Hyperfine interaction vs. spin–orbit coupling in organic semiconductors
L. B. Niu*ab,
L. J. Chena,
P. Chenc,
Y. T. Cuia,
Y. Zhangc,
M. Shaobd and
Y. X. Guan*a
aKey Laboratory of Optical Engineering, College of Physics and Electronic Engineering, Chongqing Normal University, Chongqing 400047, China. E-mail: utk_lili@126.com; niulb03@126.com
bDepartment of Materials Science and Engineering, University of Tennessee, Knoxville, TN 37996, USA
cSchool of Physical Science and Technology, MOE Key Laboratory on Luminescence and Real-Time Analysis, Southwest University, Chongqing 400715, China
dKey Laboratory of Luminescence and Optical Information, Ministry of Education, School of Science, Beijing Jiaotong University, 100044, China
First published on 9th November 2016
Abstract
Organic semiconductors have shown magnetic field responses in electrical currents. This phenomenon has been generally attributed to hyperfine interaction (HFI) and spin–orbit coupling (SOC). For the first time, we report the experimental and theoretical studies on hyperfine interaction vs. spin–orbit coupling in one thin film of organic semiconductor poly [9,9-di-n-hexyl-fluorenyl-2,7-diyl] and the dramatic influence of doping the PFO with complexes of bis [2-(2′-benzothienyl)-pyridinato-N, C3′] iridium (acetyl-acetonate). The intra-molecular HFI and SOC are only dependent on the intra-molecular interaction among electrons, nuclei and orbital field in a given molecule. Phosphorescence and fluorescence spectroscopies were used to measure the spin–orbit coupling strength. Furthermore, HFI is a short-range interaction and inter-molecular HFI is negligible. Inter-molecular SOC depends not only on molecular structure but also distance between adjacent molecules. Inter-molecular SOC strength increases strongly when the distance between adjacent molecules is decreased.
1 Introduction
Spintronics uses electron spins as quantum information carriers. Remarkable progress has been observed in the field of organic spintronics using polymers and small molecules.1–10 The report of a magnetic field influencing the emission of an organic semiconducting material was first introduced by Johnson for an anthracene crystal.11 Recently, polyfluorene has been shown to have a large magnetoresistance of over 10% at room temperature with magnetic fields of only a few mT.10 As a result, interest in the science behind this intrinsic magnetic field effects has risen sharply. Even though the exact mechanism behind magnetic field effects is still debated,12 it is widely believed to be related to hyperfine interaction (HFI) and spin–orbit coupling (SOC). HFI is the spin–spin interaction between the nucleus spin and the electron spin. As an example, in a typical organic semiconductors tris-(8-hydroxyquinoline) gallium, HFI is predominantly due to the H atoms and other nuclear atoms, such as Ga and N in tris-(8-hydroxyquinoline) gallium. SOC is the coupling between the electron orbital angle momentum and its spin momentum. Many studies suggest that HFI in simple π-conjugated molecules is dominated.13,14 On the contrary, the heavy-metal complex molecule always exhibit strong spin–orbital coupling.15 Obviously, HFI and SOC are two major contributions to magnetic field responses in organic semiconductors. However, they were investigated separately in the most published papers. To date, many experiments have been carried out to measure the HFI using the nuclear magnetic resonance and electron spin resonance (ESR) between ITO and metal electrodes.16,17 However, the SOC is not directly measurable, so there is little quantification of SOC in individual organic semiconductor. It prevents the direct understanding of spin mechanism in organic materials. It is therefore necessary to investigate hyperfine interaction and spin–orbit coupling in the same system. In the present work, intra-molecular and inter-molecular hyperfine interaction vs. spin–orbit coupling competed on the same platform. Fluorescence and phosphorescence spectroscopies were used to measure the spin–orbit coupling strength. We give the report for the first time, the theoretical and experimental studies on hyperfine interaction vs. spin–orbit coupling in one thin film of organic semiconductor poly [9,9-di-n-hexyl-fluorenyl-2,7-diyl] (PFO) and the dramatically influence of doping the PFO with complexes of bis [2-(2′-benzothienyl)-pyridinato-N, C3′] iridium (acetyl-acetonate) (BtpIr) that introduce tuning hyperfine interaction and spin–orbit coupling.2 Experimental
The polymer PFO and the phosphorescent dye BtpIr were purchased from Aldrich Chemical Company and used them as purchased without further purification. The structural formulas of these compounds are illustrated in Fig. 1(a). The BtpIr was mixed with the polymer PFO in chloroform keeping the polymer PFO concentration constant (10 mg ml−1) and altering the dye concentration. A schematic diagram of the device fabricated is shown in Fig. 1(b). The pre-patterned ITO glass substrates were cleaned ultra-sonically (sequentially) with acetone, de-ionize water, and chloroform. A PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS layer was spin coated over the ITO (having sheet resistance 12 Ω per square) keeping the spin rate at 3000 rpm for 15 s. Next, different concentration (100
PSS layer was spin coated over the ITO (having sheet resistance 12 Ω per square) keeping the spin rate at 3000 rpm for 15 s. Next, different concentration (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, 100
0.1, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 100
1, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w) of PFO
2 w/w) of PFO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BtpIr solutions were spin cast on the top of PEDOT
BtpIr solutions were spin cast on the top of PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS layer. All of these organic films were prepared under the nitrogen atmosphere protection. The samples were then baked in vacuum at 120 °C. Finally, the aluminum electrode was deposited by thermal evaporation equipment under a base pressure of 2 × 10−4 Pa at a rate of 2.0 Å s−1. The active device area was roughly 2 × 2 mm2. The magnetic field effects were measured by recording the change of the device current using a Keithley 2400 Source Meter under a parallel magnetic field to the device plane. The samples were immersed in liquid nitrogen condition which is to be taken to avoid oxygen and can keep constant temperature. The thin films for the photoluminescence (PL) measurements were characterized by a Hitachi 2700 spectrophotometer.
PSS layer. All of these organic films were prepared under the nitrogen atmosphere protection. The samples were then baked in vacuum at 120 °C. Finally, the aluminum electrode was deposited by thermal evaporation equipment under a base pressure of 2 × 10−4 Pa at a rate of 2.0 Å s−1. The active device area was roughly 2 × 2 mm2. The magnetic field effects were measured by recording the change of the device current using a Keithley 2400 Source Meter under a parallel magnetic field to the device plane. The samples were immersed in liquid nitrogen condition which is to be taken to avoid oxygen and can keep constant temperature. The thin films for the photoluminescence (PL) measurements were characterized by a Hitachi 2700 spectrophotometer.
 | ||
| Fig. 1 (a) The structural formulas of dopant and host materials that have been used in control experiments. (b) Schematic diagram of the devices fabricated for the MR measurements. | ||
3 Results and discussion
The magnetic field response, magnetoresistance (MR) is determined by measuring the change in current at a constant applied voltage with different magnetic field.
 | (1) |
Fig. 2 shows the magnetic field response on MR for all devices with various doping ratio. The MR was measured at constant voltage model with 0–320 mT magnetic field. It can be seen that all the MR is negative and the magnitude of MR rapidly increases with increasing magnetic field in the low field range and then slowly saturates in the range from 45 mT to 130 mT. It is well known that the carrier density is usually low for an organic semiconductor device. So the devices were operating in the space-charge-limited current (SCLC) regime when an electric field was applied. A series of current–voltage measurements were undertaken to experimentally verify this regime, as is shown in Fig. 2. The current density can be shown as:18
 | (2) |
| μr = ε0εrβ/2e | (3) |
On the other hand, it is seen that MR typically decreases in magnitude with the BtpIr increasing ratio of PFO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BtpIr in the Fig. 2. The overall MR is motivated by the SOC and HFI in the organic blend of PFO
BtpIr in the Fig. 2. The overall MR is motivated by the SOC and HFI in the organic blend of PFO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BtpIr (eqn (4)),
BtpIr (eqn (4)),
 | (4) |
 is the proton spin. gL and gS are the orbital and spin g-factors, respectively. μB is the electronic Bohr magneton.20
is the proton spin. gL and gS are the orbital and spin g-factors, respectively. μB is the electronic Bohr magneton.20
The ĤSOC,HFI Hamiltonian can be written20 as:
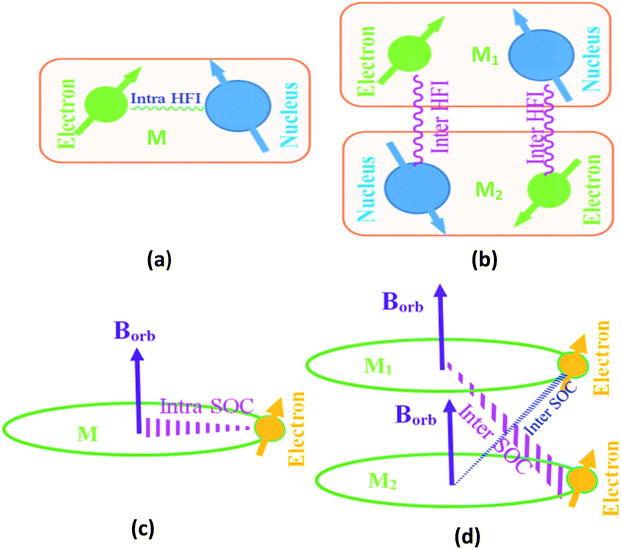
We use the first order perturbation theory (time dependent) to study the spin-dynamics.20 In our studied organic semiconductor, the electronic structure is derived from sp2 hybridization of the C atoms with the pz orbitals forming π bonds. The electrical transport properties are essentially determined by the π electrons. SOC is the coupling between the electron orbital angle momentum and its spin momentum. SOC is long-range interaction; it can be divided inter-molecular SOC (S1) and intra-molecular SOC (S2), as schematically described in eqn (4) and shown in Fig. 3. However, HFI is the spin–spin interaction between the nucleus spin and the electron spin. It is well known that the intra-molecular HFI and SOC are only dependent of intra-molecular interaction [shown in Fig. 3(a)] among electrons, nucleus and orbital field in a given molecule. HFI belongs to short-range interaction and its strength is weak, so inter-molecular HFI [shown in Fig. 3(b)] exhibits negligible, f1 ≫ f2. SOC operator is then proportional to Z4 (Z is atomic charge).20 Thus, once molecular structure is arranged, the intra-molecular HFI and SOC [shown in Fig. 3(c)] becomes a fixed quantity. However, the inter-molecular SOC [shown in Fig. 3(d)] depends not only on molecular structure but also distance between different molecules.
Inter-molecular SOC strength increases strongly when the distance between different molecules is decreased. The pure PFO only contains light atoms and consequently has the very weak SOC strength. MR effect contributes mainly from HFI. However, SOC is dominated for the pure BtpIr. External magnetic field cannot compete with the strong internal magnetic field generated by SOC. In other word, applied magnetic field cannot effectively change the recombination coefficient β under a condition controlled by SOC. As a result, the pure BtpIr shows negligible MR because of its strong SOC.
The average distance of PFO and BtpIr molecules can be calculated approximately by assuming an organic amorphous film is composed of packed cubic molecules. The average volume for a molecule occupies in an organic amorphous film can be treated as a cube by
 | (5) |
The ρ and M values for PFO and BtpIr are 1.22 g cm−3, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 (MPFO), and 1.19 g cm−3, 711.87 g mol−1 (MBtpIr). Thus the average distance of PFO21,22 and BtpIr molecules can be expressed as eqn (6)5 in PFO
000 g mol−1 (MPFO), and 1.19 g cm−3, 711.87 g mol−1 (MBtpIr). Thus the average distance of PFO21,22 and BtpIr molecules can be expressed as eqn (6)5 in PFO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BtpIr (c wt%) films.
BtpIr (c wt%) films.
 | (6) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BtpIr films are given in Table 1.
BtpIr films are given in Table 1.
PFO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BtpIr (w/w) BtpIr (w/w) |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| d (nm) | 8.622 | 7.583 | 7.740 |
Notably, the average distance decreases when guest concentration increases. That is to say, the heavy metal atom Ir becomes more and more close PFO. As a result, inter-molecular SOC strength increases with guest concentration increased.
On the basis of competing between HFI and SOC. HFI is much larger than SOC in the pure PFO thin film. So its MR response shows as large as −6.5%. On the contrary, HFI is much less than SOC in the pure BtpIr thin film and PFO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BtpIr (100
BtpIr (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) system. Therefore, theirs MR signs exhibit zero. The MR response for other architectures shows between zero and −6.5%.
1) system. Therefore, theirs MR signs exhibit zero. The MR response for other architectures shows between zero and −6.5%.
Now we further measure the inter-molecular SOC of the PFO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BtpIr thin films with different doping concentration on the basis of the spectral overlap between the phosphorescent and fluorescent emission spectra. Fig. 4 shows PL spectrum of the thin films (excitation wavelength = 370 nm). As the doping ratio increases, the value of the phosphorescence intensity (IPh) compared to the fluorescence intensity (IF) increases. For the sake of comparison, the PL spectra of neat PFO (10 mg ml−1) and neat BtpIr (1.2 mg ml−1) thin films has been shown in the inset of the Fig. 4. All the photoluminescence spectra were excited in the same condition. As in the inset, the pure PFO film PL intensity (peak) is 107 CPS (420 nm) and the pure BtpIr film PL intensity (peak) is 9 × 103 CPS (616 nm). As in the Fig. 4, the 420 nm PL intensity (peak) is 106 CPS and the 616 nm PL intensities (peak) are 4 × 104 CPS (100
BtpIr thin films with different doping concentration on the basis of the spectral overlap between the phosphorescent and fluorescent emission spectra. Fig. 4 shows PL spectrum of the thin films (excitation wavelength = 370 nm). As the doping ratio increases, the value of the phosphorescence intensity (IPh) compared to the fluorescence intensity (IF) increases. For the sake of comparison, the PL spectra of neat PFO (10 mg ml−1) and neat BtpIr (1.2 mg ml−1) thin films has been shown in the inset of the Fig. 4. All the photoluminescence spectra were excited in the same condition. As in the inset, the pure PFO film PL intensity (peak) is 107 CPS (420 nm) and the pure BtpIr film PL intensity (peak) is 9 × 103 CPS (616 nm). As in the Fig. 4, the 420 nm PL intensity (peak) is 106 CPS and the 616 nm PL intensities (peak) are 4 × 104 CPS (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1), 3.7 × 105 CPS (100
0.1), 3.7 × 105 CPS (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 3.9 × 105 CPS (100
1) and 3.9 × 105 CPS (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), respectively. Although BtpIr has its own chromophore and the same emission spectrum, only a weak 616 nm PL emission was observed for the pure BtpIr film. BtpIr has a significantly higher PL intensity for the PFO
2), respectively. Although BtpIr has its own chromophore and the same emission spectrum, only a weak 616 nm PL emission was observed for the pure BtpIr film. BtpIr has a significantly higher PL intensity for the PFO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BtpIr with the different doping concentration (100
BtpIr with the different doping concentration (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, 100
0.1, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 100
1, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w). At the same time, PFO film PL intensity shows a slight decrease for this doped system. It is well known that only singlet excitons are generated in the polymers and small molecules when they are excited optically. The triplet state can become populated through intersystem crossing. For the PFO
2 w/w). At the same time, PFO film PL intensity shows a slight decrease for this doped system. It is well known that only singlet excitons are generated in the polymers and small molecules when they are excited optically. The triplet state can become populated through intersystem crossing. For the PFO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BtpIr blend system, the emission with a peak at 420 nm originates from the radiative recombination of singlet excitons from PFO host. The emission with a peak at 616 nm, to a large extent, belongs to recombination of triplet excitons by intersystem crossing on BtpIr guest molecules for these doped films.
BtpIr blend system, the emission with a peak at 420 nm originates from the radiative recombination of singlet excitons from PFO host. The emission with a peak at 616 nm, to a large extent, belongs to recombination of triplet excitons by intersystem crossing on BtpIr guest molecules for these doped films.
The ISC rate can be written as20
 | (7) |
In other words, the ISC rate mainly depends on the strength of the spin–orbit coupling. Therefore, quite generally, the relative strength of the fluorescence and phosphorescence bands is intimately related to the spin–orbit coupling strength Tso.
 | (8) |
Furthermore,
 | (9) |
Eqn (9) therefore allows a straightforward calculation of KISC from the (spectrally integrated) phosphorescence and fluorescence intensities.20 The KISC values can be extracted using spectrally integrated method from the photoluminescence spectra (fluorescence and phosphorescence intensity) in Fig. 4 are shown in Table 2.
PFO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BtpIr (w/w) BtpIr (w/w) |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| KISC (s−1) | 2.6 × 10−10 | 7.8 × 10−10 | 8.1 × 10−10 |
So the spin–orbit coupling strength increases with increasing the doping ratio.
4 Conclusions
In summary, we have studied the magnetic field response in one doped organic polymer PFO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BtpIr solid film. From these systematic studies, the result indicates that MR attributed to hyperfine interaction and spin–orbit coupling in organic semiconductors. Intra-molecular and inter-molecular hyperfine interaction vs. spin–orbit coupling competed on the same platform. HFI belongs to short-range interaction. However, SOC is long–range interaction. The magnetic field is capable of impacting the injected current by means of influencing the recombination of the PFO
BtpIr solid film. From these systematic studies, the result indicates that MR attributed to hyperfine interaction and spin–orbit coupling in organic semiconductors. Intra-molecular and inter-molecular hyperfine interaction vs. spin–orbit coupling competed on the same platform. HFI belongs to short-range interaction. However, SOC is long–range interaction. The magnetic field is capable of impacting the injected current by means of influencing the recombination of the PFO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BtpIr system. Fluorescence and phosphorescence spectroscopes were used to measure the spin–orbit coupling strength. Clearly, our experimental studies confirmed that inter-molecular SOC strength increases strongly when the distance between adjacent molecules is decreased.
BtpIr system. Fluorescence and phosphorescence spectroscopes were used to measure the spin–orbit coupling strength. Clearly, our experimental studies confirmed that inter-molecular SOC strength increases strongly when the distance between adjacent molecules is decreased.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant No. 60806047, 11504036, 11504300 and 61475126), the Natural Science Foundation Project of CQ CSTC (Grant No CSTC2015jcyjA70001 and CSTC2015jcyjBX0032), the Chongqing science and technology personnel training program (Grant No. cstc2013kjrc-qnrc90003), and the Research Programs for Science and Technology of Chongqing Municipal Education Commission (Grant No. KJ1400509, KJ1600327 and KJ1500318) and the Foundation for the Creative Research Groups of Higher Education of Chongqing (No. CXTDX201601016). We acknowledge assistance from Dr L. Yan (University of North Carolina at Chapel Hill) and fruitful discussions with Dr H. D. Zang (Los Alamos National Laboratory).References
- H. Malissa, M. Kavand, D. P. Waters, K. J. Schooten, P. L. Burn, Z. V. Vardeny, B. Saam, J. M. Lupton and C. Boehme, Science, 2014, 345, 1487–1489 CrossRef CAS PubMed.
- D. Yuan, L. B. Niu, Q. S. Chen, W. Y. Jia, P. Chen and Z. H. Xiong, Phys. Chem. Chem. Phys., 2015, 17, 27609–27614 RSC.
- L. Yan, M. Wang, N. P. Raju, A. Epstein, L. S. Tan, A. Urbas, L. Y. Chiang and B. Hu, J. Am. Chem. Soc., 2012, 134, 3549–3554 CrossRef CAS PubMed.
- P. Chen, Y. L. Lei, Q. L. Song, Y. Zhang, R. Liu, Q. M. Zhang and Z. H. Xiong, Appl. Phys. Lett., 2009, 95, 213304 CrossRef.
- W. Y. Jia, Q. S. Chen, L. X. Chen, D. Yuan, J. Xiang, Y. B. Chen and Z. H. Xiong, J. Phys. Chem. C, 2016, 120, 8380–8386 CAS.
- J. Devkota, R. G. Geng, R. C. Subedi and T. D. Nguyen, Adv. Funct. Mater., 2016, 26, 3881–3898 CrossRef CAS.
- Y. F. Wang, K. S. Tiras, N. J. Harmon, M. Wohlgenannt and M. E. Flatté, Phys. Rev. X, 2016, 6, 011011 Search PubMed.
- B. Hu and Y. Wu, Nat. Mater., 2007, 6, 985–991 CrossRef CAS PubMed.
- F. Maciá, F. Wang, N. J. Harmon, M. Wohlgenannt, A. D. Kent and M. E. Flatté, Nat. Commun., 2014, 5, 3069 Search PubMed.
- O. Mermer, G. Veeraraghavan, T. L. Francis, Y. Sheng, D. T. Nguyen, M. Wohlgenannt, A. Kohler, M. K. A. Suti and M. S. Khan, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 205202 CrossRef.
- R. C. Johnson and R. E. Merrifield, Phys. Rev. B: Solid State, 1970, 1, 896–902 CrossRef.
- C. Boehme and J. M. Lupton, Nat. Nanotechnol., 2013, 8, 612–615 CrossRef CAS PubMed.
- P. A. Bobbert, T. D. Nguyen, F. W. A. van Oost, B. Koopmans and M. Wohlgenannt, Phys. Rev. Lett., 2007, 99, 216801 CrossRef CAS PubMed.
- Y. Wu, Z. Xu, B. Hu and J. Howe, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 035214 CrossRef.
- Z. G. Yu, Phys. Rev. Lett., 2011, 106, 106602 CrossRef CAS PubMed.
- E. Shikoh, K. Ando, K. Kubo, E. Saitoh, T. Shinjo and M. Shiraishi, Phys. Rev. Lett., 2013, 110, 127201 CrossRef PubMed.
- L. Yan, M. Shao, C. F. O. Graeff, I. Hummelgen, D. G Ma and B. Hu, Appl. Phys. Lett., 2012, 100, 013301 CrossRef.
- J. D. Bergeson, V. N. Prigodin, D. M. Lincoln and A. J. Epstein, Phys. Rev. Lett., 2008, 100, 067201 CrossRef CAS PubMed.
- V. N. Prigodin, J. D. Bergeson, D. M. Lincoln and A. J. Epstein, Synth. Met., 2006, 156, 757–761 CrossRef CAS.
- Y. G. Sheng, University of Iowa, Ph.D. Dissertations, 2008.
- S. Kilina, E. R. Batista, P. Yang, S. Tretiak, A. Saxena, R. L. Martin and D. L. Smith, ACS Nano, 2008, 2, 1381–1388 CrossRef CAS PubMed.
- P. A. Lane, L. C. Palilis, D. F. O'Brien, C. Giebeler, A. J. Cadby, D. G. Lidzey, A. J. Campbell, W. Blau and D. D. C. Bradley, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 235206 CrossRef.
| This journal is © The Royal Society of Chemistry 2016 |