High concentration levulinic acid production from corn stover
Siamak Alipour*a and
Hamid Omidvarborna b
b
aDepartment of Chemical and Environmental Engineering, The University of Toledo, Toledo, USA. E-mail: siamak.alipour@rockets.utoledo.edu
bDepartment of Civil Engineering, The University of Toledo, Toledo, USA. E-mail: hamid.omidvarborna@utoledo.edu
First published on 11th November 2016
Abstract
In this study, a novel approach is presented for high concentration levulinic acid (LA) production from biomass hydrolysate. In this process, saccharified biomass hydrolysate, prepared from acid pretreatment of corn stover, was used as the feed. Previously introduced simultaneous isomerization and reactive extraction (SIRE) and back-extraction (BE) method was implemented to convert glucose from biomass hydrolysate solution to fructose at high yield, 88%, and then fructose was transferred to an acidic aqueous reaction medium. In the acidic aqueous medium, not only fructose was converted at high yield (63 mol%) and facile reaction conditions (95 °C and autonomous pressure) to LA, but also the resulting LA was provided at a significant loading of 6.4 wt%. The required energy for LA isolation (95% purity) by evaporation was calculated by Aspen plus simulator software. Results indicated that for high LA concentration (6.4 wt%), required energy (57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 kJ kg−1 LA) is 18 times less than in the case where LA loading was 1 wt% (1
400 kJ kg−1 LA) is 18 times less than in the case where LA loading was 1 wt% (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 043
043![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kJ kg−1 LA). This dramatic reduction in the energy consumption makes the process more cost competitive. The proposed process also benefits from low energy inputs, recyclable streams and catalysts.
000 kJ kg−1 LA). This dramatic reduction in the energy consumption makes the process more cost competitive. The proposed process also benefits from low energy inputs, recyclable streams and catalysts.
Introduction
Levulinic acid (LA) is a promising building block1,2 that can be used as transportation fuels, resins, polymers, pharmaceuticals, and solvent precursors.3–5 In addition, other platform molecules such as γ-valero lactone (GVL) have been synthesized from LA.6–8 Hence, various catalytic systems, including mineral acids,9–15 Amberlyst,16 salts,17 ionic liquids,18,19 and other solid acid catalysts20–23 have been introduced to produce LA from different feedstocks.Studying one-pot LA production from carbohydrate constituent of the lignocellulosic biomass reveals two important points consistently. The first emergent theme is the low LA concentration at the end of the process. In previous attempts for direct production of LA from the biomass,3–5,24 feed loading in the reaction medium was kept deliberately low in order to avoid LA yield reduction. However, such low substrate concentrations would imply extremely low-concentration of the final product in the reaction medium. Isolating LA from these extremely dilute aqueous solutions was usually cost prohibitive.4,25 In this regard, processes that provide high loading of LA are more likely to be economically viable.
The second point is the mild reaction of the LA production from fructose. Moreover, fructose conversion to LA is more selective compared to other feed candidates. However, fructose is an expensive feed and its high yield production from the most abundant C6 sugar (e.g., glucose) is hindered by an unfavorable equilibrium reaction.3,4
In order to circumvent these challenges, an effective approach is required to use glucose from biomass hydrolysate as the feed, convert it efficiently to fructose, and finally produce a high loading LA stream at facile reaction conditions. Recently, the simultaneous isomerization and reactive extraction (SIRE) and back-extraction (BE) process was introduced.26,27 The SIRE–BE was used to overcome unfavorable aldose to ketose equilibrium and transfer fructose to a desired reaction medium.
To explain the SIRE–BE mechanism, it should be noted that aryl boronic acids (ABAs) are a class of sugar binding agents that display pH-dependent complexation with sugars. At a high-pH (>8.5), ABAs exist predominantly in their conjugate-base form (ABA−) at the organic–aqueous interface. The conjugate-base is able to bind with sugar to form tetragonal ester (ABAS−) at the organic/aqueous interface and then accumulate at the interface. Therefore, it is possible to dissolve the ABAS− in the organic phase by adding lipophilic quaternary ammonium salts (Q+Cl−) such as Aliquat® 336 to the organic phase. The lipophilic ammonium cation (Q+) pairs with the ABAS− ion form (Q+)(ABAS−), thereby pulling the tetragonal ester into the organic phase from the interface. The net result of this reactive-binding of fructose to ABA and the ion-pair formation is sugar extraction from the aqueous phase to the organic phase, whereas the ABA itself is confined to the organic phase. Due to the higher affinity of fructose to bind with ABA compared with glucose, this extraction effectively reduces the fructose concentration in the aqueous medium and shifts the fructose/glucose equilibrium in favor of fructose formation. As the solution is repeatedly contacted with the immobilized GXI enzyme and the extraction module, the extraction of fructose to organic phase continues until complete isomerization of the glucose occurs.
As discussed, ABA affinity to fructose and glucose is pH-dependent. Thus, by contacting the organic phase containing sugar complexes to ABA with the low-pH immiscible solvent, the complex will be broken and then fructose is back-extracted to the low-pH phase. In addition, conjugate base is restored to its acidic form. This step is referred to as BE step.
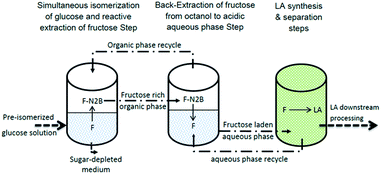
In this study, the SIRE–BE process was implemented to effectively convert glucose to fructose and prepare high concentration feed stream from biomass hydrolysate produced from dilute acid pretreatment of corn stover. Subsequently, fructose was converted to LA at high yield and mild reaction conditions (95 °C and autonomous pressure). This technique successfully addressed most of the draw backs plaguing the existing methods of high loading LA production from lignocellulosic biomass. Due to the specificity of the reactive-extraction and differences in partitioning capacities between the three media, impurities from the hydrolysate do not transfer into the low-pH reaction medium. Hence, the process has potential for implementation with hydrolysates, derived through diverse methods.26 Moreover, the SIRE–BE is a proper method for concentrating dilute hydrolysates through the use of low organic-to-hydrolysate (in SIRE) and aqueous-to-organic (in BE) volume ratios.27 The proposed process for high concentration LA production from biomass hydrolysate solution consists of SIRE, BE and LA synthesis steps. The schematic of the process is presented in Scheme 1.
Materials and methods
Chemicals and materials
Glucose, fructose, 5-(hydroxymethyl)furfural (HMF), levulinic acid (LA), Aliquat® 336, sodium acetate and naphthalene-2-boronic acid (N2B) were purchased from Sigma Aldrich Co. (St Louis, MO). Immobilized glucose isomerase (Gensweet® IGI, GXI) and Spezyme CP were a kind gift from Genencor International (Rochester, NY). Novozyme 188 (Novozyme Corp., Denmark) was used as received from Sigma-Aldrich. All other chemicals and solvents were purchased from Thermo Fisher Scientific Inc. (Pittsburgh, PA).Preparation of saccharified biomass hydrolysate
Dilute acid pre-treated corn stover biomass, which was predominantly cellulose and lignin, was received from National Renewable Energy Laboratory (NREL), in Golden, CO. The solid-containing slurry was washed twice with deionized (DI) water, and the solids were separated by filtration via type 42 of Whatman® ashless filter paper. To run saccharification step, 160 g of wet biomass was added to 500 mL of 50 mM sodium acetate buffer in DI water in an acidic medium (pH adjusted to 4.8 with HCl). 15 FPU g−1 glucan of Spezyme CP and 30 CBU g−1 glucan of Novozyme 188, as two saccharification enzyme cocktails, were added to the slurry and it was mixed continuously at 200 rpm in a shaker/incubator at 50 °C for 72 h. Centrifugation at 5000 rpm for 10 minutes, followed by filtration were conducted on the biomass hydrolysate to separate it from the remaining solids.SIRE and BE
High purity and concentrated fructose was produced from glucose via the SIRE process. A 165 mM (30 g L−1) glucose solution was prepared by diluting saccharified biomass hydrolysate, obtained from NREL, in 50 mM sodium phosphate buffer (pH adjusted to 8.5 by NaOH). The glucose solution was isomerized with 4.5 g L−1 GXI enzyme to reach an equilibrium conversion of glucose to fructose. To selectively extract fructose from the aqueous solution, the aqueous glucose/fructose solution was contacted with octanol containing 165 mM N2B and 412.5 mM Aliquat® 336 for 2 hours. During the SIRE experiment, temperature was kept at 60 °C and pH was maintained at 8.5 using NaOH. After SIRE termination, organic and aqueous phases were separated via centrifugation. In order to partially recycle non-converted sugars in the first step of BE, the organic phase was mixed with the reduced volume of aqueous phase (pH = 6) for 10 minutes at room temperature via an agitation process. Then, in the second BE step, organic phase was contacted and shaken with acidic aqueous phase (pH = 0.3) and sugars were completely back-extracted. Finally, the organic and aqueous phases were separated by centrifugation, and the fructose containing aqueous solution was used as the reaction medium for LA production.In order to make sure that there was no sugar left in the organic phase, both phases were separated by centrifugation process. Then, the organic phase was contacted (with agitation) with fresh low-pH aqueous phase. After recovering the aqueous phase, the high performance liquid chromatography (HPLC) analysis did not show any sugar presence in the aqueous phase. Thus, it was concluded that all sugars were back-extracted in the second BE stage.
Due to the cost associated with the GXI enzyme, lower concentration level of the enzyme, such as 0.45 g L−1, was tested to isomerize glucose to fructose. The results confirmed that the required time for each SIRE stage was two times higher than that of 4.5 g L−1 GXI case (more than 4 hours). Thus 4.5 g L−1 GXI enzyme loading was used for the rest of experiments.
Fructose conversion to LA
Fructose (prepared by the SIRE–BE method) conversion to LA was conducted in the acidic aqueous solution, where the fructose to DI water mass ratio was 9–35 wt%. The reaction medium was added to a 38 × 20 mm glass vial to initiate dehydration of the sugar and subsequent break down of the formed HMF to LA. Mixing was carried out by a magnetic stir bar. The vial was capped and placed in an oil bath on a stirring hotplate. Temperature was kept in a range of 85–115 °C. Reactions were conducted in specified reaction periods (5–360 minutes) and the vial was rapidly cooled by ice-water bath to quench the reaction. Humins were separated by centrifugation at 5000 rpm for 10 minutes and species concentrations in the aqueous phases were analyzed using HPLC.Analytical methods
Calibration standards for glucose, fructose, HMF, and LA were prepared in DI water. Reaction medium samples were diluted with DI water as needed and calibration standards were analyzed using an Agilent 1100 HPLC system equipped with a refractive index detector (RID). A single Shodex SH1011 column (300 × 8 mm, Showa Denko K.K., Japan) was used for the analysis of the sugar, HMF and LA, whereas a mobile phase of 5 mM H2SO4 was run at 0.6 mL min−1. For optimal peak resolution and detection, the column and RID detector temperatures were kept at 65 °C and 35 °C, respectively. All the experiments were repeated to keep the standard deviation less than 2%, whereas the reported results are the average of multiple runs. The LA and HMF yields and fructose conversion were defined as follows:In order to obtain the LA yield based on the glucose, fructose yield from glucose should be multiplied by LA yield from fructose. Moreover, in these experiments, formic acid was also produced in the stoichiometry molar ratio as LA, which was removed from the system through evaporation.
Analysis of the required energy for LA separation
LA can be separated from the aqueous reaction medium via evaporation of the solvent. To determine the required energy for the isolation of LA, a purification analysis was carried out using ASPEN Plus™ process simulator for processing of 100 kg h−1 of LA. Apart from LA, the main inputs to the process were water and HCl. The UNIQAC model was used to describe non-idealities in the liquid phase and a rigorous simulation was built for the purification system, where the properties of the pure components and their binary mixture parameters were taken from the Aspen Plus™ databank.Results and discussion
SIRE–BE
In order to conduct SIRE–BE, 30 g L−1 glucose solution (provided from saccharification of dilute acid pretreated corn stover) was prepared and its pH was raised to 8.5 by adding NaOH. This solution was isomerized by the immobilized GXI enzyme and the pre-isomerized solution was contacted with octanol containing the N2B as the ABA and Aliquat® 336 as the quaternary ammonium salts. N2B and Aliquat® 336 concentrations were adjusted in a way to optimize fructose selectivity and extracted in the SIRE steps based on the provided data.27Fructose was recovered by running multistage BE steps. In the first stage, octanol containing sugars was contacted with the reduced volume of an aqueous phase (pH = 6) to partially recover non-converted glucose and purify the fructose stream. Then, in the second BE stage, organic phase was contacted with highly acidic aqueous phase (HCl concentration: 0.5 M) at room temperature for 10 minutes and consequently fructose was completely extracted to the aqueous phase. The SIRE–BE results are summarized in Fig. 1. As reported by Alipour et al. (2014), this process benefits from recycling streams.26
Fructose conversion to LA
The acidic aqueous solution is a proper solvent for fructose recovery in the BE step; in addition, it has been reported as a suitable reaction medium for LA synthesis from fructose.3–5 In an acidic aqueous environment, fructose first undergoes dehydration to form HMF. HMF reacts with water molecules and produces LA and formic acid. The breakdown of HMF to LA also takes place readily in the presence of an acid catalyst. Moreover, HMF participates in other reactions involving condensation, which leads to HMF loss in the aqueous medium.3,4 Ideally, the reaction conditions should maximize the formation of HMF and its subsequent rehydration to LA and suppress the undesired reactions at the same time. Hence, different parameters such as reaction duration and temperature in addition to acid catalyst and substrate loading should be investigated to produce LA in high yields.Acid catalyst loading effect on the LA yield
Fig. 2 shows the effect of HCl concentration in the reaction medium on the LA yield. In the acidic aqueous medium used in the BE step, HCl concentration was kept at 0.5 M. The results indicated that 0.5 M HCl loading was not efficient for 9 wt% fructose conversion to LA at 95 °C (see Fig. 2). As the HCl concentration was raised from 0.5 to 2 M, LA yield was dramatically improved and almost all of the fructose was converted (>95%) in highly acidic condition (2 M). Furthermore, a major portion of the reacted fructose was converted to LA and HMF, as indicated by the yields of these compounds in Fig. 2.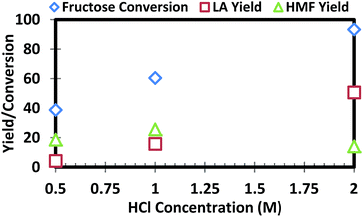 | ||
| Fig. 2 Acid catalyst concentration effect on fructose conversion, LA and HMF yields, reaction conducted at 95 °C for 90 minutes, fructose loading was 9 wt% (fructose mass ratio to aqueous mass). | ||
Reaction duration and temperature effects on the LA yield
In order to evaluate the effect of reaction temperature on fructose conversion, HMF and LA yields, the experiments were also conducted at different temperatures (85 °C, 95 °C, 105 °C and 115 °C), as shown in Fig. 3.The results demonstrate that lowering the reaction temperature to 85 °C reduced the products yield significantly (Fig. 3a). Moreover, reaction kinetics became slow, and for complete fructose conversion very long reaction duration was required (e.g., 360 minutes) at this temperature. HMF also did not fully convert at these conditions.
As it was established in the previous section, near-to-complete fructose conversion takes place in about 90 minutes at 95 °C. Thus, the rehydration of the HMF to LA beyond 90 minutes was investigated by allowing the reaction to proceed up to 180 minutes (Fig. 3b) continuously. Fig. 3b shows that fructose was completely converted when the reaction was allowed to proceed till 120 minutes, and the LA and HMF yields reached 60% and 5%, respectively. Further continuing the process consumed the entire HMF and increased LA yield slightly.
Although complete conversion of fructose and HMF took very long time at 85 °C, their reaction kinetics seemed highly temperature dependent with the same performance as they were consumed much faster at the higher temperatures (Fig. 3c and d at 105 °C and 115 °C, respectively).
Fructose loading effect on the LA yield
In kinetic studies, it has been reported that the initial substrate concentration has a substantial effect on the LA yield and concentration at the end of the reaction.14,28–30 In order to provide high LA loading, increasing initial fructose concentration is imperative. However, LA yield is decreased by increasing feed concentration due to the formation of undesired products, such as humins. Fig. 4 presents the initial fructose loading effect on the product yield. The results show that by increasing the fructose concentration from 9 to 36 wt%, LA yield dropped from 65% to 23%, respectively. This is in agreement with the findings of Girisuta et al.28 that LA yield drop was more significant when the initial feed concentration was high. Although in the cases where fructose initial concentration was 13.5 or 18 wt%, LA had slightly lower yield compared to 9 wt% and LA loading was considerably higher (e.g., 5.1 and 6.4 wt%) compared to 3.7 wt% as shown in Fig. 4. The results indicated that by implementing the SIRE–BE, high initial fructose loading such as 18 wt% can be used and considerable LA concentration (e.g., 6.4 wt%) can be reached without significant LA yield scarification. | ||
| Fig. 4 Fructose loading effects on fructose conversion, maximum LA yield, and LA loading (reaction conducted at 95 °C and HMF was completely consumed). | ||
LA loading effects on required energy for product isolation
Recently, different methods for LA separation and purification including distillation, adsorption, membrane separation, and extraction by an immiscible solvent have been summarized.5 Each method has its own benefits and limitations; however, LA concentration, a parameter with vital effects on the product separation process economy, needs to be explored.To highlight the importance of the LA loading in the product purification process, required energy per mass of produced LA for three different loadings was calculated via Aspen process simulator. The energy was calculated based on the LA separation by two evaporators in series (see Fig. 5). Results summarized in Table 1 present that for increasing LA loading from 1 to 6.4 wt%, the required amount of energy to produce 95% pure LA stream dramatically reduced from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 043
043![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 57
000 to 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 kJ kg−1 LA, respectively.
400 kJ kg−1 LA, respectively.
| Fructose loading (wt%) | LA loading (wt%) | Required amount of energy (kJ kg−1 LA) | LA puritya (wt%) | HCl concentration |
|---|---|---|---|---|
| a Remaining water was considered as impurity; however, it is also possible to completely remove water. | ||||
| <5.0 | 1.0 | 355![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–1 000–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 043 043![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.90–0.95 | Trace |
| 13.5 | 5.1 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500–85 500–85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
0.90–0.95 | Trace |
| 18.0 | 6.4 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300–57 300–57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
0.90–0.95 | Trace |
It is worth noting that in one-pot thermochemical methods, such as Biofine process,24 the biomass loading was less than 5 wt%, which resulted in LA concentration of about 1 wt% at the end of the process.25 However, in this study, as shown in Fig. 4, LA can be produced in an appreciable yield (55%) up to fructose loading of 18 wt% and LA concentration of 6.4 wt%, which would imply a 6 fold increase in the LA concentration in the final reaction mixture and more than 18 times reduction in the energy required for LA isolation (95 wt% LA purity) by evaporation.
Furthermore, the proposed method not only benefits from a higher percentage of LA loading and lower consumption of water, but also the acid catalyst has a lower boiling point than that of LA (b.p. 245 °C). Thus, this is a less energy intensive separation process as well. Moreover, the acid catalyst did not accompany LA in the product stream (Table 1), which made the neutralization and downstream applications less complex.
Conclusion
To summarize, a novel multistep method is developed that converts glucose from biomass hydrolysate to LA at high yield and provides significant loading of LA (6.4 wt%) at the end of the process. In this method, the SIRE–BE process was implemented for efficient glucose conversion to fructose (yield > 88%) and transferred fructose to low-pH aqueous medium. Then, fructose was dehydrated to HMF and produced HMF was converted subsequently to LA at high yield (>60%).The major advantage of this process is the facile reaction conditions that reduce energy inputs. Moreover, in this technology, process streams can be easily recycled, which benefits the process economy. Furthermore, by implementing this approach, it is possible to achieve high fructose loadings in the reaction medium even when the starting biomass hydrolysates are diluted in sugar concentrations. Accommodating high sugar loadings in the reaction medium in addition to effective fructose conversion to LA led to a significant LA concentration at the end of the reaction. This consequently decreased LA separation and isolation cost and made the process more cost competitive than the already available methods.
Acknowledgements
This study was supported by the Chemical Engineering Department at the University of Toledo. Authors want to appreciate Dr S. Varanasi and P. Relue's helpful discussions.References
- T. Werpy and G. Petersen, NREL/TP-510–35523, National Renewable Energy Laboratory, Golden, CO, 2004 Search PubMed.
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539 RSC.
- D. W. Rackemann and W. O. S. Doherty, Biofuels, Bioprod. Biorefin., 2011, 5, 198 CrossRef CAS.
- A. Mukherjee, M. Dumont and V. Raghavan, Biomass Bioenergy, 2015, 72, 143 CrossRef CAS.
- F. D. Pileidis and M. M. Titirici, ChemSusChem, 2016, 9, 562 CrossRef CAS PubMed.
- I. T. Horváth, H. Mehdi, V. Fábos, L. Boda and L. T. Mika, Green Chem., 2008, 10, 238 RSC.
- S. G. Wettstein, D. M. Alonso, Y. Chong and J. A. Dumesic, Energy Environ. Sci., 2012, 5, 8199 CAS.
- D. M. Alonso, S. G. Wettstein and J. A. Dumesic, Green Chem., 2013, 15, 584 RSC.
- G. Novodárszki, N. Rétfalvi, G. Dibó, P. Mizsey, E. Cséfalvay and L. T. Mik, RSC Adv., 2014, 4, 2081 RSC.
- A. Victor, I. N. Pulidindi and A. Gedanken, RSC Adv., 2014, 4, 44706 RSC.
- T. Flannelly, M. Lopes, L. Kupiainen, S. Dooley and J. J. Leahy, RSC Adv., 2016, 6, 5797 RSC.
- J. Li, D. Ding, L. Xu, Q. Guo and Y. Fu, RSC Adv., 2014, 4, 14985 RSC.
- D. W. Rackemann, J. P. Bartley, M. D. Harrison and W. O. S. Doherty, RSC Adv., 2016, 6, 74525 RSC.
- J. Shen and C. E. Wyman, AIChE J., 2012, 58, 236 CrossRef CAS.
- K. Dussan, B. Girisuta, D. Haverty, J. J. Leahy and M. H. B. Hayes, Bioresour. Technol., 2013, 149, 216 CrossRef CAS PubMed.
- D. M. Alonso, J. M. R. Gallo, M. A. Mellmer, S. G. Wettstein and J. A. Dumesic, Catal. Sci. Technol., 2013, 3, 927 CAS.
- K. Qin, Y. Yan, Y. Zhang and Y. Tang, RSC Adv., 2016, 6, 39131 RSC.
- Z. Sun, M. Cheng, H. Li, T. Shi, M. Yuan, X. Wang and Z. Jiang, RSC Adv., 2012, 2, 9058 RSC.
- H. Ren, Y. Zhou and L. Liu, Bioresour. Technol., 2013, 129, 616 CrossRef CAS PubMed.
- Q. Liu, F. Yang, H. Yin and Y. Du, RSC Adv., 2016, 6, 49760 RSC.
- N. A. S. Ramli and N. A. S. Amin, Appl. Catal., B, 2015, 163, 487 CrossRef CAS.
- C. H. Kuo, A. S. Poyraz, L. Jin, Y. Meng, L. Pahalagedara, S. Y. Chen, D. A. Kriz, C. Guild, A. Gudz and S. L. Suib, Green Chem., 2014, 16, 785 RSC.
- S. Van de Vyver, J. Thomas, J. Geboers, S. Keyzer, M. Smet, W. Dehaen, P. A. Jacobs and B. F. Sels, Energy Environ. Sci., 2011, 4, 3601 CAS.
- S. W. Fitzpatrick, US Pat., 5608105, 1997.
- R. Weingarten, W. C. Conner and G. W. Huber, Energy Environ. Sci., 2012, 5, 7559 CAS.
- S. Alipour, B. Li, S. Varanasi, P. Relue and S. Viamajala, US Pat., 063661, 2014.
- S. Alipour, Green Chem., 2016, 18, 4990 RSC.
- B. Girisuta, L. P. B. M. Janssen and H. J. Heeres, Green Chem., 2006, 8, 701 RSC.
- B. Girisuta, B. Danon, R. Manurung, L. P. B. M. Janssen and H. J. Heeres, Bioresour. Technol., 2008, 99, 8367 CrossRef CAS PubMed.
- R. Weingarten, J. Cho, R. Xing, W. C. Conner Jr and G. W. Huber, ChemSusChem, 2012, 5, 1280 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |