www.heart.org/HEARTORG/HealthcareResearch/Healthcare-Research_UCM_001093_SubHomePage.jsp, accessed March 2015.
- R. Hachamovitch, D. S. Berman, L. J. Shaw, H. Kiat, I. Cohen, J. A. Cabico, J. Friedman and G. A. Diamond, Circulation, 1998, 97, 535–543 CrossRef CAS PubMed.
- R. S. Gibson, D. D. Watson, G. B. Craddock, R. S. Crampton, D. L. Kaiser, M. J. Denny and G. A. Beller, Circulation, 1983, 68, 321–336 CrossRef CAS PubMed.
- S. Liu, Dalton Trans., 2007, 1183–1193 RSC.
- L. Maria, C. Fernandes, R. Garcia, L. Gano, A. Paulo, I. C. Santos and I. Santos, Dalton Trans., 2009, 603–606 RSC.
- C. F. Ramogida and C. Orvig, Chem. Commun., 2013, 49, 4720–4739 RSC.
- E. W. Price and C. Orvig, Chem. Soc. Rev., 2014, 43, 260–290 RSC.
- T. J. Wadas, E. H. Wong, G. R. Weisman and C. J. Anderson, Chem. Rev., 2010, 110, 2858–2902 CrossRef CAS PubMed.
- B. Y. Yang, J. M. Jeong, Y. J. Kim, J. Y. Choi, Y. S. Lee, D. S. Lee, J. K. Chung and M. C. Lee, Nucl. Med. Biol., 2010, 37, 149–155 CrossRef CAS PubMed.
- M. Tarkia, A. Saraste, T. Saanijoki, V. Oikonen, T. Vähäsilta, M. Strandberg, C. Stark, T. Tolvanen, M. Teräs, T. Savunen, M. A. Green, J. Knuuti and A. Roivainen, Nucl. Med. Biol., 2012, 39, 715–723 CrossRef CAS PubMed.
- B. W. Tsang, C. J. Mathias, P. E. Fanwick and M. A. Green, J. Med. Chem., 1994, 37, 4400–4406 CrossRef CAS PubMed.
- E. Boros, C. L. Ferreira, B. O. Patrick, M. J. Adam and C. Orvig, Nucl. Med. Biol., 2011, 38, 1165–1174 CrossRef CAS PubMed.
- I. Velikyan, Med. Chem., 2011, 7, 345–379 CrossRef CAS.
- Y. M. Hsiao, C. J. Mathias, S. P. Wey, P. E. Fanwick and M. A. Green, Nucl. Med. Biol., 2009, 36, 39–45 CrossRef CAS PubMed.
- E. Boros, C. L. Ferreira, J. F. Cawthray, E. W. Price, B. O. Patrick, D. W. Wester, M. J. Adam and C. Orvig, J. Am. Chem. Soc., 2010, 132, 15726–15733 CrossRef CAS PubMed.
- C. F. Ramogida, J. F. Cawthray, E. Boros, C. L. Ferreira, B. O. Patrick, M. J. Adam and C. Orvig, Inorg. Chem., 2015, 54, 2017–2031 CrossRef CAS PubMed.
- C. F. Ramogida, J. Pan, C. L. Ferreira, B. O. Patrick, K. Rebullar, D. T. T. Yapp, K.-S. Lin, M. J. Adam and C. Orvig, Inorg. Chem., 2015, 54, 4953–4965 CrossRef CAS PubMed.
- E. Price, B. Zeglis, J. F. Cawthray, C. F. Ramogida, N. Ramos, J. S. Lewis, M. J. Adam and C. Orvig, J. Am. Chem. Soc., 2013, 135, 12707–12721 CrossRef CAS PubMed.
- R. Ferreiros-Martinez, D. Esteban-Gomez, C. Platas-Iglesias, A. de Blas and T. Rodriguez-Blas, Dalton Trans., 2008, 5754–5765 RSC.
- K.-S. Lin, J. Pan, G. Amouroux, G. Turashvili, F. Mesak, N. Hundal-Jabal, M. Pourghiasian, J. Lau, S. Jenni, S. Aparicio and F. Benard, Cancer Res., 2015, 75, 387–393 CrossRef CAS PubMed.
- M. Regueiro-Figueroa, B. Bensenane, E. Ruscsák, D. Esteban-Gómez, L. J. Charbonnière, G. Tircsó, I. Tóth, A. De Blas, T. Rodríguez-Blas and C. Platas-Iglesias, Inorg. Chem., 2011, 50, 4125–4141 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H, 13C, 1H-1H COSY and variable temperature NMR spectra of selected precursors, ligands, and complexes. See DOI: 10.1039/c6ra24070d |
| This journal is © The Royal Society of Chemistry 2016 |