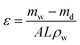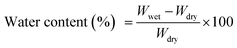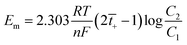Electrochemical behavior of a membrane based on zirconium(IV) phosphoborate nanocomposite and its application in dye removal
Sandeep Kaushala,
Rahul Badru*a,
Sanjeev Kumarb,
Pushpender K. Sharmac,
Susheel K. Mittald and
Pritpal Singh*a
aDepartment of Chemistry, Sri Guru Granth Sahib World University, Fatehgarh Sahib, Pb, India. E-mail: dhillonps2003@gmail.com; rahulbadru@gmail.com; Fax: +91-1763-234236; Tel: +91-84270-00415 Tel: +91-99884-79134
bDepartment of Physics, Sri Guru Granth Sahib World University, Fatehgarh Sahib, Pb, India
cDepartment of Biotechnology, Sri Guru Granth Sahib World University, Fatehgarh Sahib, Pb, India
dSchool of Chemistry & Biochemistry, Thapar University, Patiala 147004, India
First published on 18th November 2016
Abstract
A new hybrid ion-exchange membrane was prepared from a novel composite obtained by dispersion of polyaniline into an inorganic matrix of zirconium(IV) phosphoborate employing a sol–gel method. The composite was characterized by FTIR, XRD, SEM, TEM and TGA/DTA analysis. Membrane potential, transport number, permselectivity and fixed charge density were studied as a function of electrolytic concentration, in order to explore the electrochemical behavior of the prepared membrane. Values for all these electrochemical properties were found to increase upto a mean concentration for each electrolyte and declined thereafter. All these electrochemical properties have higher values for monovalent alkali metal cations than those for bivalent alkaline earth metal cations. The nanocomposite worked well to hamper the growth of both G− and G+ bacterial cultures of Escherichia coli and Bacillus strains to a remarkable level. The nanocomposite was also evaluated for adsorptive removal of methylene blue from aqueous solutions. Maximum adsorption was achieved at pH 11 with an adsorbent dosage of 20 mg for a 1.6 mg/100 mL dye solution in an interval of 3.5 h. Up to 99% dye was recovered upon elution and the regenerated column was efficiently used a number of times. Promising results obtained in dye removal by adsorption and antimicrobial activity encourage the use of such composites in water purification.
1. Introduction
Inorganic ion exchangers have been comprehensively used in pollution control, sensors, separation of radioisotopes and water purification.1 The low mechanical and chemical stability of these ion exchangers are the main constraints. In addition, fine powdered form makes them unsuitable for column operations. The low pollutant removal capacity and lower stability in high radiation fields are the major drawbacks of organic ion exchangers which make them unsuitable as alternatives for inorganic exchangers.2 Hybrid (organic–inorganic) nanocomposite ion-exchangers prepared by various methods are advanced materials which have attracted a good deal of attention3 due to their stability and reproducible analytical and electroanalytical applications. The specificity, selectivity and extensive range of applicability of nano domain advanced composite ion exchangers have made them most promising agents for environmental remediation. Recently, Yao et al. and Bradder et al. have reported the use of ferric oxide and graphene oxide nanoparticles based nanocomposites in waste water remediation via adsorptive removal of dyes on the composite surface.4 Adsorption is gaining much more attention in water remediation over other conventional techniques as it possesses advantageous features of easy mode of operation, low cost and versatility without need of any highly sophisticated instrument.5 Although the hybrid nano-composites are advancing in waste water remediation through adsorption now a days,6 but increasing toxicity of water due to discharge of synthetic organic dyes by textile and paper industries still demands the development of efficient and low cost adsorptive composite materials that can sustain water for future use. In present work, polyaniline (PANI) doped zirconium phosphoborate (ZrPB) nanocomposite has been synthesized by sol–gel method. The composite has been used to fabricate a membrane in order to explore the electrochemical properties like membrane potential, transport number, permselectivity and fixed charge density. The real life application of nanocomposite has also been explored by employing it in adsorptive removal of methylene-blue dye from aqueous solution and as antibacterial agent against Escherichia coli and Bacillus strains of bacteria.PANI was selected as the organic matrix as it is a low cost conducting polymer with suitable biological and chemical resistance.7 Among various inorganic ion exchangers, ZrPB is matter of choice as it is reported to be an excellent ion exchanger with better electrochemical properties.8 Till date, none of the papers reports the doping of PANI into zirconium phosphoborate inorganic matrix, although a few publications report incorporation of PANI into other similar inorganic matrix.9 A detailed electrochemical characterization of IEMs prepared using PANI is still desirable.
2. Experimental
2.1. Reagents and instruments
The reagents used for synthesis were of analytical grade and used as received, without any further purification. Thermal analysis was performed by heating nanocomposite material up to 700 °C at a constant increment of 10 °C per minute in nitrogen atmosphere, using Hitachi SGA7400 thermogravimetric analyzer. IR spectra were recorded on a Perkin Elmer RX I FTIR spectrophotometer. X-ray diffraction patterns were obtained using PAN analytical system DY 3190 X-ray diffractometer. The spectrum was recorded between 5° to 40° at 2θ, using Cu Kα radiation. The topography and elemental composition of the synthesized particles were recorded using JEOL scanning electron microscope and JSM 6510LV energy dispersive X-ray detector. Transmission electron microscopy (TEM) studies were done using model MIC JEM 2100. Potentiometric studies were done on Systronic digital potentiometer (model 318). Elico pH meter (model 1012) was employed to perform pH studies. Membrane thickness was measured using screw gauge.2.2. Preparation of nanocomposite
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio, maintaining the pH ∼ 1.10 Appearance of green colour from the initial brown colour indicated the completion of reaction. Stirring was further continued for 30 minutes. The polyaniline gel obtained was used as such for the next step.
1 volume ratio, maintaining the pH ∼ 1.10 Appearance of green colour from the initial brown colour indicated the completion of reaction. Stirring was further continued for 30 minutes. The polyaniline gel obtained was used as such for the next step.2.3. Ion-exchange capacity (IEC)
The ion exchange capacity of the dry PANI–ZrPB nanocomposite ion-exchanger in H+ form was determined by column method, using sodium nitrate solution (0.1 M) as an eluent. H+ ions eluted from the column were determined titrimetrically against standard solution of sodium hydroxide. Ion exchange capacity (IEC) was calculated by the formula:where N and V are normality and volume in mL of NaOH, respectively, and W is weight in gram of PANI–ZrPB nanocomposite.
2.4. Membrane preparation
The nanocomposite was ground to a fine powder (approximately 200 micrometer in a sieve), and then mixed thoroughly with araldite [PANI–ZrPB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) epoxy resin = 80
epoxy resin = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/w)]. The slurry was spread between the folds of a glossy paper and a pressure of 2.0 kg cm−2 was applied over the glass plates containing the glossy paper for 24 hours. The glossy paper was removed by dipping the membrane in distilled water. Circular membranes were obtained by cutting with sharp knife.
20 (w/w)]. The slurry was spread between the folds of a glossy paper and a pressure of 2.0 kg cm−2 was applied over the glass plates containing the glossy paper for 24 hours. The glossy paper was removed by dipping the membrane in distilled water. Circular membranes were obtained by cutting with sharp knife.
2.5. Physical characterization of fabricated nanocomposite membrane
where mw and md are the mass (g) of wet and dry membrane, respectively. L is the thickness of the membrane, A is the area of the membrane, and ρw is the density of water.
where Wwet and Wdry are the masses in gram of the wet membrane and the dry membrane, respectively.
2.6. Electrochemical properties of fabricated nanocomposite membrane
| Hg|Hg2Cl2, Cl−‖solution C1|membrane|solution C2‖Cl−, Hg2Cl2|Hg |
Potential measurements were made for different concentrations of same electrolyte on two sides of the membrane in such a way that the concentration ratio δ(C2/C1) is 10. During measurements, the electrode assembly was kept immersed in water thermostat maintained at 27 ± 0.1 °C.
The transport number ![[t with combining macron]](https://www.rsc.org/images/entities/i_char_0074_0304.gif) + was calculated by the following equation:11
+ was calculated by the following equation:11
Ps = (![[t with combining macron]](https://www.rsc.org/images/entities/i_char_0074_0304.gif) + − t+)/(1 − t+) + − t+)/(1 − t+) |
![[t with combining macron]](https://www.rsc.org/images/entities/i_char_0074_0304.gif) + and t+ refer to the transport numbers of counter ion in the membrane and in free solution at the same concentration, respectively.13
+ and t+ refer to the transport numbers of counter ion in the membrane and in free solution at the same concentration, respectively.13![[X with combining macron]](https://www.rsc.org/images/entities/b_i_char_0058_0304.gif) ). The electrical character of a membrane is expressed in terms of fixed charge density. The fixed charge density of the prepared membrane for 1
). The electrical character of a membrane is expressed in terms of fixed charge density. The fixed charge density of the prepared membrane for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 electrolytes has been evaluated by Kobatake equation.14
2 electrolytes has been evaluated by Kobatake equation.14where
 &
& 
where Em is membrane potential difference; u & v are ionic mobilities of cation and anion, respectively; K is a constant depending on the solution viscosity and F is Faraday constant.
2.7. Antibacterial study
The antibacterial effects of inorganic part (ZrPB), organic part (PANI) and hybrid nanocomposite (PANI–ZrPB) were investigated individually. Initially, the cultures for both the E. coli and Bacillus were grown overnight aseptically, in an incubator maintained at 37 °C with a shaking speed of 220 rpm. The 1% overnight grown culture was transferred to new flasks having 20 milliliter Luria Broth. A control experiment was set for both E. coli and Bacillus without the addition of any chemical. In another control experiment, 500 μL DMSO (dimethyl sulfoxide) was added into each E. coli and Bacillus culture. In the test experiments, all chemical agents viz. inorganic part, organic part and nano-composite were added at final concentration of 50 μg in each of the six flasks. Following one hour of growth in shaker, one milliliter culture was withdrawn from respective flask at an interval of two hours. The optical density (OD600 nm) was recorded for each sample and OD600 nm versus time (h) graph was plotted to measure the rate of growth inhibition.2.8. Adsorption studies
A series of volumetric flasks of 100 mL capacity containing 25 mL of adsorbate solution of different concentrations were prepared. The pH of the solution was adjusted by addition of 1% NaOH solution. Ion exchange composite material (20 mg) was then added as adsorbent in each flask. The solution was stirred for 4 hours employing magnetic stirrer. The filtrate was analyzed for final concentration of the dye by measuring the absorbance.2.9. Column studies
Fixed bed adsorbent columns of the synthesized nanocomposite were prepared to find the percentage removal of adsorbed dye and to check the reusability of spent column. A specific amount of nanocomposite was added to the glass column [30 cm length and 1 cm internal diameter]. Adsorbate solution of known concentration was percolated through the column at a constant flow rate of 0.5 mL min−1. In each case, 10 mL aliquots of effluents were collected and the effluent concentration was analysed spectrophotometrically. Regeneration and recovery of the adsorbent was also performed on the exhausted column by elution with 10% HCl solution.3. Results and discussion
To meet the requirements of synthesizing an electro-active material that possesses better transport number, permselectivity, fixed charge density and ion-exchange phenomenon than the already reported ZrPB inorganic component, five different samples (S1–S5, Table 1) were prepared by varying the volume ratio of inorganic exchanger, aniline and potassium persulphate. All the samples of nanocomposite possessed enhanced IEC for Na+ ions than its inorganic counterpart ZrPB under identical conditions. The binding of organic material (PANI) into the inorganic matrix may account for the increased IEC of nanocomposite over the inorganic counterpart ZrPB under identical conditions.6c The organic part also enhances the mechanical strength and surface area of the nanocomposite material.15 Since IEC provides information about charge density in the membrane, so it was selected as preliminary criteria that decides the other electrochemical parameters like transport number, permselectivity and fixed charge density in one way or the other. So, the sample with maximum IEC of 0.67 meq g−1 for Na+ ion (sample S-4, Table 1) in comparison to an IEC of 0.38 meq g−1 of ZrPB,8 was selected for fabrication of membrane. The prepared nanocomposite has been characterized through FESEM, EDX, TEM, FTIR, and TGA studies.| S. no. | Mixing volume ratio (v/v) | Mixing volume ratio (v/v) | Colour | Ion exchange capacity (meq g−1) | |||
|---|---|---|---|---|---|---|---|
| 0.1 M zirconyl oxychloride | 0.1 M boric acid | 0.1 M phosphoric acid | 10% aniline in 1 M HCl | 0.1 M potassium persulphate | |||
| S-1 | 1 | 0.5 | 0.5 | 0 | 0.0 | White | 0.38 |
| S-2 | 1 | 0.5 | 0.5 | 0.10 | 0.10 | Green | 0.43 |
| S-3 | 1 | 0.5 | 0.5 | 0.25 | 0.25 | Green | 0.51 |
| S-4 | 1 | 0.5 | 0.5 | 0.50 | 0.50 | Green | 0.67 |
| S-5 | 1 | 0.5 | 0.5 | 1.00 | 1.00 | Green | 0.54 |
3.1. Structural and morphological characterization of PANI–ZrPB composite
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× shows the agglomerates and aggregates of ZrPB particles. The particles are found to be quasi spherical (spherical marked in Fig. 3a) and further form the clusters (marked region P). The nanosize of the ZrPB particles has been confirmed from 500 nm scale bar on the micrograph. Fig. 3b shows the aggregates of the PANI–ZrPB composite taken at 200
000× shows the agglomerates and aggregates of ZrPB particles. The particles are found to be quasi spherical (spherical marked in Fig. 3a) and further form the clusters (marked region P). The nanosize of the ZrPB particles has been confirmed from 500 nm scale bar on the micrograph. Fig. 3b shows the aggregates of the PANI–ZrPB composite taken at 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×. The cluster size of the PANI–ZrPB composite is larger as compared to ZrPB (marked regions in Fig. 3b). It is clearly revealed from the micrographs that after binding of PANI with ZrPB, the topography of the ZrPB has changed. EDX spectrum of PANI–ZrPB nanocomposite (Fig. 3c) confirms the presence of Zr, P, B, C, O, N.
000×. The cluster size of the PANI–ZrPB composite is larger as compared to ZrPB (marked regions in Fig. 3b). It is clearly revealed from the micrographs that after binding of PANI with ZrPB, the topography of the ZrPB has changed. EDX spectrum of PANI–ZrPB nanocomposite (Fig. 3c) confirms the presence of Zr, P, B, C, O, N.
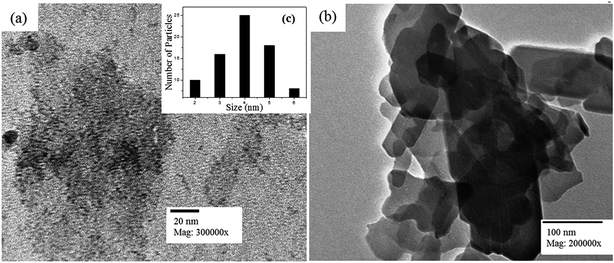
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× (Fig. 4a) shows the homogenous distribution of nearly spherical particles. The 20 nm scale bar in the image confirms the nanometer size of the ZrPB particles. The size of ZrPB particles varies from 2–6 nm range with average size of 4 nm (Fig. 4c – histogram of size variation). Fig. 4b shows the TEM image of PANI–ZrPB composite taken at 200
000× (Fig. 4a) shows the homogenous distribution of nearly spherical particles. The 20 nm scale bar in the image confirms the nanometer size of the ZrPB particles. The size of ZrPB particles varies from 2–6 nm range with average size of 4 nm (Fig. 4c – histogram of size variation). Fig. 4b shows the TEM image of PANI–ZrPB composite taken at 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×. It shows the aggregation in-between PANI–ZrPB composites. The nanosize of the composite has been confirmed by comparing the particle size with 100 nm scale bar in the image. PANI has a great influence on the morphology of ZrPB. The image depicts the morphological changes i.e. from spherical to rod shape, which occurred after binding of inorganic precipitate with the organic polymer matrix. These morphological changes are also reflected in the SEM micrograph of the composite.
000×. It shows the aggregation in-between PANI–ZrPB composites. The nanosize of the composite has been confirmed by comparing the particle size with 100 nm scale bar in the image. PANI has a great influence on the morphology of ZrPB. The image depicts the morphological changes i.e. from spherical to rod shape, which occurred after binding of inorganic precipitate with the organic polymer matrix. These morphological changes are also reflected in the SEM micrograph of the composite.
3.2. Membrane preparation and physiochemical characterization
The nanocomposite was ground to a fine powder (approximately 200 μm). Araldite and the nanocomposite were mixed in the ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 2
9, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and 3
8 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 for fabrication of three membranes of different compositions. An ideal membrane should have less thickness, moderate porosity and water content and high ion exchange capacity. It can be seen from Table 2 that the water content and ion exchange capacity of the membrane decreased with increasing thickness of the prepared membranes. Membrane M-2 was selected for electrochemical studies due to its better ion exchange capacity than membrane M-3. Membrane M-1 having even higher ion exchange capacity than M-2 was not selected for further studies as it was too thin to work, and suffered from frequent leakage during experiments.
7 for fabrication of three membranes of different compositions. An ideal membrane should have less thickness, moderate porosity and water content and high ion exchange capacity. It can be seen from Table 2 that the water content and ion exchange capacity of the membrane decreased with increasing thickness of the prepared membranes. Membrane M-2 was selected for electrochemical studies due to its better ion exchange capacity than membrane M-3. Membrane M-1 having even higher ion exchange capacity than M-2 was not selected for further studies as it was too thin to work, and suffered from frequent leakage during experiments.
| Sample number | Thickness (mm) | Water content (%) | Porosity | Na+ ion exchange capacity (meq g−1) |
|---|---|---|---|---|
| M-1 | 0.38 | 11.57 | 2.4 × 10−2 | 0.22 |
| M-2 | 0.46 | 7.65 | 6.3 × 10−3 | 0.15 |
| M-3 | 0.50 | 5.82 | 8.4 × 10−3 | 0.11 |
3.3. Electrochemical studies of PANI–ZrPB nanocomposite for different electrolytes
The transport number (mobility) of the electrolyte in membrane phase increases with increase in concentration upto a certain limit for all the electrolytes (Fig. 6b). A gradual drop in the values was observed with further increase in concentration of electrolyte. The increase in transport numbers initially, may be attributed to increase in the number of cations (counter ions) available for transport with increase in concentration of the electrolyte. With further increase in concentration of electrolyte beyond a certain limit, increased inter-ionic interactions and concentration polarization phenomenon lead to lower values of transport numbers. The permselectivity exhibits the same trend as that of transport numbers (Fig. 7). Fall in permselectivity with increasing concentration of electrolyte is also supported by the Donnan exclusion principle.24
 | ||
| Fig. 7 Permselectivity of prepared cation exchange membrane for different concentrations of monovalent and bivalent ionic solutions. | ||
The ion selectivity of ion exchange membranes is quantitatively expressed in terms of membrane permselectivity, which measures the ease with which the counter ions migrate through an ion exchange membrane. The membrane potentials, transport numbers (Fig. 6a and b) and permselectivity (Fig. 7) of the membrane for monovalent alkali metal cations were found to be higher than those for bivalent alkaline earth metal cations. It may be due to stronger electrostatic interactions of bivalent cations with fixed ion exchange functional groups on the ion exchange membrane.25 Moreover, the bivalent cations have larger hydrated ionic radii as compared to monovalent cations, leading to their lesser penetration into the membrane. Among the alkali metal and alkaline earth metal ions, potassium(I) and barium(II) ions, respectively, have higher values of membrane potential owing to their smallest hydrated ionic radii in their respective groups.
3.4. Antibacterial studies on PANI–ZrPB nanocomposite
Investigation of the antibacterial activity revealed that all compounds viz. ZrPB, PANI, PANI–ZrPB were able to hamper the growth of both G− and G+ bacterial culture to a remarkable level. The control experiments however demonstrated normal growth. Interestingly, the inhibitory effect was observed in the order ZrPB < PANI < ZrPB–PANI, and was increasing with time as depicted in Fig. 9. | ||
| Fig. 9 Growth curves of (a) E. coli (b) Bacillus bacteria in the presence of, control, DMSO, ZrPB, PANI and PANI/ZrPB nanocomposite ion exchanger. | ||
The nano-composite particles having ∼30 nm size (as validated by TEM) can easily interact with the cell membrane and may disrupt the cellular and molecular functions of the cell, primarily by interfering with its permeability. Similar observations were reported previously where the nano size particles of composite were suggested to be interfering with the cellular and molecular machinery of the cell.26 Recently Li et al.27 also reported similar antibacterial effects of silver nano particles. Their study has shown that the nanoparticles may enter the inner bacterial membrane and can inactivate number of respiratory enzymes that may result in retardation of normal growth of the cells. This may finally result in collapsing of membrane structure that eventually causes death of cells. The preferred selectivity of the nano composite exchanger towards K+ ion (Fig. 8) substantiates our observation of enhanced antibacterial effects in presence of exchanger as K+ ions are involved in membrane transport under heading Na+–K+ pump.28 It can be hypothesized that K+ ions may impair the permeability processes across the membrane and may further retard the bacterial growth.
3.5. Adsorption studies of methylene blue over PANI–ZrPB nanocomposite
Preliminary studies like the effect of pH, adsorbent dosage and contact time were performed before carrying out batch adsorption of methylene blue dye from aqueous solutions over nanocomposite surface.The adsorption conditions were optimized to obtain maximum adsorption of methylene blue over nanocomposite surface and results are reported in Table 3. The results indicate that the extent of adsorption of the dye increased rapidly with increasing pH from 3 to 11 (Table 3, 1–9) and thereafter decreases or remains constant. The higher adsorption of the dye at high pH is apparently due to the effect of pH on surface binding sites of the adsorbent, and on the ionization process of the dye molecules. This leads to greater accessibility of the dye to the active sites. An optimum pH of 11 was selected for further investigations as the nanocomposite reflected maximum adsorption at this pH value.
| S. no. | Dye Conc. (mg/100 mL) | pHa | Adsorbent dosage (mg) | Contact time (min) | % adsorptionb |
|---|---|---|---|---|---|
| a pH was varied by addition of 1% NaOH.b Calculations were made from absorbance measurements using UV spectrophotometer. | |||||
| 1 | 1.6 | 3 | 25 | 240 | 6.25 |
| 2 | 1.6 | 4 | 25 | 240 | 9.3 |
| 3 | 1.6 | 5 | 25 | 240 | 15.6 |
| 4 | 1.6 | 6 | 25 | 240 | 19.2 |
| 5 | 1.6 | 7 | 25 | 240 | 28.9 |
| 6 | 1.6 | 8 | 25 | 240 | 66.4 |
| 7 | 1.6 | 9 | 25 | 240 | 83.6 |
| 8 | 1.6 | 10 | 25 | 240 | 92.3 |
| 9 | 1.6 | 11 | 25 | 240 | 95.0 |
| 10 | 1.6 | 11 | 20 | 240 | 93.2 |
| 11 | 1.6 | 11 | 15 | 240 | 83.1 |
| 12 | 1.6 | 11 | 10 | 240 | 64.4 |
| 13 | 1.6 | 11 | 5 | 240 | 39.2 |
| 14 | 1.6 | 11 | 20 | 210 | 92.5 |
| 15 | 1.6 | 11 | 20 | 180 | 83.7 |
| 16 | 1.6 | 11 | 20 | 120 | 66.5 |
| 17 | 1.6 | 11 | 20 | 60 | 40.1 |
As expected, the percentage of dye adsorption increased with increasing adsorbent dosage at the same initial dye concentration in all cases [Table 3, 9–13]. The increase in dye removal with the adsorbent dose can be attributed to the increased surface area and the adsorption sites. Optimum dosage of nanocomposite for dye removal has been observed to be 20 mg as no significant change was observed with further increase in the amount of adsorbent.
The contact time required for establishment of equilibrium suggests the effectiveness of the composite employed for wastewater treatment. In order to determine the equilibrium time for maximum dye uptake, percentage adsorption was recorded at different time intervals with a fixed adsorbent dose of 20 mg added to 1.6 mg/100 mL (Table 3, 10, 14–17) dye solution at pH 11. After one, two and three hours of contact, about 40.5%, 66.6% and 84.3% of the dye was adsorbed, respectively. Results reflect that the adsorption reaches maximum value of 92.5% in three and half hours, and attains a saturation value with further increase in time.
Langmuir isotherm. The Langmuir isotherm assumes that the surface of any adsorbent material contains a fixed number of active sites and saturation of these active sites stops further adsorption of the adsorbate. This indicates that the adsorption occurs until a monolayer of adsorption is completed and no further adsorption occurs at that site. The equation is stated as:
where qe is the amount adsorbed (in mol g−1), Ce denotes the equilibrium concentration of the dye (in mol L−1), Q0 is the adsorption capacity (mol g−1), and b is the energy of adsorption (L mol−1). Straight line obtained between 1/qe and 1/Ce (Fig. 10a) shows that the adsorption data fits into Langmuir adsorption isotherm. Langmuir constants can be calculated from the plot.
 | ||
| Fig. 10 (a) Langmuir adsorption isotherm and; (b) Freundlich adsorption isotherm for methylene blue. | ||
Freundlich isotherm. The Freundlich model is based on the assumption that adsorption occurs on a heterogeneous adsorption surface having unequally available sites with different energies of adsorption and is given by the relation:
 ; where qe is the amount adsorbed (mol g−1), and Ce is the equilibrium concentration of the adsorbate (mol L−1). The plot of log
; where qe is the amount adsorbed (mol g−1), and Ce is the equilibrium concentration of the adsorbate (mol L−1). The plot of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe with log
qe with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce for the nanocomposite adsorbent along with R2 value is presented in Fig. 10b. Straight line obtained for the Freundlich isotherm models was used to calculate different Freundlich constants.
Ce for the nanocomposite adsorbent along with R2 value is presented in Fig. 10b. Straight line obtained for the Freundlich isotherm models was used to calculate different Freundlich constants.
4. Conclusions
In summary, the prepared polyaniline doped zirconium phosphoborate nanocomposite featured to be a better ion exchange material than its inorganic counterpart zirconium phosphoborate. The membrane potential, transport number and permselectivity values studied with the membrane fabricated from the composite were found to be higher for monovalent cations than those for bivalent cations. The nanocomposite worked well to hamper the growth of both G− and G+ bacterial cultures of Escherichia coli and Bacillus strains to a remarkable level. The nanocomposite was also evaluated for adsorptive removal of methylene blue from aqueous solutions. Remarkable results obtained in dye removal by adsorption and antimicrobial activity encourage the use of such nanocomposites in water purification for environmental remediation.Acknowledgements
SK, RB, PKS and PPS gratefully acknowledge Sri Guru Granth Sahib World University, Fatehgarh Sahib, Punjab (India) for support and lab facilities. SKM is thankful to Director, Thapar University, Patiala for support.References
- (a) S. A. Nabi, S. A. Ganai and A. M. Khan, J. Inorg. Organomet. Polym. Mater., 2011, 21, 25–35 CrossRef; (b) T. Liang and C. Hsu, Radiochim. Acta, 1993, 61, 105–108 CrossRef; (c) H. Lopez, M. T. Olguin, P. Bosch and S. Bulbulian, J. Radioanal. Nucl. Chem. Lett., 1995, 200(1), 19–23 CrossRef.
- (a) W. A. Siddiqui, S. A. Khan and Inamuddin, Colloids Surf., A, 2007, 295, 193–199 CrossRef; (b) R. Niwas, A. A. Khan and K. G. Varshney, Colloids Surf., A, 1999, 150, 7–14 CrossRef.
- (a) K. G. Varshney, N. Tayal, A. A. Khan and R. Niwas, Colloids Surf., A, 2001, 181, 123–129 CrossRef; (b) K. G. Varshney and N. Tayal, Langmuir, 2001, 25, 89–93 Search PubMed; (c) K. G. Varshney and P. Gupta, Indian J. Chem., Sect. A: Inorg., Bio-inorg., Phys., Theor. Anal. Chem., 2003, 42, 2974–2977 Search PubMed; (d) A. A. Khan, M. M. Alam and Inamuddin, Mater. Res. Bull., 2005, 40, 289–305 CrossRef; (e) M. D. A. Khan, A. Akhtar, S. A. Nabi and M. A. Khan, Ind. Eng. Chem. Res., 2014, 53, 15253 CrossRef; (f) S. Kaushal, R. Badru, S. Kumar, S. Mittal and P. Singh, RSC Adv., 2016, 6, 3150–3158 RSC.
- (a) Y. Yao, S. Miao, S. Liu, L. P. Ma, H. Sun and S. Wang, Chem. Eng. J., 2012, 184, 326–332 CrossRef; (b) P. Bradder, S. Ling, S. Wang and S. Liu, J. Chem. Eng. Data, 2011, 56, 138–141 CrossRef.
- K. E. Noll, G. Vassilios and W. S. Hou, Adsorption Technology for Air and Water Pollution Control, Lewis Publishers, Chelsea, MI, USA, 1992 Search PubMed.
- (a) V. K. Gupta, S. Agarwal, I. Tyagi, D. Pathania, B. S. Rathore and G. Sharma, Ionics, 2015, 21(7), 2069–2078 CrossRef; (b) G. Sharma, D. Pathania and Mu. Naushad, Ionics, 2015, 21, 1045–1055 CrossRef; (c) G. Sharma, D. Pathania, Mu. Naushad and N. C. Kothiyal, Chem. Eng. J., 2014, 251, 413–421 CrossRef.
- V. K. Gupta, D. Pathania, N. C. Kothiyal and G. Sharma, J. Mol. Liq., 2014, 190, 139–144 CrossRef.
- (a) P. S. Thind, S. K. Mittal and S. Gujral, Synth. React. Inorg. Met.–Org. Chem., 1988, 18, 593–607 CrossRef; (b) S. Kaushal, P. P. Singh and S. K. Mittal, J. Electrochem. Sci. Eng., 2014, 4, 55–65 Search PubMed.
- (a) Z. A. Alothman, M. M. Alam, M. Naushad and R. Bushra, Int. J. Electrochem. Sci., 2015, 10, 2663–2684 Search PubMed; (b) A. A. Khan and S. Shaheen, J. Electroanal. Chem., 2014, 714–715, 38–44 CrossRef; (c) S. A. Nabi and Mu. Naushad, Colloids Surf., A, 2008, 316, 217–225 CrossRef.
- Z. Alam, Inamuddin and S. A. Nabi, Desalination, 2010, 250, 515–522 CrossRef.
- N. Lakshiminarayanaiah, Transport phenomena in membranes, Academic Press, New York, 1969 Search PubMed.
- (a) G. S. Gohil, V. V. Binsu and V. K. Shahi, J. Membr. Sci., 2006, 280, 210–218 CrossRef; (b) R. K. Nagarale, V. K. Shahi, S. K. Thampy and R. Rangarajan, React. Funct. Polym., 2004, 61, 131–138 CrossRef.
- D. R. Lide, CRC Handbook of Chemistry and Physics, CRC press, Taylor & Francis Group, Boca Raton, Florida, 87th edn, 2006 Search PubMed.
- Y. Kobatake, N. T. Toyoshima and H. Futiza, J. Phys. Chem., 1965, 69, 3981–3988 CrossRef.
- G. Crinic, Bioresour. Technol., 2006, 97, 1061–1085 CrossRef PubMed.
- (a) Y. Furukawa, F. Ueda, Y. Hyodo, I. Harada, T. Nakajima and T. Kawagoe, Macromolecules, 1988, 21, 1297 CrossRef; (b) Z. Ping, J. Chem. Soc., Faraday Trans., 1996, 92, 3063 RSC.
- E. T. Kang, K. G. Neoh and K. L. Tan, Prog. Polym. Sci., 1998, 23, 277 CrossRef.
- (a) C. N. R. Rao, Chemical Applications of Infrared Spectroscopy, Academic Press, New York, 1963, p. 53 Search PubMed; (b) V. K. Gupta, D. Pathania, N. C. Kothiyal and G. Sharma, J. Mol. Liq., 2014, 190, 139–145 CrossRef.
- G. Socrstes, Infrared Characteristics Group Frequencies, Wiley, NJ, 1980, p. 145 Search PubMed.
- C. Duval, Inorganic Thermogravimetric Analysis, Elsevier, Amsterdam, 2nd edn, 1963 Search PubMed.
- S. A. Innamuddin, A. A. Siddiqui and A. A. Khan, Synthesis, characterization and ion-exchange properties of a new and novel ‘organic–inorganic’ hybrid cation-exchanger: Nylon-6,6, Zr(IV) phosphate, Talanta, 2007, 71, 841–847 CrossRef PubMed.
- Y. Kobatake, N. T. Toyoshima and H. Futiza, J. Phys. Chem., 1965, 69, 3981–3988 CrossRef.
- K. Singh and A. K. Tiwari, Proc. Indian Natl. Sci. Acad., Part A, 2004, 70, 477–484 Search PubMed.
- S. M. Hosseini, S. S. Madaeni and A. R. Khodabakhshi, Sep. Sci. Technol., 2010, 45, 2308–2321 CrossRef.
- R. K. Nagarale, G. S. Gohil, V. K. Shahi and R. Rangarajan, Colloids Surf., A, 2004, 251, 133–140 CrossRef.
- E. M. Grainger, H. S. Kim, J. P. Monk, S. A. Lemeshow, M. Gong, R. R. Bahnson and S. K. Clinton, Urol. Oncol., 2008, 26, 125–132 CrossRef CAS PubMed.
- W. R. Li, X. B. Xie, Q. S. Zeng, Y. Sheng, O. Yang and Y. B. Chen, Appl. Microbiol. Biotechnol., 2010, 85, 1115–1122 CrossRef CAS PubMed.
- M. S. P. Samsom, I. H. Srivatava, J. N. Bright, J. Tate, C. E. Capener and P. C. Biggin, Biochim. Biophys. Acta, 2002, 1565, 294–307 CrossRef.
| This journal is © The Royal Society of Chemistry 2016 |