Characterization of a Chinese lignite and the corresponding derivatives using direct analysis in real time quadrupole time-of-flight mass spectrometry†
Xing Fan*ab,
Chu-Fan Wanga,
Chun-Yan Youa,
Xian-Yong Weia,
Lu Chena,
Jing-Pei Caoa,
Yun-Peng Zhaoa,
Wei Zhaoa,
Yu-Gao Wang*c and
Jin-Li Lud
aKey Laboratory of Coal Processing and Efficient Utilization, Ministry of Education, China University of Mining & Technology, Xuzhou, Jiangsu 221116, China. E-mail: fanxing@cumt.edu.cn; Tel: +86-516-83885951
bKey Laboratory for Mass Spectrometry and Instrumentation, East China Institute of Technology, Nanchang, Jiangxi 330013, China
cCollege of Chemistry and Chemical Engineering, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China. E-mail: wyg_cumt@163.com; Tel: +86-13653602168
dNews Center of CUMT, China University of Mining & Technology, Jiangsu 221116, China
First published on 27th October 2016
Abstract
Direct analysis in real time (DART) ionization technique coupled with a quadrupole time-of-flight mass spectrometry (Q-TOF MS) with high resolving power was applied to characterize raw coal and coal derivatives in the solid state. Desorption and ionization under optimized parameters allowed for the detection of small molecules scattered inside pores of a macromolecular network of raw coal. Use of the second-stage MS (Q-TOF) significantly contributed to the structural elucidation of compounds in coal and coal derivatives.
Introduction
Although lignite accounts for about 40% of global coal reserves,1 the utilization of lignite is not diversified and it has been mostly used as a fuel. Nevertheless, lignite is regarded as an inferior source of fuel due to its high ash yields, high content of moisture, and low calorific value.2 The high content of organic oxygen in lignite limits its application in thermoelectricity generation.3 In the meantime, most oxygen-containing organic compounds are value-added chemicals.4 It is necessary to explore the clean and efficient utilization of lignite, but insufficient knowledge of the composition and structure of lignite limits such exploration.5Mass spectrometry (MS) has been developed for characterizing components of coals and has proven to be a powerful tool for identifying organic species.6–8 In a previous study, pyrolysis-field ionization MS was applied to acquire the molecular mass distribution and compositional data for major components of coal.9 A variety of hydrocarbons such as alkylated and non-alkylated hydroaromatics, hydrocarbons, alicyclic and paraffinic hydrocarbons, and derivatives of aromatic hydrocarbons containing one or two oxygen atoms were identified. Size-exclusion chromatography and matrix-assisted laser desorption/ionization MS have also been used to characterize heavy hydrocarbons in coal, and have shown the presence of hydrocarbons with molecular mass over thousands of Da.10,11
Ambient ionization coupled with MS has provided rapid and simple approaches for noncontact, in situ, and high-throughput analysis with minimal sample preparation.12–14 Desorption electrospray ionization (DESI) MS was in a previous study applied to analyze petroleum distillates containing saturated hydrocarbons.15 In that study, betaine aldehyde was used as the reactive reagent to improve the sensitivity of the detection of oxidation products with long-chain alcohols. Eckert et al. reported extracting water-soluble components from a sample of crude oil, and analyzing these components by selectively ionizing them and using nanospray DESI MS.16 Atmospheric solids analysis probe (ASAP) MS has been reported to be used to analyze coal-related model compounds17 and coal derivatives.18 Ionic fragments were produced from bridge bond breaking of precursor ions, rearrangement reactions, and elimination of neutral fragments.17 ASAP-time of flight (TOF) MS was used to realize a rapid and in situ analysis of compounds with medium levels of polarity in high-temperature coal tar without sample pretreatment.18
In another ambient ionization method, direct analysis in real time (DART), ionization is triggered by exposing the sample to a stream of metastable species containing atoms and molecules in their excited states.19 The use of DART coupled with an ion trap mass analyzer has been reported in the analysis of coals and coal derivatives.20,21 However, the resolving power of the mass analyzer needs to be improved to fully characterize the composition of the coals at the molecular level.
Methanolysis is considered to be a promising way to depolymerize lignite into its subunit chemicals, and accomplishes this depolymerization by breaking the oxygen bridge bonds of lignite.22 In this work, a sample of Chinese lignite and the corresponding methanolysis product were dried and analyzed using a hybrid quadrupole time-of-flight (Q-TOF) MS coupled with a DART ion source. Analysis of the ionic fragments of precusors created by collision-induced dissociation (CID) improved our understanding of the structure of coal.
Experimental
The lignite sample was obtained from Xilinhaote Mine located in Inner Mongolia, China. The sample was pulverized and passed through a 200-mesh sieve followed by drying at 80 °C for 24 h in near-vacuum conditions. Proximate and ultimate analyses of the coal sample are shown in Table 1. The commercial analytical reagents, methanol, acetone and carbon disulfide (CS2) were employed and purified by distillation using a rotary evaporator (R-134, Büchi Labortechnik AG, Switzerland) prior to use.| Sample | Proximate analysis | Ultimate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Ad | VMdaf | Cdaf | Hdaf | Ndaf | St,d | Odiffb | H/C | |
| a Mad = moisture (air-dried base); Ad = ash (dried base, i.e., moisture-free base); VMdaf = volatile matter (dried and ash-free base); St,d = total sulfur (dry base); daf = dry and ash-free base.b By difference (dried and ash-free base). | |||||||||
| Raw coal | 20.41 | 19.00 | 45.99 | 72.20 | 4.95 | 0.30 | 1.10 | 21.45 | 0.8227 |
| Residue | 6.98 | 22.80 | 33.94 | 79.66 | 5.89 | 1.35 | 0.98 | 12.11 | 0.8811 |
As shown in Fig. S1,† 73 g of a dehydrated coal sample and 330 mL of methanol were placed into a stainless-steel, magnetically stirred autoclave. The autoclave filled with nitrogen was heated to 310 °C for 2 h (with an interior pressure of 9.6 MPa) followed by hot filtration of the reaction mixture to obtain the filtrate and residue. The residue dried in the near-vacuum conditions was extracted with a CS2/acetone (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, vol/vol) mixture at room temperature repeatedly to obtain the extract and the inextractable portion. The raw coal, residue, extract, and inextractable portion were dried to solid states and analyzed using a Q-TOF MS (G6530, Agilent Technologies, USA) coupled with a DART ion source (SVP 100, IonSense Inc., USA).
1, vol/vol) mixture at room temperature repeatedly to obtain the extract and the inextractable portion. The raw coal, residue, extract, and inextractable portion were dried to solid states and analyzed using a Q-TOF MS (G6530, Agilent Technologies, USA) coupled with a DART ion source (SVP 100, IonSense Inc., USA).
The DART source was operated in positive ion mode at 150 °C, 350 °C, and 450 °C, respectively, using helium (99.999%) as the discharge gas with a flow rate of 5 L min−1. Tuning and mass calibration of the Q-TOF MS were performed every day using the Agilent ESI tuning mixture. Samples were introduced onto the surface of a melting point capillary and manually placed at the gap between the outlet of the DART source and the inlet of the MS. The total ion chromatograms (TICs) were monitored for ionized molecules with m/z values between 50 and 1700.
Results and discussion
Parameter optimization for DART in the process of desorption and ionization plays a crucial role for acquiring accurate data. A high flow rate of helium can promote the desorption of analyte molecules from the sample surface into the gas phase, inducing an enhancement of sensitivity. But high helium flow also blows sample clusters and particulates directly into the inlet of the mass analyzer, resulting in contamination of the system.23 Therefore, a flow rate of 5 L min−1 was used.The temperature of the gas heater has to be optimized for samples with different characteristics. Fernández and co-workers reported that temperatures between 150 and 200 °C were chosen for the DART gas heater because thermal desorption of silylated metabolites was too fast to acquire data at temperatures above 250 °C.24 Zhao et al. examined the influence of the temperature of the gas heater on the sensitivity of the detection of small molecules in plasma.23 High temperatures (over 400 °C) would cause biological samples to dry and char immediately on the surface, and would hence further obstruct the desorption of analyte molecules. Petucci et al. monitored synthetic organic reactions in a drug discovery study and showed 350 °C to be the temperature that yielded the strongest response signal for [M + H]+ ions without degrading the analyte.25 The effects of gas temperature on signal intensity are shown in Fig. 1. Although the molecular mass distributions were different at 150, 350, and 450 °C, most of the detected organic species showed molecular masses between 200 and 500 u. As the temperature was increased from 150 °C to 450 °C, the average relative content of small molecules (<200 u) decreased gradually and that of compounds over 500 u increased gradually. Small molecules are easily desorbed and ionized at 150 °C because of their relatively low vaporization temperatures. In the meantime, organic species with relatively high molecular masses do not desorb easily into the gas phase due to their low volatilization rates at 150 °C. Increasing the gas temperature has been shown to speed up the transfer of analytes to the gas phase,12 especially for compounds with high molecular masses. However, the use of high temperatures might lead to thermal decomposition of the analyte and destroy the corresponding original structure, and has been shown to be an ineffective approach for understanding the analyte structure.23–25 Therefore, 350 °C, a medium temperature, was considered to be the optimum temperature, and was used for the analysis of coal and coal derivatives.
 | ||
| Fig. 1 Molecular mass distributions of the organic species detected in the inextractable portion of lignite sample at different gas temperatures. | ||
The structure of coal is complex, and includes a three-dimensional macromolecular network and compounds with low molecular masses scattered inside the pores of the network.26 Low molecular mass compounds can be separated from the macromolecular network by using solvent extraction, especially for compounds on or close to the surface.27 Grinding coal into powders increases the specific surface area of the coal and hence increases the extraction rate. Shown in Fig. 2 are the DART mass spectra of the raw coal, methanolysis residue, and inextractable portion. Scattered small molecules were desorbed from the surface of solid samples by the helium stream and transferred into the gas phase where they were ionized.12,28 Similar compounds were identified at the surface of the raw coal, methanolysis residue, and inextractable portion, indicating the consistency of the coal structure after methanolysis and extraction. However, compounds with molecular masses between 400 and 520 u were only detected in the raw coal powders. The macromolecules, which formed the skeletal network were hard to desorb under the DART conditions. It is possible that the embedded small compounds including the ones on or close to the surface were separated from the network during the methanolysis and extraction processes, and were not detected in the methanolysis residue and inextractable portion.
 | ||
| Fig. 2 DART mass spectra of molecules detected in (a) the raw coal, (b) the methanolysis residue, and (c) the inextractable portion. | ||
Fig. 3 shows the organic species detected in the dried extract. Since the species were separated from the three-dimensional macromolecular network and combined through unconsolidated accumulation (without sufficient intermolecular forces), the MS spectral signal of the dried extract was much more intense than the signals of the raw coal, methanolysis residue, and inextractable portion. Many dimers and trimers were formed through interactions between extractable molecules in lignite, with these interactions including hydrogen bonds, π–π interactions between aromatic rings, and electrostatic and charge–transfer interactions other than van der Waals interactions.17,21,29 Loosely accumulated molecules in the dried extract were easily desorbed by the heated helium gas, inducing a high level of gaseous molecules around the ionization area, which significantly facilitated the interactions. Although small compounds (400 to 520 u in Fig. 2a) were also found to be scattered in the raw coal, the corresponding signals were not strong. The structure of coal indicated that small molecules at the surface were embedded in the macromolecular network. Therefore, fewer small molecules were located on the surface of the raw coal than on the surface of the dried extract. In the meantime, small molecules can be influenced by chain entanglements in the raw coal network, with these entanglements hampering the desorption and ionization of the small molecules.
In comparison to raw coal, the residue showed a greater H/C ratio, higher contents of C, H, and N, and lower relative amounts of S and O. As shown in Table 1, the increase of the H/C ratio and contents of C and H may be related to the incorporation of methyl groups through the reaction with the methanol solvent. S and O were released as volatile molecules, such as H2S and CO2, respectively, inducing the decrease of their contents.30
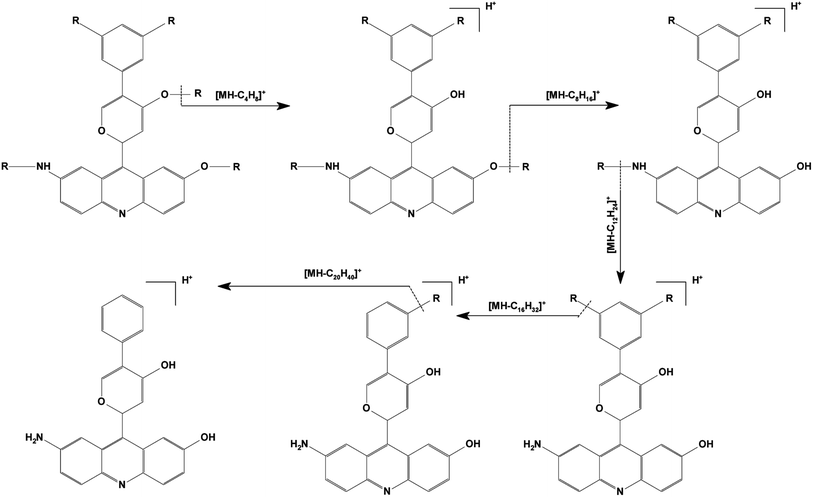
The second stage of MS has been shown to significantly contribute to the structural elucidation of organic compounds.31,32 Wang et al. found two kinds of sulfur compounds in the oxidation products of coal by using GC/Q-TOF MS.33 Fig. 4(a) shows the DART Q-TOF mass spectrum of a compound, one with the parent ion at 944.73 m/z, detected in the inextractable portion. The ionic fragment with an m/z of 680.48 was chosen as the precursor ion to be analyzed using the second stage of MS with a collision energy of 30 eV, and the corresponding CID mass spectrum is displayed in Fig. 4(b). A series of ionic fragments whose values differed from one another by 56 m/z were identified; this result indicated the possible presence of C4H8 groups. The CID mass spectral information of the m/z 680.48 precursor ion is included in Table S1.† The proposed fragmentation pathway for C44H58N2O3 (m/z at 680.48 [M + NH4]+) is shown in Fig. 5 where R denotes tert-butyl. The possible molecular structures of C44H58N2O3 and other ionic fragments are shown in Fig. 4(b).
Conclusions
DART was shown to be a powerful tool for identifying scattered species in coal. A comparison of the DART MS spectra of raw coal, its methanolysis residue, and its inextractable portion provided indirect evidence for the model of the coal structure consisting of a macromolecular network with small molecules scattered inside the pores of the network. Use of the second-stage MS (Q-TOF) was shown to significantly contribute to the structural elucidation of organic species in coal and coal derivatives.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant 21676293), the Fund from China University of Mining and Technology (Grant 2015XKQY06) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.References
- V. Taseska-Gjorgievskaa, A. Dedinec, N. Markovska, J. Pop-Jordanov, G. Kanevce, G. Goldstein and S. Pye, Therm. Sci., 2014, 18, 721–730 CrossRef.
- K. Ouchi, K. Shiraishi, H. Itoh and M. Makabe, Fuel, 1981, 60, 471–473 CrossRef CAS.
- P. Nolan, A. Shipman and H. Rui, Eur. Manag. J., 2004, 22, 150–164 CrossRef.
- I. Omae, Coord. Chem. Rev., 2012, 256, 1384–1405 CrossRef CAS.
- M. Wang, X. Fan, X. Y. Wei, J. P. Cao, Y. P. Zhao, S. Z. Wang, C. F. Wang and R. Y. Wang, Fuel, 2016, 183, 115–122 CrossRef CAS.
- T. Yoshida, Y. Maekawa, T. Higuchi, E. Kubota, Y. Itagaki and S. Yokoyama, Bull. Chem. Soc. Jpn., 1981, 54, 1171–1175 CrossRef CAS.
- A.-L. Zheng, X. Fan, F.-J. Liu, X.-Y. Wei, S.-Z. Wang, Y.-P. Zhao, Z.-M. Zong and W. Zhao, Fuel Process. Technol., 2014, 117, 60–65 CrossRef CAS.
- F. J. Liu, X. Y. Wei, Y. G. Wang, P. Li, Z. K. Li and Z. M. Zong, RSC Adv., 2015, 5, 7125–7130 RSC.
- A. Marzec and H.-R. Schulten, Fuel, 1994, 73, 1294–1305 CrossRef CAS.
- M.-J. Lázaro, A. A. Herod, M. Cocksedge, M. Domin and R. Kandiyoti, Fuel, 1997, 76, 1225–1233 CrossRef.
- M. Millan, T. J. Morgan, M. Behrouzi, F. Karaca, C. Galmes, A. A. Herod and R. Kandiyoti, Rapid Commun. Mass Spectrom., 2005, 19, 1867–1873 CrossRef CAS PubMed.
- E. S. Chernetsova, G. E. Morlock and I. A. Revelsky, Russ. Chem. Rev., 2011, 80, 235–256 CrossRef CAS.
- X. Fan, J. L. Zhu, A. L. Zheng, X. Y. Wei, Y. P. Zhao, J. P. Cao, W. Zhao, Y. Lu, L. Chen and C. Y. You, J. Anal. Appl. Pyrolysis, 2015, 115, 16–23 CrossRef CAS.
- V. P. Sica, H. A. Raja, T. El-Elimat and N. H. Oberlies, RSC Adv., 2014, 4, 63221–63227 RSC.
- C. P. Wu, K. N. Qian, M. Nefliu and R. G. Cooks, J. Am. Soc. Mass Spectrom., 2010, 21, 261–267 CrossRef CAS PubMed.
- P. A. Eckert, P. J. Roach, A. Laskin and J. Laskin, Anal. Chem., 2012, 84, 1517–1525 CrossRef CAS PubMed.
- S.-Z. Wang, X. Fan, A.-L. Zheng, Y.-G. Wang, Y.-Q. Dou, X.-Y. Wei, Y.-P. Zhao, R.-Y. Wang, Z.-M. Zong and W. Zhao, Fuel, 2014, 117, 556–563 CrossRef CAS.
- J.-L. Zhu, X. Fan, X.-Y. Wei, S.-Z. Wang, T.-G. Zhu, C.-C. Zhou, Y.-P. Zhao, R.-Y. Wang, Y. Lu and L. Chen, Fuel Process. Technol., 2015, 138, 65–73 CrossRef CAS.
- R. B. Cody, J. A. Laramee and H. D. Durst, Anal. Chem., 2005, 77, 2297–2302 CrossRef CAS PubMed.
- D.-L. Shi, X.-Y. Wei, X. Fan, Z.-M. Zong, B. Chen, Y.-P. Zhao, Y.-G. Wang and J.-P. Cao, Energy Fuels, 2013, 27, 3709–3717 CrossRef CAS.
- X. Fan, L. Chen, S.-Z. Wang, Y. Qing, X.-Y. Wei, Y.-P. Zhao, A.-L. Zheng, J.-L. Zhu and C.-Y. You, Anal. Lett., 2014, 47, 2012–2022 CrossRef CAS.
- S. Saka, D. Kusdiana and E. Minami, J. Sci. Ind. Res., 2006, 65, 420–425 CAS.
- Y. P. Zhao, M. Lam, D. L. Wu and R. Mak, Rapid Commun. Mass Spectrom., 2008, 22, 3217–3224 CrossRef CAS PubMed.
- M. S. Zhou, J. F. McDonald and F. M. Fernández, J. Am. Soc. Mass Spectrom., 2010, 21, 68–75 CrossRef CAS PubMed.
- C. Petucci, J. Diffendal, D. Kaufman, B. Mekonnen, G. Terefenko and B. Musselman, Anal. Chem., 2007, 79, 5064–5070 CrossRef CAS PubMed.
- A. Marzec, Fuel Process. Technol., 1986, 14, 39–46 CrossRef CAS.
- K.-C. Xie, Structure and Reactivity of Coal, Springer, Berlin, Heidelberg, 2015 Search PubMed.
- E. S. Chernetsova and G. E. Morlock, Bioanal. Rev., 2011, 3, 1–9 CrossRef.
- M. Iino, Fuel Process. Technol., 2000, 62, 89–101 CrossRef CAS.
- H. Y. Lu, X. Y. Wei, R. Yu, Y. L. Peng, X. Z. Qi, L. M. Qie, Q. Wei, J. Lv, Z. M. Zong, W. Zhao, Y. P. Zhao, Z. H. Ni and L. Wu, Energy Fuels, 2011, 25, 2741–2745 CrossRef CAS.
- N. Ochiai, K. Mitsui, K. Sasamoto, Y. Yoshimura, F. David and P. Sandra, J. Chromatogr. A, 2014, 1358, 240–251 CrossRef CAS PubMed.
- M. R. Khan, M. A. Khan, Z. A. Alothman, I. H. Alsohaimi, M. Naushad and N. H. Al-Shaalan, RSC Adv., 2014, 4, 34037–34044 RSC.
- Y. G. Wang, X. Y. Wei, H. L. Yan, Z. K. Li, S. K. Wang, F. J. Liu, L. Peng, F. Xing and Z. M. Zong, Fuel, 2014, 135, 188–190 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra23899h |
| This journal is © The Royal Society of Chemistry 2016 |