Direct conversion of chitosan to 5-hydroxymethylfurfural in water using Brønsted–Lewis acidic ionic liquids as catalysts†
Abstract
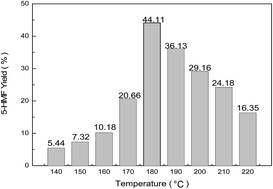
The dehydration of chitosan to 5-hydroxymethylfurfural (HMF) via hydrothermal conversion was investigated in the presence of Brønsted–Lewis acidic ionic liquids. The catalytic effect of different Brønsted- and Lewis-acidic sites and the molar fraction of Lewis-acidic sites were investigated in detail. It was found that Brønsted–Lewis acidic ionic liquids ([Hmim][HSO4]–0.5FeCl2) exhibited good catalytic performance, furnishing HMF in 44.11% yield. Furthermore, the effects of reaction temperature, time, and amount of catalyst were also investigated. The optimal reaction conditions were obtained for a 1.25 wt% aqueous solution of the catalyst under hydrothermal conditions (180 °C for 4 h). A high HMF yield of 44.11% was obtained starting from 50 mg of chitosan. The Brønsted–Lewis acidic ionic liquid catalysts could be recycled by simple separation and exhibited constant activity in four successive trials.

 Please wait while we load your content...
Please wait while we load your content...