The sorption of the nonsteroidal anti-inflammatory drugs diclofenac and naproxen onto UV and/or H2O2 treated MWCNT-COOH and MWCNT-OH†
B. Czech
Department of Environmental Chemistry, Faculty of Chemistry, Maria Curie-Skłodowska University, Pl. Marii Curie-Skłodowskiej 3, 20-031 Lublin, Poland. E-mail: bczech@hektor.umcs.lublin.pl; Fax: +48 81 537 55 65; Tel: +48 81 537 55 54
First published on 2nd November 2016
Abstract
The release of carbon nanotubes (CNTs) with treated wastewater is one of the major sources of CNTs in the environment. The great sorption capacity of pristine CNTs can be changed during wastewater processing. CNTs in the environment can interact with different components and pollutants. The aim of this study is the determination of the effect of treatment methods of wastewater containing functionalized CNTs on the sorption of new emerging contaminants. The process of sorption of diclofenac sodium (DCF) and naproxen (NPX) was tested using carbon nanotubes released after treatment by UV and/or H2O2. The results revealed that sorption on non-treated CNTOHs was governed by chemisorption, with chemisorption being rate limiting step (pseudo-second order regime). Treatment of the CNTOHs changed the kinetics of NPX sorption to a pseudo-first order (CNTOH-UV and CNTOH-H2O2) or an intraparticle diffusion model (CNTOH-UV + H2O2). DCF sorption was described by pseudo-first or pseudo-second order kinetics. NPX sorption onto treated CNTOHs and CNTCOOHs was ascribed to the Freundlich or Temkin models. The Freundlich and Dubinin–Radushkevich models revealed the best fitting for the description of DCF sorption onto CNTOHs and CNTCOOHs. Although treatment affected the physicochemical properties of the CNTs only slightly, it did change the sorption affinity and capacity. NPX sorption was favored over all tested CNTs, and CNTCOOHs revealed a higher sorption capacity for all tested adsorbates. The mechanism of sorption was connected with π–π interactions between the NPX and the CNTOHs, and the functional groups of the CNTCOOHs. DCF sorption onto CNTOHs was governed by electrostatic forces, with adsorption onto CNTCOOHs governed by functionalization.
Introduction
The unique mechanical, optical and surface properties of carbon nanotubes (CNTs)1 has resulted in their wide examination and application. Pristine CNTs are used for studies of stabilization2–4 and sorption of heavy metals5 or organic pollutants.6,7 Pristine CNTs are prone to aggregation via van der Waals interaction forces and therefore are difficult to use in treatment and other applications.8 After introduction of surface functional groups (–OH, –COOH, –CO, –NH2) , increased dispersion and reactivity is observed. The improved solubility results from the side chain functionalization of the CNT surface.9 CNT dispersion dictates both sorption behavior and environmental fate.10,11 Studies on the sorption behavior of surface functionalized CNTs however are still scarce.9,10,12CNTs are classified as a group of emerging contaminants that will be noted in the environment at 0.003–0.02 ng L−1.13 In wastewater during treatment CNTs will be subjected to many physicochemical processes.14 Treatment will change their adsorptive properties.15,16 Currently applied wastewater treatment methods, however, are not designed to remove new pollutants such as CNTs completely.17,18
The problem of the removal of new emerging contaminants from the environment is vital.13,19–21 Pharmaceuticals and personal care products (PPCPs),22 pesticides,23 and engineered nanomaterials24 are present in relatively low concentrations (ng L−1 up to μg L−1)21 in environmental matrices but they may disturb the life cycles of many organisms.20,25 The consumption of pharmaceuticals is growing26 and their release into the environment seems to be enhanced.27 PPCPs are introduced into the environment during usage, through landfills, or with wastewater.24 Their presence in wastewater treatment (WWT) plant effluents28,29 indicates their low susceptibility to conventional treatments.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are frequently used as over-the-counter drugs in most countries.30 Among these NSAIDs, diclofenac sodium salt (DCF), 2-(2,6-dichloranilino)phenylacetic acid sodium salt, and naproxen (NPX, (+)-(S)-2-(6-methoxynaphthalen-2-yl)propanoic acid) have been mainly noted in WWT effluent.31 DCF was noted in WWT effluents at concentrations <0.001–0.69 μg L−1 and its removal efficiency was broad, ranging from 0 to 81.4%. NPX effluent concentrations were similar to DCF (<0.002–5.09 μg L−1). Removal of NPX was however enhanced (43.3–98.6%).32 PPCPs introduced into the environment could impact the productivity, reproduction, and/or health of many organisms.30
It is interesting though to study the behavior of CNTs in treated wastewater, in particular their sorption of new emerging contaminants. For the treatment of –OH or –COOH functionalized CNTs in wastewater UV (254 nm, 5 h) and/or (0.35 wt%) H2O2 was applied. DCF and NPX were used as test molecules. The aim of this study was (1) to determine the kinetics of DCF or NPX sorption onto UV and/or H2O2 treated functionalized CNTs, and (2) to evaluate the mechanism of sorption of selected PPCPs onto treated functionalized CNTs.
Results and discussion
CNTOH and CNTCOOH properties after treatment are presented in the ESI in Table S1.† Treatment generally decreased both SBET and the pore volume of the CNTOHs and the CNTCOOHs. It was found that H2O2 had the greatest effect on the CNTOHs, while UV had the greatest effect on the CNTCOOHs. The percentage amount of oxygen increased for the CNTOHs, and decreased for the CNTCOOHs. Both the zeta potential and the mobility were also reduced.NPX
 | ||
| Fig. 1 Kinetics of the sorption of NPX onto (a) CNTOH and CNTCOOH, (b) treated CNTOHs, and (c) treated CNTCOOHs. | ||
| Kinetics | Parameter | CNTOH | CNTOH-UV | CNTOH-H2O2 | CNTOH-UV + H2O2 |
|---|---|---|---|---|---|
| a Qe – mg g−1, k1 – h−1, t1/2 – h, Qe – mg g−1, k2 – g mg−1 h−1. | |||||
| Pseudo-first order | Qe1 | 1.245 | 3.041 | 3.060 | 3.484 |
| k1 | 0.0088 | 0.0153 | 0.0127 | 0.0173 | |
| t1/2 | 78.8 | 45.3 | 54.6 | 40.1 | |
| R2 | 0.9117 | 0.9609 | 0.8622 | 0.9352 | |
| Pseudo-second order | Qe2 | 34.13 | 23.87 | 25.19 | 36.50 |
| k2 | 2740.8 | 500.4 | 460.6 | 1996.1 | |
| R2 | 0.9989 | 0.8628 | 0.8082 | 0.9182 | |
| Elovich | α | 2 × 1015 | 4.056 | 4.374 | 6.676 |
| β | 1.208 | 0.265 | 0.260 | 0.177 | |
| R2 | 0.8739 | 0.7995 | 0.7222 | 0.8722 | |
| Intraparticle diffusion | Kip | 0.346 | 1.664 | 1.713 | 2.400 |
| R2 | 0.9103 | 0.9312 | 0.8620 | 0.9410 | |
| Kinetics | Parameter | CNTCOOH | CNTCOOH-UV | CNTCOOH-H2O2 | CNTCOOH-UV + H2O2 |
|---|---|---|---|---|---|
| Pseudo-first order | Qe | 2.9838 | 3.8019 | 1.0462 | 2.9294 |
| k1 | 0.0216 | 0.0224 | 0.0237 | 0.0117 | |
| t1/2 | 32.1 | 30.9 | 29.2 | 59.2 | |
| R2 | 0.9686 | 0.8105 | 0.8318 | 0.8392 | |
| Pseudo-second order | Qe | 40.65 | 85.47 | 15.58 | 22.62 |
| k2 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 353.7 353.7 |
1662.2 | 2498.7 | 338.8 | |
| R2 | 0.9915 | 0.8022 | 1 | 0.9249 | |
| Elovich | α | 222.3 | 7.7 | 1756.0 | 8.4 |
| β | 0.232 | 0.245 | 0.750 | 0.410 | |
| R2 | 0.9332 | 0.8888 | 0.9117 | 0.9368 | |
| Intra-particle diffusion | Kip | 1.777 | 1.683 | 0.475 | 0.990 |
| R2 | 0.9463 | 0.9100 | 0.6950 | 0.9255 |
Adsorption of NPX onto the non-treated CNTOHs proceeded according to the pseudo-second order model, indicating chemisorption as a rate limiting step. The highest k2 value (2740.79 g mg−1 h−1) was obtained for the CNTOHs. Similarly, the pseudo-second order regime was observed for the sorption of antibiotics onto the CNTs.15 Generally, treatment resulted in a decrease in adsorbed NPX (Fig. 1b) and the observed Qe2 values were lower by 26–30%.
Treatment of the CNTOHs changed the kinetics to pseudo-first order (CNTOH-UV and CNTOH-H2O2) indicating a physical character of sorption. The adsorption rate was determined by the NPX concentration. k1 increased with treatment (from 0.0088 to 0.0127 and 0.0153 h−1 for CNTOH, CNTOH-H2O2 and CNTOH-UV, respectively).
The greatest effect of NPX sorption onto CNTOHs occurred when the CNTOHs were treated with UV and H2O2 simultaneously. Adsorption onto CNTOH-UV + H2O2 and CNTOH-H2O2 was ascribed to the intraparticle diffusion model and the highest Kip was obtained for CNTOH-UV + H2O2. However, similar values of R2 were obtained for a pseudo-first and intraparticle diffusion model for sorption onto CNTOH-H2O2. These data indicate that the process of NPX sorption is complex, and involved both external mass transfer and intraparticle diffusion through micropores.33
To identify the effect of the physicochemical parameters of the CNTOHs and CNTCOOHs (SBET, elemental composition, pore radius and volume, zeta potential and mobility of) on NPX sorption, Pearson correlations (p < 0.05 0.9500) were calculated. Statistically significant correlations were obtained only for k2 with SBET (0.9588) and micropore volume (0.9464) indicating the obvious role of surface area in sorption onto CNTOH. Adsorption occurred in micropores. It needs to be emphasized that the porosity of nanotubes should be considered not as the pores in the nanotubes as such but as the distances between nanotube agglomerates. Therefore, NPX sorption proceeded with the inclusion of the sieve effect. NPX sorption onto highly mobile CNTOHs was favored (0.9590). A significantly lower and statistically insignificant correlation was observed for Kip with SBET (−0.8012) and the micropore volume (−0.8556) confirming however the role of reduced surface area and pore volume in the diffusion of NPX.
Treatment of CNTCOOHs resulted in a decrease of NPX sorption by about 50% (Table 1, Fig. 1c). NPX sorption proceeded according to the pseudo-second order model (Table 1) indicating that chemisorption is the rate limiting step. Adsorption onto CNTCOOH-UV and CNTCOOH-UV + H2O2 proceeded differently however, and the best fitting (R2 0.9368) was obtained with the intraparticle diffusion and Elovich models describing chemical adsorption onto heterogeneous surfaces. Qe obtained over the treated CNTCOOH was significantly (44–62%) lower. Similarly k2 was also reduced: 4 times for CNTCOOH-H2O2 and 6 times for CNTCOOH-UV.
According to the calculated correlations it was observed that an increase in the pore radius of the CNTCOOHs enhanced the value of k2 (0.9644). In the Elovich model, the increase of β (desorption coefficient) was connected with larger aggregates (0.9701). The diffusion rate coefficient, Kip, increased with the decrease in the hydrogen content (−0.9753), zeta potential (−0.9329) and mobility (−0.9327) of the CNTCOOHs. The increase of CNTCOOH aromaticity hindered sorption indicating that NPX was sorbed, but not onto the exposed walls and not with the participation of functional groups. This indicates that oxygen groups did not participate in the formation of bonds between NPX and the CNTCOOHs.
Mechanism of sorption of NPX
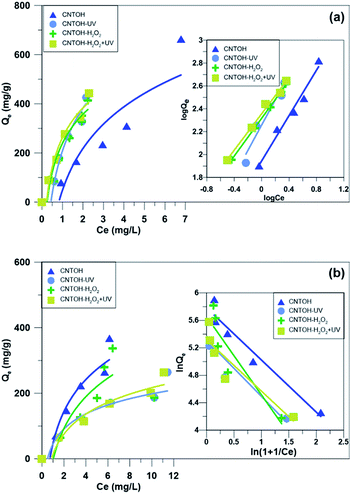
The description of NPX sorption on nanotubes involved the use of the Freundlich, Langmuir, Temkin and Dubinin–Radushkevich models (Fig. 2 and S1†). The process of NPX sorption onto the CNTOHs was described by a Freundlich isotherm (Table 2). The other isotherms revealed lower fittings (Table S2†). The Freundlich constant QF is an indicator of adsorption capacity, while 1/n is a function of the strength of adsorption during the adsorption process. Treatment of the CNTOHs reduced QF, with treatment by H2O2 yielding the greatest effect. The lowest obtained value of 1/n for CNTOH-UV + H2O2 indicates the highest heterogeneity of its surface. A value of 1/n > 1 for CNTOH-H2O2 implies cooperative sorption. The sorption of NPX onto the other treated CNTOHs was physical (1/n < 1) confirming that H2O2 treatment yielded the greatest impact on CNTOH. As the 1/n values were reduced for the treated CNTs (from 0.934 to 0.6), the process of adsorption is changed from cooperative (chemical) (CNT) to normal (physical) (CNT-UV, CNT-H2O2).34 The as-obtained 1/n values were in the range 1–2 or <1, which confirmed moderately difficult or poor adsorption characteristics, respectively.35 Pearson correlations revealed that the adsorption capacity, QF, was reduced for broader pores (−0.9914). The increase of surface area (0.9588), micropore volume (0.9464) and mobility (0.9590) enhanced the strength of adsorption. | ||
| Fig. 2 The Freundlich isotherm of NPX sorption over the treated CNTOHs. Lines represent the model predicted data fitting. Inset shows the linearized form of the adsorption isotherms. | ||
| CNTOHs | Freundlich | ||||
|---|---|---|---|---|---|
| QF | STE | 1/n | STE | R2 | |
| a QF – mg g−1, STE standard error. | |||||
| CNTOH | 15.180 | 0.5050 | 0.934 | 0.031 | 0.9558 |
| CNTOH-UV | 12.557 | 0.6104 | 0.649 | 0.031 | 0.9614 |
| CNTOH-H2O2 | 10.016 | 0.1888 | 1.091 | 0.021 | 0.9560 |
| CNTOH-UV + H2O2 | 13.193 | 0.3665 | 0.609 | 0.017 | 0.9910 |
In the literature there are several proposed models of sorption of organic compounds onto carbon nanotubes: heterogeneous adsorption, hydrophobic or π–π interactions, hydrogen bonds, and electrostatic interactions.12 It seems that the π–π interactions of NPX with the graphene nanotube walls described the sorption. Nevertheless, the nanotube functionalization, or rather its changes after the modification, had an indirect effect on the sorption of NPX. The variations in the CNT functionalization (expressed indirectly as changes in the percentage content of O) altered the wettability of the nanotube surface and its affinity for hydrophobic organic contaminants.12 The hydrophobic interactions were crucial only in the final phase of the sorption.36
The data obtained indicate that the process of NPX sorption onto CNTOHs is not directly related to the presence of functional oxygen groups (no correlation with the percentage of O), but rather indirectly. Oxygen groups, determining the surface charge of the CNTOHs, slightly affected the partitioning (−0.567), which indicates that the mechanism of NPX sorption by means of hydrogen bonds cannot be considered.12 A notably stronger effect was exerted by parameters related to porosity, which would indicate a physical rather than chemical character of the sorption.34 The sorption of NPX onto the CNTOHs took place on diverse sites in terms of energy, such as the outermost surfaces, interstitial channels, inner cavities and grooves.11
NPX sorption onto the treated CNTCOOHs (Fig. 3 and S2,† Tables 3 and S3†) was ascribed to the Freundlich model. Sorption according to the Temkin model proceeded for CNTCOOH-H2O2. Generally, treatment reduced the adsorption capacity of the CNTCOOHs. Both oxidizing factors (UV + H2O2) revealed only a small change in comparison to CNTCOOH in terms of both the QF and the 1/n values from the Freundlich isotherm. For CNTCOOH-H2O2 an increase in the adsorption capacity was observed. However, the better fitting of the Temkin model revealed that an increase of B was connected with the physical character of NPX sorption. The linear decrease in the heat of adsorption resulted in greater coverage of NPX on the surface layer.
 | ||
| Fig. 3 Isotherms of NPX sorption over the treated CNTCOOHs: (a) Freundlich, (b) Temkin. Lines represent the model predicted data fittings. Insets show the linearized form of the adsorption isotherms. | ||
| Freundlich | |||||
|---|---|---|---|---|---|
| QF | STE | 1/n | STE | R2 | |
| a QF – mg g−1, STE standard error. | |||||
| CNTCOOH | 21.09 | 0.81 | 0.8607 | 0.0331 | 0.9951 |
| CNTCOOH-H2O2 | 24.27 | 0.39 | 0.5859 | 0.0095 | 0.9251 |
| CNTCOOH-UV | 15.35 | 0.13 | 0.8311 | 0.0070 | 0.9728 |
| CNTCOOH-H2O2 + UV | 21.03 | 0.67 | 0.6466 | 0.0207 | 0.9853 |
| Temkin | |||||
|---|---|---|---|---|---|
| KT | STE | B | STE | R | |
| CNTCOOH | 1.761 | 0.068 | 162.07 | 6.236 | 0.9628 |
| CNTCOOH-H2O2 | 1.161 | 0.019 | 173.95 | 2.831 | 0.9553 |
| CNTCOOH-UV | 1.239 | 0.010 | 141.75 | 1.187 | 0.853 |
| CNTCOOH-H2O2 + UV | 2.240 | 0.072 | 115.72 | 3.712 | 0.8896 |
Sorption onto the CNTCOOHs depended on their functionalization, and thus on the amount of surface oxygen, indicating a notable share of chemical character for the sorption process. An increase in the oxygen percentage resulted in easier generation of hydrogen bonds, as the benzene rings of NPX can act as an H-bond donor and thus participate in the formation of hydrogen bonds with oxygen functional groups on the surface of the CNTCOOHs.12,15
A weak correlation was observed between sorption and aggregate size indicating that NPX adsorbed better on large agglomerates of CNTCOOH. Access to sorption sites with the highest energy could have been difficult not only due to the size exclusion phenomenon, but also due to the presence of amorphous carbon or metals (residues after the process of synthesis). The presence of water clusters formed around the oxygen functional groups could also hinder sorption.11
Hydrophobic properties play a key role in the adsorption of organic compounds onto CNTs: the lower the hydrophobicity, the lower the adsorption of hydrophobic compounds. As the Qe value of NPX sorption onto treated CNTCOOHs and CNTOHs was lower than the values of DCF sorption at the same initial concentration, the lower hydrophobicity of NPX than DCF (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow of NPX and DCF is 3.3 and 4.4, respectively)38 explains the results obtained. The data clearly indicate different mechanisms of NPX sorption onto the CNTCOOHs and the CNTOHs.
Kow of NPX and DCF is 3.3 and 4.4, respectively)38 explains the results obtained. The data clearly indicate different mechanisms of NPX sorption onto the CNTCOOHs and the CNTOHs.
DCF
 | ||
| Fig. 4 Kinetics of the sorption of DCF onto (a) CNTOH and CNTCOOH, (b) treated CNTOHs and (c) treated CNTCOOHs. | ||
| Kinetics | Parameter | CNTOH | CNTOH-UV | CNTOH-H2O2 | CNTOH-UV + H2O2 |
|---|---|---|---|---|---|
| a Qe – mg g−1, k1 – h−1, t1/2 – h, Qe – mg g−1, k2 – g mg−1 h−1. | |||||
| Pseudo-first order | Qe | 1.409 | 3.758 | 3.926 | 2.600 |
| k1 | 0.026 | 0.022 | 0.023 | 0.024 | |
| t1/2 | 27.0 | 31.5 | 30.1 | 28.9 | |
| R2 | 0.9053 | 0.7947 | 0.8119 | 0.9544 | |
| Pseudo-second order | Qe | 11.25 | 40.65 | 43.67 | 19.12 |
| k2 | 89.81 | 2005.16 | 2037.07 | 942.73 | |
| R2 | 0.9879 | 0.6468 | 0.6458 | 0.9867 | |
| Elovich | α | 208307.7 | 11.66 | 11.01 | 6.65 |
| β | 1.716 | 0.199 | 0.198 | 0.171 | |
| R2 | 0.5865 | 0.5082 | 0.4918 | 0.6077 | |
| Intraparticle diffusion | Kip | 0.271 | 2.439 | 2.522 | 2.762 |
| R2 | 0.757 | 0.7151 | 0.7393 | 0.8108 | |
| Kinetics | Parameter | CNTCOOH | CNTCOOH-UV | CNTCOOH-H2O2 | CNTCOOH-UV + H2O2 |
|---|---|---|---|---|---|
| Pseudo-first order | Qe | 3.109 | 4.265 | 3.802 | 6.788 |
| k1 | 0.020 | 0.039 | 0.022 | 0.209 | |
| t1/2 | 34.7 | 17.8 | 0.2 | 3.3 | |
| R2 | 0.9187 | 0.7846 | 0.8105 | 0.9502 | |
| Pseudo-second order | Qe | 25.25 | 42.74 | 38.46 | 42.74 |
| k2 | 768.6 | 2040.5 | 1388.5 | 2040.5 | |
| Rad2 | 0.9166 | 0.6514 | 0.6191 | 0.6514 | |
| Elovich | α | 8.10 | 7.26 | 12.52 | 16.10 |
| β | 0.293 | 0.206 | 0.204 | 0.200 | |
| R2 | 0.8067 | 0.5232 | 0.4853 | 0.5598 | |
| Intraparticle diffusion | Kip | 1.523 | 2.383 | 2.448 | 2.423 |
| R2 | 0.9611 | 0.7538 | 0.7229 | 0.7885 |
DCF sorption over treated CNTOHs was notably increased (Fig. 4b). The amount of adsorbed DCF was similar for each of the treated CNTOHs ∼260 mg g−1. After an initial rapid stage of the sorption (up to 8 hours) a slow-down of the sorption and sorption equilibrium onto CNTOH was attained. To a certain extent, this may be due to external mass transfer (diffusion of DCF) through the film covering the CNTOHs, to the external surfaces of the nanotubes.36
The sorption kinetics for CNTOH-UV + H2O2, as in the case of CNTOH, were described by the pseudo-second order regime. The values of k2 increased for all CNTOHs (Table 4). Treating by UV or H2O2 separately resulted in changed kinetics, with the best fitting given by the pseudo-first order model, however the R2 values were rather low.
k1 increased with decreasing mobility (−0.9762) indicating that an increase in the surface area of the CNTOHs reduces the value of the adsorption constant. The calculated Pearson correlations between the values of k2 and the physicochemical parameters of the nanotubes showed that the porous structures (inner pores and interstitial channels) did not participate in DCF sorption onto the CNTOHs. This was confirmed by the negative values of the correlation of k2 with Qe (−0.8557). A similar correlation was obtained in a study on the sorption of 17α-ethinyl estradiol and bisphenol A sorption onto MWCNTs (diameter 8–15 nm).37 Therefore, the stage limiting the rate of the sorption was not diffusion but chemisorption of the DCF.39,40 In the case of the other correlations no statistically significant results were obtained, but the high values of R2 for the correlation between k2 and SBET (−0.9086) and micropore volume (−0.6195) indicated that the process was controlled by the surface properties of the nanotubes, similar to the sorption of other PPCPs onto CNTs.17 With increasing CNTOH aggregate diameters, access for DCF was easier and the sorption was enhanced (0.9041). The stability of the nanotubes and their initial state determined the DCF sorption. An increase of zeta potential (0.9377) and at the same time mobility (0.9067) improved the sorption of DCF, showing that the surface charge of the CNTOHs determined the sorption.
DCF sorption over treated CNTCOOHs increased (Fig. 4c); Qe differed only slightly. The kinetics observed for non-treated CNTCOOHs was ascribed to the intraparticle diffusion model (Table 4). Treatment changed the kinetics and sorption onto treated CNTCOOHs proceeded according to the pseudo-first order regime. The obtained kinetics run contrary to literature data.41 The treatment of CNTCOOHs results in a significant increase of k1 and the values were much higher than for CNTOHs.
The correlations calculated showed that SBET had no effect on the sorption of DCF (−0.6480), and that the process was dependent to a greater extent on the other properties of the nanotubes. The character of DCF sorption was related to the chemical structure of the CNTCOOH surface. It was observed that an increase in the percentage of hydrogen inhibited sorption (0.9764). The process proceeded without any participation of oxygen functional groups, which was evident in the lack of correlation with the oxygen percentage (0.5429). The key parameter, however, was access to the surface sites, which was confirmed by a positive correlation with dispersion (0.8243). DCF was adsorbed better on small and well dispersed CNTOH aggregates. Increased surface charge and mobility (−0.9682) also caused a reduction of DCF sorption, which resulted from greater repulsive forces. In the intraparticle diffusion model, Kip increased with increasing pore volume (0.9668) and pore diameter (0.9892) stressing the role of porosity in CNTCOOHs.
Mechanism of DCF sorption
Among the tested models, the Freundlich and Dubinin–Radushkevich isotherms yielded better fittings (Tables 5 and S4†). QF calculated from the Freundlich isotherm for treated CNTOHs increased by 2-fold, similar to 1/n (20–24%) except for CNTOH-UV. The low value of 1/n calculated for CNTOH-H2O2 (<1) indicated the physical character of the sorption mechanism.42 The Dubinin–Radushkevich model was applicable only for the description of DCF sorption onto CNTOH-UV. QDR decreased slightly. The calculated low value of mean free energy (less than 8 kJ g−1) confirmed the physical character of DCF sorption.
| DCF | Freundlich | ||||
|---|---|---|---|---|---|
| QF | STE | 1/n | STE | R2 | |
| a QF – mg g−1, STE standard error, QDR – mg g−1, E – kJ g−1. | |||||
| CNTOH | 19.633 | 0.755 | 1.009 | 0.039 | 0.9750 |
| CNTOH-UV | 34.818 | 0.567 | 0.829 | 0.013 | 0.9349 |
| CNTOH-H2O2 | 38.621 | 0.323 | 1.234 | 0.010 | 0.9998 |
| CNTOH-UV + H2O2 | 41.767 | 1.340 | 1.264 | 0.041 | 0.9849 |
| Dubinin–Radushkevich | |||||
|---|---|---|---|---|---|
| QDR | STE | E | STE | R2 | |
| CNTOH | 6 × 10−7 | 2 × 10−8 | 0.0245 | 13.93 | 0.8199 |
| CNTOH-UV | 3 × 10−7 | 1.46 × 10−8 | 0.0223 | 24.25 | 0.9810 |
| CNTOH-H2O2 | 1 × 10−7 | 1.89 × 10−9 | 0.0256 | 7.21 | 0.9186 |
| CNTOH-UV + H2O2 | 1 × 10−7 | 2.78 × 10−9 | 0.0250 | 11.09 | 0.9292 |
In the literature, DCF sorption is described by means of Freundlich or Langmuir isotherms.41,43,44 Khatem et al.41 described DCF sorption according to the Freundlich model using a 0.3 mmol L−1 solution; at higher concentrations the process was described better by the Langmuir model. The Langmuir model was also applicable for DCF sorption onto regenerable granular carbon nanotube/alumina hybrid adsorbents.43 DCF sorption onto magnetic MWCNTs proceeded also according to the Langmuir model with a maximum adsorption capacity of 33.37 mg g−1,44 which was higher than the results obtained for non-treated CNTOHs and CNTCOOHs, but lower than for treated ones (Table 4). Nevertheless, DCF sorption was determined not by the physical properties of the carbon nanotubes but rather by their surface composition. Therefore, modification of carbon nanotubes does not always lead to an increase in the sorption of organic contaminants.
The mechanism of DCF sorption onto CNTs differed from that of NPX. The process was complicated and the effects of π–π interactions, hydrogen bonds and electrostatic interactions were not uniform. Increased aromaticity of the CNTOHs (0.9145) caused an increase in DCF sorption, which may indicate a certain contribution of π–π interactions in the mechanism of DCF adsorption onto CNTOHs.12 Considering the π–π interactions, the chemical character of DCF should have a notable effect.11 The presence of chlorine in a DCF molecule usually reduces the electron density, thus increasing adsorptive affinity. Similarly, the presence of an unpaired pair of nitrogen electrons in the amino group results in the enriching of the benzene ring electron density, which should enhance interactions with electron-poor groups of the CNTOHs more strongly than with water. This would suggest that the results of DCF sorption should be higher than those of NPX. However, the results do not support this, which may result from the fact that the number of electron depleting regions is limited, and thus the highest sorption should be observed at low concentrations. On the other hand, the introduction of functional groups onto CNTOHs causes a weakening of π–π dispersion, lowering the sorption of DCF.
A positive correlation with pore diameter (0.9914) was observed, which confirmed that the process was controlled by the nanotube’s porosity. With an increase in the aggregate diameter of the CNTOHs, access for DCF was more difficult and the sorption decreased (−0.9167). This indicates that the nanotube’s stability, and their initial status, directly determine DCF sorption. The ability of CNTs to aggregate affects their stability, and indirectly affects the sorption of contaminants. The aggregation results in a reduction in the accessibility for organic compounds on the surface of the CNTs. Hence the bundle structure of the CNTs and the molecular configuration of DCF were of key importance.11
The surface charge determined the sorption of DCF, as an increase in the zeta potential (−0.6873) lowered the sorption. The charge of the CNTOHs results from the presence of oxygen on the surface of the sp3 carbon,9,45–47 primarily in the form of carbonyl moieties. According to the literature,48 an increase of the number of hydroxyl groups results in the formation of a greater number of water clusters and more hydrogen bonds. That, however, can reduce the sorption when the bonds are created only within the nanotubes, as it was observed in these studies. An excess of the oxygen percentage (−0.8753) and increased polarity (−0.8663) both hindered sorption. Nonetheless, the reactivity of functional oxygen groups is not uniform. On the other hand, the lower the oxygen content in the CNTs, the less stable they are.46,49 As the percentage of oxygen in the CNTOHs changed, their sorption abilities and sorptive capacity were also altered.
An increase in the zeta potential reduced the amount of adsorbed DCF, which confirmed that oxygen did not participate in DCF bonding. It was observed that the larger the aggregates of the CNTOHs were, the lower the sorption of DCF was, which was caused by problems with the penetration of relatively large DCF molecules through the CNTOHs. On the other hand, an increase of the surface charge of nanotubes affected the electrostatic interactions between the CNTOHs and DCF. At the pH level at which the measurements were conducted, both the CNTOHs and the DCF are dissociated (mainly as deprotonated carboxylic acid groups). Hence, the repelling forces were greater and the sorption was more difficult.50
Sorption occurred mainly at the sites of defects (Pearson correlation with ID/IG −0.7458). The graphitic surfaces of the CNTs have regions which are both rich and poor in π-electrons, with the interaction of π electrons influencing adsorption. Therefore, the key element in the sorption of DCF onto CNTOHs was access to adsorption sites such as corners, pores, and defects.
| DCF | Freundlich | ||||
|---|---|---|---|---|---|
| QF | STE | 1/n | STE | R2 | |
| a QF and QDR – mg g−1, STE – standard error, E – kJ g−1. | |||||
| CNTCOOH | 27.437 | 1.056 | 1.361 | 0.052 | 0.8014 |
| CNTCOOH-H2O2 | 40.530 | 0.660 | 1.333 | 0.022 | 0.9970 |
| CNTCOOH-UV | 39.992 | 0.335 | 1.324 | 0.011 | 0.9987 |
| CNTCOOH-H2O2 + UV | 39.453 | 1.266 | 1.363 | 0.044 | 0.9953 |
| Dubinin–Radushkevich | |||||
|---|---|---|---|---|---|
| QDR | STE | E | STE | R2 | |
| CNTCOOH | 5 × 10−7 | 2 × 10−8 | 0.022 | 0.0008 | 0.8724 |
| CNTCOOH-H2O2 | 1 × 10−7 | 2 × 10−9 | 0.026 | 0.0004 | 0.9222 |
| CNTCOOH-UV | 1 × 10−7 | 8 × 10−10 | 0.026 | 0.0002 | 0.8840 |
| CNTCOOH-H2O2 + UV | 1 × 10−7 | 3 × 10−9 | 0.026 | 0.0008 | 0.9010 |
No statistically significant correlations were obtained among the analyzed correlations of QF with the physicochemical parameters of CNTCOOHs, nevertheless the high values of R2 indicate that QF decreases with increasing pore diameter (−0.9914).
In contrast to the CNTOH adsorption mechanism, a totally different mechanism for DCF sorption onto CNTCOOHs is proposed. The key parameter is the percentage content of oxygen. With increases in the level of the oxygen percentage in the structure of the CNTs, the adsorption affinity decreased as the water clusters formed around the functional groups could prevent electron donor–acceptor interactions through competition with DCF molecules.10,11 The role of oxygen in the sorption of organic contaminants onto CNTs is described in two ways. An increase in the content of oxygen can affect the sorption through changes in the location of π electrons, which reduces the π–π interactions between the graphitic surface of the CNT and the benzene rings of the aromatic compounds, or though the formation of water clusters that block the access to adsorption sites.11 Such strong interactions between aromatic carbon and DCF have been described in a study on the sorption of DCF by modified biochars.51 That mechanism may be also applicable for the description of DCF sorption onto CNTs.43 The size of the DCF molecules was also a factor that affected the process of sorption (size exclusion phenomenon), as the access for the relatively large DCF molecules, and the steric hindrance to the inner regions of the CNTs, was difficult.11
The affinity of nanotubes to DCF results from the aromaticity and polarity of those materials. Polarity increases DCF adsorption due to the relatively high molecular polarizability and π-energy values of the DCF compared to NPX.51 This was confirmed in this study as the lower polarity of the treated CNTCOOHs52 resulted in reduced sorption. Thermodynamic data show that the energy of DCF bonding is −20 kcal mol−1, and that it is lower for the bonds between aryl carbons and DCF (−18.8 kcal mol−1). Although non-aromatic carbons such as aliphatic carbons and carbonyls have higher values of energy of bonding with DCF, their small amounts in the adsorbent mean that they do not play any key role.51 Therefore, DCF sorption onto CNTCOOHs occurred on the graphitic walls of the CNTs.
Experimental
Chemicals and CNTs
Diclofenac sodium (C14H10Cl2NNaO2, pKa 4.2) and naproxen (C14H14O3, pKa 4.2) were purchased from Sigma-Aldrich, Poland (http://www.poch.com.pl). Multi-walled carbon nanotubes with –COOH groups (referred as CNTCOOH) and –OH groups (referred as CNTOH) were supplied by Timesnano, China (http://www.timesnano.com). The CNTs possessed >95% purity, 10–20 nm outer diameter and 10–30 μm length. The surface area of all studied materials was in the range of 159.1 to 208.8 m2 g−1.52 The content of surface groups (XPS & titration): –OH in CNTOH or –COOH in CNTCOOH was 3.06 wt% and 2.00 wt%, respectively. The procedure of CNT wastewater treatment was described in a previous paper.52 For the treatment of wastewater containing functionalized CNTs, 0.35 wt% addition of H2O2 (POCH, Poland) and/or 5 hours of UV irradiation (254 nm, 15 W) were applied. CNTs after treatment were labelled as follows: CNTOH-UV or CNTCOOH-UV – indicating UV irradiated CNT; CNTOH-H2O2 or CNTCOOH-H2O2 – indicating H2O2 treatment of the CNTs and CNTOH-UV + H2O2 or CNTCOOH-UV + H2O2 – indicating UV and H2O2 treatment.Sorption kinetics
Sorption kinetics were measured using 2 mg of CNTOH or CNTCOOH and 2.5 mg L−1 DCF or NPX in methanol (<0.1%) and biocide solution (0.01 mol L−1 CaCl2, 200 mg L−1 NaN3) at 23 ± 1 °C and pH 7.0 ± 0.2 (0.01 M HCl or NaOH for correction). Samples were shaken on a rotary shaker (150 rpm) and analyzed after 2, 4, 8, 24, 48, 72 and 144 hours. Centrifuged samples (20 min) were analyzed spectrophotometrically (Varian Carry 4000, λDCF = 276 nm, λNPX = 230 nm) and the amount of adsorbed PPCPs was calculated based on the decrease of the solute concentration in the aqueous phase. A blank test without PPCPs was also conducted. LODNPX was estimated at 1.54 mg L−1,53 and LODDCF – at 1.35 × 10−5 mol L−1.54 For the sorption of PPCPs four of the most popular mathematical models of kinetics: pseudo-first and pseudo-second order, Elovich and intraparticle diffusion were applied. The kinetic parameters are presented in Tables 1 and 4.Sorption experiment
A batch equilibration technique at 23 ± 1 °C using 40 mL Teflon centrifuge tubes was applied for sorption studies. The process was conducted in a biocide solution (0.01 M CaCl2, 200 mg L−1 NaN3). Nanotubes (2 mg) were placed in the biocide solution for 24 h at pH 7.0 ± 0.2 (0.01 M HCl or 0.01 M NaOH for correction). The next step was the addition of DCF or NPX (1.25–25 mg L−1) in methanol (<0.1%). After 5 days the solutions were centrifuged (1000g for 30 min) and filtered (0.45 μm) to separate the CNT from the supernatant.A UV-Vis Varian Cary 4000 spectrophotometer was used for the determination of the concentration of DCF and NPX in the solution (276 nm and 232 nm, respectively; scan rate 600 nm min−1; time response: 0.1 s; spectral band 2 nm). The linear correlation between absorbance and concentration was tested using tested concentrations with the linear response over the whole range of considered concentrations. Each concentration point, including blanks, was run in duplicate. Freundlich, Langmuir, Temkin, and Dubinin–Radushkevich55 isotherms were applied for sorption studies (ESI†).
Conclusions
From the obtained data it can be concluded:(1) Adsorption of NPX onto non-treated CNTOHs proceeded according to the pseudo-second order model indicating chemisorption as the rate limiting step. Treatment changed the kinetics to a pseudo-first order (CNTOH-UV and CNTOH-H2O2) or an intraparticle diffusion model (CNTOH-UV + H2O2).
(2) Heterogeneous sorption of NPX onto CNTOHs (Freundlich model) was observed. NPX sorption onto treated CNTCOOHs was ascribed to the Freundlich or the Temkin model.
(3) Sorption of NPX onto CNTOHs was governed by π–π interactions, and that onto CNTCOOHs was governed by functionalization.
(4) CNTCOOHs revealed higher sorption capacity for DCF and NPX than CNTOHs.
(5) DCF sorption onto CNTOHs proceeded according to the pseudo-second order regime. Treatment by UV or H2O2 resulted in changed kinetics (the pseudo-first order model).
(6) DCF sorption kinetics observed over non-treated CNTCOOHs were ascribed to the intraparticle diffusion model. Treatment changed the kinetics, and the sorption onto treated CNTCOOHs proceeded according to the pseudo-first order regime.
(7) Freundlich and Dubinin–Radushkevich models revealed the best fitting for description of DCF sorption onto CNTOHs and CNTCOOHs.
(8) The process of DCF sorption onto CNTOHs was governed by electrostatic forces or functionalization of CNTCOOHs.
References
- F. Gottschalk, T. Sonderer, R. W. Scholz and B. Nowack, Environ. Sci. Technol., 2009, 43, 9216–9222 CrossRef CAS PubMed.
- D. Lin and B. Xing, Environ. Sci. Technol., 2008, 42, 5917–5923 CrossRef CAS PubMed.
- D. Lin, T. Li, K. Yang and F. Wu, J. Hazard. Mater., 2012, 241–242, 404–410 CrossRef CAS PubMed.
- S. Ma, C. Liu, K. Yang and D. Lin, Sci. Total Environ., 2012, 439, 123–128 CrossRef CAS PubMed.
- X. Tian, T. Li, K. Yang, Y. Xu, H. Lu and D. Lin, Chemosphere, 2012, 89, 1316–1322 CrossRef CAS PubMed.
- S. Li, U. Turaga, B. Shrestha, T. A. Anderson, S. S. Ramkumar, M. J. Green, S. Das and J. E. Cañas-Carrell, Ecotoxicol. Environ. Saf., 2013, 96, 168–174 CrossRef CAS PubMed.
- I. Lerman, Y. Chen, B. Xing and B. Chefetz, Environ. Pollut., 2013, 182, 169–176 CrossRef CAS PubMed.
- S. Mukherjee, B. Kundu, A. Chanda and S. Sen, Ceram. Int., 2015, 41, 3766–3774 CrossRef CAS.
- X. Li, H. Zhao, X. Quan, S. Chen, Y. Zhang and H. Yu, J. Hazard. Mater., 2011, 186, 407–415 CrossRef CAS PubMed.
- M. Kah, X. Zhang and T. Hofmann, Sci. Total Environ., 2014, 497–498, 133–138 CrossRef CAS PubMed.
- O. G. Apul and T. Karanfil, Water Res., 2015, 68, 34–55 CrossRef CAS PubMed.
- B. Pan and B. Xing, Environ. Sci. Technol., 2008, 42, 9005–9013 CrossRef CAS PubMed.
- F. Gottschalk and B. Nowack, J. Environ. Monit., 2011, 13, 1145–1155 RSC.
- I. Schwyzer, R. Kaegi, L. Sigg, A. Magrez and B. Nowack, Environ. Pollut., 2011, 159, 1641–1648 CrossRef CAS PubMed.
- P. Oleszczuk, P. Bo and X. Baoshan, Environ. Sci. Technol., 2009, 43, 9167–9173 CrossRef CAS PubMed.
- P. Oleszczuk and B. Xing, Chemosphere, 2011, 85, 1312–1317 CrossRef CAS PubMed.
- B. Nowack, J. F. Ranville, S. Diamond, J. A. Gallego-Urrea, C. Metcalfe, J. Rose, N. Horne, A. A. Koelmans and S. J. Klaine, Environ. Toxicol. Chem., 2012, 31, 50–59 CrossRef CAS PubMed.
- R. Das, S. B. Abd Hamid, M. E. Ali, A. F. Ismail, M. S. M. Annuar and S. Ramakrishna, Desalination, 2014, 354, 160–179 CrossRef CAS.
- J. B. Ellis, Environ. Pollut., 2006, 144, 184–189 CrossRef CAS PubMed.
- E. Diamanti-Kandarakis, J.-P. Bourguignon, L. C. Giudice, R. Hauser, G. S. Prins, A. M. Soto, R. Thomas Zoeller and A. C. Gore, Endocr. Rev., 2009, 30, 293–342 CrossRef CAS PubMed.
- J. Xu, L. Wu and A. C. Chang, Chemosphere, 2009, 77, 1299–1305 CrossRef CAS PubMed.
- T. A. Ternes, Water Res., 1998, 32, 3245–3260 CrossRef CAS.
- M. Hu, X. Liu, F. Dong, J. Xu, S. Li, H. Xu and Y. Zheng, Food Chem., 2015, 175, 395–400 CrossRef CAS PubMed.
- A. A. Keller and A. Lazareva, Environ. Sci. Technol. Lett., 2014, 1, 65–70 CrossRef CAS.
- S. H. Joo and B. Tansel, J. Environ. Manage., 2015, 150, 322–335 CrossRef CAS PubMed.
- H. Maegawa, T. Nakamura and K. Saito, J. Ethnopharmacol., 2014, 158, 511–515 CrossRef PubMed.
- A. Cincinelli, T. Martellini, E. Coppini, D. Fibbi and A. Katsoyiannis, J. Nanosci. Nanotechnol., 2015, 15, 3333–3347 CrossRef CAS PubMed.
- C. I. Kosma, D. A. Lambropoulou and T. A. Albanis, J. Hazard. Mater., 2010, 179, 804–817 CrossRef CAS PubMed.
- A. Nikolaou, S. Meric and D. Fatta, Anal. Bioanal. Chem., 2007, 387, 1225–1234 CrossRef CAS PubMed.
- W. Schmidt and C. H. Redshaw, Ecotoxicol. Environ. Saf., 2015, 112, 212–222 CrossRef CAS PubMed.
- E. N. Evgenidou, I. K. Konstantinou and D. A. Lambropoulou, Sci. Total Environ., 2015, 505, 905–926 CrossRef CAS PubMed.
- Y. Luo, W. Guo, H. H. Ngo, L. D. Nghiem, F. I. Hai, J. Zhang, S. Liang and X. C. Wang, Sci. Total Environ., 2014, 473–474, 619–641 CrossRef CAS PubMed.
- D. Kołodyńska, R. Wnętrzak, J. J. Leahy, M. H. B. Hayes, W. Kwapiński and Z. Hubicki, Chem. Eng. J., 2012, 197, 295–305 CrossRef.
- M. B. Desta, J. Thermodyn., 2013, 2013, 1–6 CrossRef.
- O. Hamdaoui and E. Naffrechoux, J. Hazard. Mater., 2007, 147, 381–394 CrossRef CAS PubMed.
- S. Zhang, T. Shao, H. S. Kose and T. Karanfil, Environ. Toxicol. Chem., 2012, 31, 79–85 CrossRef CAS PubMed.
- I. Mobasherpour, E. Salahi and M. Pazouki, Arabian J. Chem., 2012, 5, 439–446 CrossRef CAS.
- M. Cleuvers, Ecotoxicol. Environ. Saf., 2004, 59, 309–315 CrossRef CAS PubMed.
- B. Pan, K. Sun and B. Xing, J. Soils Sediments, 2010, 10, 845–854 CrossRef CAS.
- P. S. Kumar, K. Ramakrishnan, S. D. Kirupha and S. Sivanesan, Braz. J. Chem. Eng., 2010, 27, 347–355 CAS.
- R. Khatem, R. O. Miguel and A. Bakhti, Eurasian Journal of Soil Science, 2015, 4, 126–136 CrossRef.
- A. O. Dada, A. P. Olalekan, A. M. Olatunya and O. Dada, IOSR J. Appl. Chem., 2012, 3, 38–45 CrossRef.
- H. Wei, S. Deng, Q. Huang, Y. Nie, B. Wang, J. Huang and G. Yu, Water Res., 2013, 47, 4139–4147 CrossRef CAS PubMed.
- Z.-H. Xiong, L. Wang, J.-G. Zhou and J.-M. Liu, Acta Phys.-Chim. Sin., 2006, 26, 2890–2898 Search PubMed.
- A. V. Ellis, S. Wallace and W. M. Arnold, Mater. Forum, 2007, 31, 110–115 CAS.
- M. Li, T.-C. Hsieh, R.-A. Doong and C. P. Huang, Sep. Purif. Technol., 2013, 117, 98–103 CrossRef CAS.
- D. Lin, N. Liu, K. Yang, L. Zhu, Y. Xu and B. Xing, Carbon, 2009, 47, 2875–2882 CrossRef CAS.
- F. Yu, Y. Wu and J. Ma, J. Hazard. Mater., 2012, 237–238, 102–109 CrossRef CAS PubMed.
- X. Qu, P. J. J. Alvarez and Q. Li, Environ. Sci. Technol., 2013, 47, 14080–14088 CrossRef CAS PubMed.
- B. Smith, J. Yang, J. L. Bitter, W. P. Ball and D. H. Fairbrother, Environ. Sci. Technol., 2012, 46, 12839–12847 CrossRef CAS PubMed.
- C. Jung, L. K. Boateng, J. R. V. Flora, J. Oh, M. C. Braswell, A. Son and Y. Yoon, Chem. Eng. J., 2015, 264, 1–9 CrossRef CAS.
- B. Czech, P. Oleszczuk and A. Wiącek, Environ. Pollut., 2015, 200, 161–167 CrossRef CAS PubMed.
- S. R. Dharmalingam, S. Ramamurthy, K. Chidambaram and S. Nadaraju, Int. J. Anal., Pharm. Biomed. Sci., 2013, 2, 49–55 CAS.
- E. G. Ciapina, A. O. Santini, P. L. Weinert, M. A. Gotardo, H. R. Pezza and L. Pezza, Ecletica Quim., 2005, 30, 29–36 CrossRef CAS.
- M. Ahmad, S. S. Lee, A. U. Rajapaksha, M. Vithanage, M. Zhang, J. S. Cho, S.-E. Lee and Y. S. Ok, Bioresour. Technol., 2013, 143, 615–622 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Description of chemicals and CNTs, sorption models, sorption kinetics (figures and tables), isotherms and linear parameters of isotherms. See DOI: 10.1039/c6ra23732k |
| This journal is © The Royal Society of Chemistry 2016 |