Towards the electrochemical diagnosis of cancer: nanomaterial-based immunosensors and cytosensors
Ülkü Anik*a and
Suna Timur*b
aMugla Sitki Kocman University, Faculty of Science, Chemistry Department, 48000 Mugla, Turkey. E-mail: ulkuanik@yahoo.com; ulkuanik@gmail.com
bEge University, Faculty of Science, Biochemistry Department, 35100 Bornova, İzmir, Turkey. E-mail: suna.timur@ege.edu.tr
First published on 21st November 2016
Abstract
In this review, nanomaterial based electrochemical biosensors including electrochemical immunosensors and cytosensors towards cancer detection are covered. Brief definitions of biosensors together with nanomaterials are presented. Electrochemical biosensors for cancer biomarkers and cytosensors are listed. Also, the potential of adaptation of these biosensors to point of care systems is discussed.
General approach to electrochemical biosensors
Biosensors can be described as analytical devices which use a recognition element, generally a biomolecule, to bind an analyte and some transduction mechanisms to detect this binding event. Since biosensors are specific, economical and practical, they provide advantages in many areas including clinical applications.1a–e If an electrode system is used in transduction mechanism as a transducer, then an electrochemical biosensor is obtained. Nowadays, in order to increase sensitivity and selectivity of electrochemical applications, chemical modification and functionalization of electrodes have been conducted while in the old years, the development of new electrochemical techniques was important for the same manner.2 In terms of modification, nanomaterials including nanotubes, metallic nanoparticles, nanoballs and nanolaminates have been introduced into electrode structure.3–7Nanomaterials exhibit novel behaviors including quantum size effect, optical, catalytical and electrochemical properties which largely differ from the bulk materials due to their small size.8–13a,b Recent developments about attractive properties of nanomaterials increase interest about their applicability in biosensing areas that results with great progress in development of nanomaterial based biosensors. The use of nanostructured materials for the organization of electrochemical sensing devices is an extremely promising prospect because they provide electrocatalytic effect together with higher surface area which is very important in terms of enzyme immobilization in electrochemical biosensors. Besides, the conductivity properties of nanoparticles at nanoscale dimensions allow the electrical contact of redox-centers in proteins with electrode surfaces.14,15 On the other hand, early diagnosis of cancer is crucial for patient survival hence successful treatment of the disease. Sensitive and specific methods are required for early cancer diagnosis. Existing diagnostic tests (e.g., ELISA) are not sensitive enough and detect proteins at levels corresponding to advanced stages of the disease. Practical, faster and economic methods are needed. For this reason, usage of electrochemical techniques like electrochemical biosensors for cancer diagnosis is meaningful.1a,b Also the potential of including biosensors in point of care (POC) systems is another promising aspect. There are wide usage areas of application of biosensors for cancer detection. Recently, bioanalytical approaches have experienced unprecedented progress, driven in huge part by the necessity for rapid, more sensitive, portable-POC-systems to analyse tumour related biomarkers and cancer cells for clinical diagnosis. Electrochemical detection strategies in conjunction with immunosensors and cytosensors, offer the possibility of design of fast, simple, low cost and effective systems.1b In this review, only part of them, electrochemical immunosensors for detection of cancer biomarkers as well as electrochemical cytosensors will be discussed. Also the potential of usage of biosensors in cancer diagnostic POC systems will be mentioned.
Electrochemical detection strategies towards cancer biomarkers
Nanomaterial based electrochemical immunosensors
Immunosensors are kind of biosensors where interactions between antigen and antibody are monitored by means of an electrochemical transducer. In the case of electrochemical immunosensing, an electrode is used as a transducer where an electrical signal is obtained. On the other hand, a biomarker can be described as a kind of indicator for normal biological processes, pathogenic processes, or pharmacological responses to therapeutic intervention.16 Various strategies using secondary antibodies (Ab2) labelled with nanoparticles in sandwich type immunosensors containing nanomaterials as well as nanoparticles with multiple biomolecular labels or redox probes are well documented in the literature.17Depending on the bio-functionalization of nanomaterials with proper and selective bio-recognition elements during the fabrication process, nanomaterial-based immunoassay platforms yield high signal amplification in electrochemical reactions resulting with higher sensitivity in analyte detection (Fig. 1).
 | ||
| Fig. 1 Amplification strategies for electrochemical immunosensors using nanomaterials or other labels combined to secondary antibody (Ab2). Images (a)–(g) are reproduced with permission from ref. 17, Copyright Royal Society of Chemistry (UK), 2010. | ||
The abnormal concentrations of these kinds of proteins which are mainly overexpressed due to the physiological conditions can indicate the presence of various cancers. The development of fast and sensitive methodologies towards tumour-associated antigens is important for evaluating their roles in cancer immunology as well as clinical diagnostics. Therefore, detection of serum protein biomarker level provides the existence of various tumors besides following the tumor re-occurrence.18 For instance, carcinoembryonic antigen (CEA), is typically a cancer biomarker and detection of CEA levels in adults is very important in terms of early cancer diagnosis.19 Various types of methods have been developed for detecting cancer biomarkers, like ELISA,20 radioimmunoassay,21 electrophoretic immunoassay22 and mass spectrometry-based proteomics.23 However, these techniques require complicated instrumentation, qualified personnel, significant sample volumes, and they are expensive as well as have limited sensitivity. For this reason, electrochemical techniques are preferred since they are simple, rapid, sensitive and economical. Various kinds of voltammetric techniques such as linear sweep, differential pulse (DPV), squarewave, stripping, cyclic voltammetry (CV), amperometry and also electrochemical impedance spectroscopy (EIS) techniques are the most widely used electrochemical detection methods in these biosensor applications. In immunosensors, formation of antibody–antigen complex is probed with an electrochemical compound, enzyme or nanomaterial.18 Briefly, the principle of the fabrication and operation procedures as follows: (i) a tracer antibody is labelled with an proper electroactive species or enzyme; (ii) the tracer is allowed to capture the target analyte (a primary antibody is preferred), and thus, immobilized on the electroactive surface; (iii) the concentration of the targeted biomarker is quantified by applying a potential and measuring the resulting current at the electrode. In principle, the applied potential drives a redox reaction of the labelled electroactive species and provides a current signal that is proportional to the amount of antibody-captured analyte.1b Moreover, the combination of electrochemical platforms with nanomaterials, such as gold nanoparticles (AuNP), carbon nanotubes (CNT), magnetic particles, and quantum dots (QD), offers multiplexing capability for simultaneous measurements of multiple cancer biomarkers. An example for the possible surface modifications, construction of biorecognition interfaces for the electrochemical detection is summarized in Scheme 1.
 | ||
| Scheme 1 Possible surface modifications, biorecognition interfaces for the electrochemical biomarker detection. | ||
In one study, AuNP and nickel hexacyanoferrates nanoparticles (NiHCFNPs) were used for determination of CEA in clinical immunoassay.19 As a type of nanomaterials, AuNPs are one of the most utilized metallic nanoparticles in electroanalytical applications. AuNP labels have numerous advantages in compared to radioisotopic and enzyme labels. They are chemically stable or less hazardous, their fabrication is more straightforward, and their biocompatibility allows them not to interfere with the labelled biomolecule.13b They also provide suitable microenvironment similar to that of redox protein with more freedom in orientation. Besides these important features, it is claimed that this nanoparticle reduces the insulating effect of the protein shell by providing direct electron transfer through the conducting tunnels of gold nanocrystals. It is stated that the nanomeric edges of AuNPs penetrate the insulating shell of enzyme. By this way, the distance between the electrode and biomolecular redox sites for electron transfer is decreased.11,14,24–31 In the above mentioned study, a non-composite carbon based electrode, glassy carbon electrode (GCE) was used as a transducer. Then AuNP was immobilized on the surface of bare GCE by means of electrochemical reduction of HAuCl4 solution. NiHCFNPs, an electroactive substance was immobilized on the layer of AuNP. After that, AuNP was immobilized on the surface of NiHCFNPs. As electrochemical techniques, EIS and CV were utilized. Under optimal conditions, the peak current of CV of the immunosensor decreased linearly with increasing CEA concentration in two ranges from 0.5 to 10 ng mL−1 and from 10 to 160 ng mL−1, with a limit of detection (LOD) of 0.1 ng mL−1.19 In the other work; a practical electrochemical immunosensor design for the human lung cancer-associated antigen, α-enolase (ENO1) was reported. In that platform, a polyethyleneglycol layer and subsequently primary monoclonal anti-ENO1 antibody were immobilized on the screen printed carbon electrode. Anti-ENO1-tagged AuNP was used as bioprobes as signal amplifiers to improve the sensitivity of the assay.13b A schematic representation for the operation of ENO1 immunoassay which is also one of the examples for signal amplification by AuNP bioprobes, is provided in Fig. 2.
 | ||
| Fig. 2 A schematic representation for the operation of ENO1 immunoassay. Images are reproduced with permission from ref. 13b, Copyright American Chemical Society (US), 2010. | ||
Carboxylated single-wall carbon nanotubes (SWNT) bundles onto the Nafion–iron oxide decorated conductive surfaces was also reported for the fabrication two different immunosensors.32 In the first design, a primary antibody on the SWNT captures antigen in the sample, which in turn, interacts a peroxidase (HRP)-labelled antibody. Amperometric signals are occurred as a result of adding hydrogen peroxide to the medium (Fig. 3A). In the other design; the immunosensor was treated with HRP–CNT–Ab2 instead of HRP labelled Ab2 to obtain signal amplification (Fig. 3B).
 | ||
| Fig. 3 Representation of detection strategies of SWNT immunosensors: on the bottom left is a tapping mode atomic force microscope image of a SWNT forest onto the electroactive surface as immunosensor platform. Above this on the left is a schematic representation of a SWNT immunosensor (HRP is the enzyme label). At (A) on the right shows the immunosensor after treating with HRP–Ab2. At (B) on the right shows the immunosensor after treating with HRP–CNT–Ab2 to obtain signal amplification. Images are reproduced with permission from ref. 32, Copyright American Chemical Society Chemistry (US), 2006. | ||
In another study, an electrochemical biosensor based on a graphene platform was prepared.18 For this immunosensor, graphene was combined with magnetic beads (MBs) and enzyme labeled antibody–AuNP bioconjugate. Graphene is an excellent nanomaterial for electrochemical applications since it provides excellent conductivity and electrocatalytic activity.33–36 It has two-dimensional sheets of sp2-hybridized C atoms in hexagonal configuration.37 Because of these sheets, very suitable, a two dimensional environment for electron transfer has been offered. Also they give fast electron transfer at the edges. For this reason, graphene has been widely used for construction of cancer biomarkers biosensors.38,39 On the other hand, nanoscale structures of MBs provide fast reaction kinetics, good stability and due to their small diameter, high surface area. Since they can be easily modified with proper functional groups, MBs have extensively been used in variety of electrochemical immunoassay systems.40,41 In another study by Jin et al., MBs were coated with capture antibodies (primary antibody; Ab1) and they were attached to graphene sheets by means of an external magnetic field.18 Target antibody (Ab2) was then, combined with HRP modified AuNPs and Ab2–AuNPs–HRP bio-conjugate was formed. By the usage of “multi-nanomaterial”, it was believed that electron transfer between the electrode and analyte target was accelerated. As a result, LOD value in that study for CEA was calculated as 5.0 ng mL−1.18 Considering graphene with combination with Au and Ag, Huang et al. developed an immunoassay based on chemically functionalized Ag/Au nanoparticles coated on graphene for the detection of CEA. In that work, formerly synthesized Ag/Au nanoparticles provide electrocatalytic activity which provides signal amplification for the fabrication of the “Sandwich-type” immunosensor. Under optimal experimental conditions, the linear range was found between 10 to 1.2 × 105 pg mL−1 with a LOD of 8.0 pg mL−1.42
The other Huang et al., have been developed an amperometric immunosensor for the detection of α-fetoprotein (AFP), which is a kind of glycoprotein cancer biomarker. TiO2–graphene (TiO2–Gr), chitosan and AuNPs composite film modified GCE was served as the developed immunosensors' transducer. AFP antibody was immobilized onto the negatively charged AuNPs, which were adsorbed on the positively charged chitosan/TiO2–Gr composite film by using electrostatic interactions. The more antigens were attached onto the antibody, the more DPV signal decrease was observed due to the inhibition of electron transfer reactions. All the process was monitored in DPV mode by using Fe(CN)63−/4− as a water soluble redox probe. As a result under optimal experimental conditions, wide linear range like 0.1–300 ng mL−1 with sensitive LOD value, 0.03 ng mL−1 was obtained.43 Besides graphene, the other nanoparticles, like metallic nanoparticles have also been used for these systems. Another CEA biosensor was developed by our group which included a nanomaterial based electrochemical immunosensor towards the detection of CEA.44 For this purpose, one of the most popular carbon composite electrodes, carbon paste electrode (CPE) was modified with titanium(IV) oxide micro-particles by adding the proper amount during paste electrode formation. AuNPs were dispersed in chitosan and immobilized on CPE surface via chitosan membrane. As a result a nanostructure modified composite electrode was obtained. Then CEA antibody was attached onto the electrode surface and the binding of CEA antigen was followed via [Fe(CN)6]3−/4− redox pair by means of CV and EIS. Two linear ranges of 0.01–1.0 ng mL−1 and 1.0–20 ng mL−1 CEA with corresponding correlation coefficients R2 = 0.986 and R2 = 0.990, were found respectively. LOD was calculated as 0.01 ng mL−1 with 2.8% relative standard deviation, (n = 5).44 In the other report, human epidermal growth factor receptor 2 (HER2) and HER2-overexpressing breast cancer cells were determined by using an electrochemical immunosensor where hydrazine and aptamer-conjugated AuNPs were used in surface design. The hydrazine–AuNP–aptamer bioconjugate, in which the hydrazine was directly attached to AuNPs to prevent the non-specific deposition of silver on the surface, was prepared and used to reduce silver ion for signal amplification. The silver-stained target cells were then, quantitatively analyzed using stripping voltammetry. The surface can discriminate HER2-positive and HER2-negative cell lines with a LOD of 26 cells per mL.45
The simultaneous detection of multiple biomarkers that could be termed as ‘Multiprotein detection’ protocols using multiple labels as well as multiple electrodes are also well reported in the previous works.1b For instance, in one of the works, MBs coated with various primary antibodies are used to recognize their antigens. Then, secondary antibodies labelled with different inorganic nanocrystal labels bind to the corresponding antigens. Subsequent acid dissolution of the nanocrystals, the resultant ions are detected by electrochemical stripping technique.1c Also, MBs labelled with multi-enzymes and secondary antibodies were used to capture specific analytes on a sandwich immunoassay using an eight-electrode array platform.1d Some of the other nanomaterial based electrochemical biosensors that were used for cancer biomarker detection are summarized in Table 1.46–67
| Cancer marker | Nanomaterial | Electrochemical method | LOD | Linear range | References |
|---|---|---|---|---|---|
| a DPV: differential pulse voltammetry; CV: cyclic voltammetry; EIS: electrochemical impedance spectroscopy; SWV: square wave voltammetry; AuNPs: gold nanoparticles. | |||||
| Mucin 1 | Poly(o-phenylene diamine)–AuNPs hybrid film as carrier and AuNPs functionalized silica/multiwalled carbon nanotubes core–shell nanocomposites as tracing tag | DPV, CV and EIS | 1.0 pM | 1.0–100 nM | 65 |
| Carcinoembryonic antigen (CEA) and cancer antigen 125 (CA125) | Au-nanorods modified paper working electrode | DPV | 0.08 pg mL−1 for CEA and 0.06 mU per mL for CA125 | 0.1 pg mL−1 to 50 ng mL−1 for CEA and 0.1 mU per mL to 50 U per mL for CA125 | 66 |
| Carbohydrate antigen 72-4 | Nanoporous gold film as the sensor platform and polyaniline–Au asymmetric multicomponent nanoparticles as labels | EIS | 0.10 U per mL−1 | 2.0–200 U per mL−1 | 67 |
| CEA and squamous cell carcinoma antigen (SCCA) | Reduced graphene oxide–tetraethylene pentamine and Au@mesoporous carbon CMK-3 | CV, DPV and EIS | 0.013 ng mL−1 for CEA and 0.010 ng mL−1 for SCCA | 0.05–20 ng mL−1 for CEA and 0.03–20 ng mL−1 for SCCA | 61 |
| α-Fetoprotein (AFP) | Mesoporous silica nanoparticles loaded with ferroferric oxide nanoparticles | CV | 4.0 pg mL−1 | 0.01–25 ng mL−1 | 62 |
| AFP | Functionalized single-walled carbon nanohorns | CV and EIS | 0.33 pg mL−1 | 0.001–60 ng mL−1 | 63 |
| CA125 and carcinoma antigen 199 (CA199) | Cuboid silver modified paper working electrode/different metal ions-coated nanoporous silver–chitosan | SWV | 0.08 U per mL−1 for CA125 and 0.10 U per mL−1 for CA199 | 0.1–100 U per mL−1 for both | 64 |
| CEA | AuNP decorated graphene composites | DPV | 0.04 ng mL−1 | 0.10–80 ng mL−1 | 58 |
| CA125 | AuNP modified screen printed graphite electrode | EIS | 6.7 U per mL−1 | 0–100 U per mL−1 | 59 |
| Prostate-specific antigen (PSA) | Ag hybridized mesoporous silica nanoparticles | CV | 15 pg mL−1 | 0.05–50.0 ng mL−1 | 60 |
| PSA | Nanocomposite film of graphene sheets–methylene blue–chitosan | CV | 13 pg mL−1 | 0.05–5.00 ng mL−1 | 54 |
| CEA | Hollow Pt nanospheres | DPV, CV | 1.0 pg mL−1 | 0.001–100 ng mL−1 | 55 |
| Neuron-specific enolase (NSE) | Nickel hexacyanoferrates nanoparticles | CV | 0.3 pg mL−1 | 0.001–100 ng mL−1 | 56 |
| PSA free prostate specific antigen (fPSA) | AuNPs modified Prussian blue nanoparticles decorated onion-like mesoporous graphene sheets | CV, DPV | 6.7 pg mL−1 for fPSA and 3.4 pg mL−1 for PSA | 0.02–10 ng mL−1 for fPSA and 0.01–50 ng mL−1 for PSA | 57 |
| Progastrin releasing-peptide (ProGRP) | Spherical AuNP capped nano-TiO2 composite nanoparticles and nano-Au embedded cysteine/Nafion–graphene | CV | 3.0 pg mL−1 | 10.0–500 pg mL−1 | 51 |
| AFP | Horseradish peroxidase labelled carbon nanotubes | CV | 0.067 ng mL−1 | 0.2–200 ng mL−1 | 52 |
| Human serum chorionic gonadotropin | Nanoporous Au foils and graphene sheets | CV | 0.034 ng mL−1 | 0.5–40.00 ng mL−1 | 53 |
| AFP | Magnetic Fe3O4@Au composite nanomaterial | EIS | 0.13 ng mL−1 | 0.4–50 ng mL−1 | 48 |
| PSA | Fe3O4 nanoparticles loaded poly(ethylene glycol)–poly(lactic acid) | CV | 2.0 pg mL−1 | 0.005–10 ng mL−1 | 49 |
| CEA | Nano Au-enwrapped graphene nanocomposites | EIS | 0.01 ng mL−1 | 0.05–350 ng mL−1 | 50 |
| AFP | AuNPs and carbon nanotubes doped chitosan film | CV, EIS | 0.6 ng mL−1 | 1.0–55 ng mL−1 | 47 |
| PSA | CdSe@ZnS | SWV | 0.02 ng mL−1 | 0.05–4.0 ng mL−1 | 46 |
Nanomaterial based electrochemical cytosensors
Cytosensors are kind of biosensors that use living cells as the recognition elements. Besides detection of cancer biomarkers, direct electrochemical detection of cancer cell is another way to use the electrochemical biosensors in these areas. Polymeric matrices which could be easily covered on the desired surfaces in various geometry by electrochemical deposition have been reported as attractive and interesting platforms for the investigation of cell–surface interactions.68,69 The development of rapid and simple techniques for the detection of cancer cells with high sensitivity is of great importance in preclinical testing studies. For these reasons novel approaches like nanomaterial based electrochemical cytosensors have been developed. In one study, gold electrode was modified with folic acid-functionalized AuNP for detecting folate receptor-rich HeLa cells. For this cytosensor, after the immobilization of AuNP onto Au electrode, 11-mercaptoundecanoic acid (MUA) was attached onto this modified surface. Folic acid, which recognizes HeLa cells, was then linked to the MUA layer and as a result Au electrode/AuNP/MUA/folic acid was formed. This structure was used to detect folic acid-rich HeLa cells. The measurement was done via EIS. The principle behind the EIS measurement was that the HeLa cells essentially prevents charge transfer from the electrode to the bulk electrolyte when they were adsorbed on the gold electrode/AuNP/MUA/folic acid electrode surface. The HeLa cells adsorbed on the electrode resulted in an increase in the electrode impedance. It was reported that with this cytosensor, LOD of 6.0 cells per mL was reached.70In another study, a sandwich-type electrochemical aptamer cytosensor which includes AuNP together with hybrid nanoelectrocatalysts, was developed for detection of human liver hepatocellular carcinoma cells (HepG2). First, AuNP was formed electrochemically onto GCE and thiolated TLS11a aptamers were attached onto AuNPs. For, the electrochemical nanoprobes Au@Pd core–shell nanoparticle-modified magnetic Fe3O4/MnO2 beads (Fe3O4/MnO2/Au@Pd) were used and G-quadruplex/hemin/aptamer complexes and HRP was immobilized on these nanomaterials. The cancer cells were captured onto thiolated aptamers and then the hybrid nanoprobes were attached onto these cells to obtain an aptamer–cell-nanoprobes sandwich-like system on the electrode surface. It is stated that hybrid nanoprobe was used to catalyse the oxidation of hydroquinone with H2O2 and getting more sensitive results. As a result, a wide detection range of 1.0 × 102 to 1.0 × 107 cells per mL and LOD value of 15.0 cells per mL were obtained.71 The other two HeLa cells based cytosensors were conducted by our group.6,72 For these two cytosensors, glassy carbon paste electrode (GCPE) was used as supporting electrode and AuNP, cysteamine (Cys), glutaraldehyde (Glu), polyamidoamine (PAMAM) dendrimer and folic acid were immobilized onto it, respectively. However, in one work this system was combined with centrifuge and cytocentri-voltammetry was developed.72 Centri-voltammetry was a novel method and developed by our group. It involves the combination of centrifugal force with voltammetry in a specifically fabricated centri-voltammetric cell and with the application of this method; more sensitive results were obtained.73 Under the optimal experimental conditions the GCPE/AuNP/Cys/Glu/PAMAM/folic Acid cytosensor without any centrifugation showed a linear range of between 102 cells per mL and 106 cells per mL with LOD value of 100 cells per mL.6 When this system was combined with centrifuge, in another word, when cytocentri-voltammetry was applied, the linear range was found between 8.0 cells per mL and 5.0 × 106 cells per mL with LOD value of 8 cells per mL.72 Carbon nanotubes (CNT) were also used for cytosensor design. In terms of electroanalytical and bioanalytical applications, CNTs have extensively been used as electrode material or as a modifier of electrodes because of their electrocatalytic activities.74 Wang and his colleagues used folate conjugated poly ethylenimine–carbon nanotubes (folate–PEI–CNT) in an impedimetric cytosensor fabrication for HeLa cells and they obtained very sensitive results. It is reported the LOD value and a linear detection range were found as 90 cells per mL and from 2.4 × 102 to 2.4 × 105 cells per mL.75 Additionally, design, synthesis and modification of cell selective surfaces that provides for both electrochemical and optical sensing of cancerous cells are also reported in the literature. The selective surfaces based on the folic acid modified clay/polymer nanocomposites have been used for discrimination of folate receptor positive and negative cell lines.76 Similar strategies were also used to verify cell selective adhesion by using bio-active block copolymers prepared by CuAAC/thiol–ene double click reactions.77 In the other study, an electrochemical cytosensor was fabricated for drug-resistant leukemia K562/ADM cells based on the P-glycoprotein (P-gp) expression level on cell membrane. The nanocomposite interface of the gold nanoparticles/polyaniline nanofibers was used in the surface design and then, anti-P-glycoprotein molecules that could provide a biomimetic interface for the immunosensing of cell surface P-glycoprotein was used biomodifier to selective capture of the over-expression P-gp cells.1e The schematic representation of nanocomposite/biomimetic interface and the operation procedure is shown in Fig. 4. The other selected and recently reported electrochemical cytosensors are given in Table 2.78–84
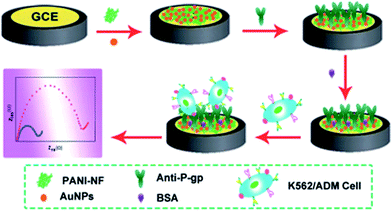 | ||
| Fig. 4 Schematic illustration of label-free cytosensor reproduced with permission from ref. 1e, Copyright Royal Society of Chemistry (UK), 2014. | ||
| Cells | Nanomaterial | Electrochemical method | LOD | Linear range | References |
|---|---|---|---|---|---|
| Leukemia cells (HL-60) | AuNP | EIS and DPV | 350 cells per mL | 5.0 × 102 to 7.5 × 107 cells per mL | 84 |
| HeLa cells | AuNP | CV and DPV | 10 cells per mL | 1.0 × 101 to 1.0 × 106 cells per mL | 82 |
| HeLa cells | Polyaniline nanofibers (PANI-NF), AuNP | CV and EIS | 2000 cells per mL | 1.0 × 104 to 6.4 × 106 cells per mL | 81 |
| K-562 leukemic cells | AuNP | CV and EIS | 73 cells per mL | 1.0 × 102 to 1.0 × 107 cells per mL | 80 |
| Breast cancer cells (MCF-7) | Cadmium sulphur nanoparticle (CdS) | SWV and EIS | 3.3 × 102 cells per mL | 1.0 × 104 to 1.0 × 107 cells per mL | 79 |
| Gastric carcinoma cells (BGC-823) | Single-walled nanotubes (SWNTs) | CV and DPV | 620 cells per mL | 1.0 × 103 to 1.0 × 107 cells per mL | 78 |
| Leukemia cells (HL-60) | Graphene | CV and EIS | 500 cells per mL | 5.0 × 102 to 5.0 × 106 cells per mL | 83 |
Point of care cancer diagnostic systems
POC systems are considered as integrated systems that could analyze physiological samples or fluids with different types of biomarkers in clinical laboratories and even at home. Principally, POC systems provide the clinician the capability to have access to a wealth of molecular information to identify cancer profiles via novel and efficient technologic platforms that have been found at only major cancer centres in the past.85,86 POC systems cover the rapid, economical and practical diagnostic tests that can be applied on site by anyone without needing any specialist. Considering the electrochemical biosensors which provide easy, sensitive and rapid detections, it is obvious that these systems are good candidates to be used in POC analytical platforms. These are extremely useful for providing bio-diagnostic data in a practical and low cost manner in connection to POC analysers. For obtaining these integrated systems, biosensors are usually combined with Lab-on-a-Chips (LOC), microfluidics and other techniques like arrays.17 Schematic illustration of an immuno-array strategy designed to detect cancer biomarkers is shown in Fig. 5. | ||
| Fig. 5 Illustration of an immuno-array strategy based on gold nanoparticles providing high spot areas, for cancer biomarkers (PSA: prostate specific antigen). After washing step, a labelled secondary antibody, or a multi-labelled nanoparticle with secondary antibody, is added to the medium. This species binds to another site on the captured analytes. After additional washing step, either electrical or optical detection is used to observe the amount of bound labels which is proportional to analyte concentration. Images are reproduced with permission from ref. 17, Copyright Royal Society of Chemistry (UK), 2010. | ||
The i-STAT hand-held battery-operated and user-friendly clinical analyser, integrating various electrochemical biosensors and potentiometric on a single disposable cartridge, has been extensively used for quick POC analyses of metabolites in emergency settings (http://www.i-STAT.com). In sight of cancer diagnosis, it is obvious that nanomaterial based biosensors included POC systems provide early, practical and fast detection87,88 which is very important for the early cure of this disease. Fabricating a biosensor integrated POC system needs to provide information about presence of cancer cells as well as the level of this cells.88 Nanoscale materials offer excellent prospects for designing powerful bioanalytical procedures as well as tools with significant sensitivity and multiplexing/coding ability.85 The huge signal amplification related with the usage of nanomaterial based labels and with the formation of nanoscale materials–biomolecule assemblies provides the base of electrochemical detection with higher sensitivity. Moreover, affinity based bioassays on nanowire transducers offer great potential for label-free detection of biomolecular interactions.89,90 In this part, we examined nanomaterial based electrochemical arrays only. Microfluidics and LOC based systems will not be covered in this review.
As it is well known, breast cancer has been widely seen in women. For this reason, it is very important to detect this cancer type at the early stages. Swischer et al., developed nanoelectrode arrays for detecting level of malignancy cancer cells based on measurements of cathepsin B activities in normal and breast cancer cells. For preparing nanoelectrode arrays, ferrocene-labelled-tetrapeptides were immobilized onto vertically aligned carbon nanofibers and alternating current voltammetric signals were monitored. Based on the obtained remarkable results, the authors claimed that this system has potential to be converted into portable multiplex diagnostic kit for cancer detection.91
In another work, AuNPs and enzymatic catalysis have been used to prepare the sandwich based biosensor for CEA detection. Screen printed carbon electrodes were used as supporting array electrodes where AuNPs were immobilized on it. Then self-assembly of Cys was formed for covalent attachment of lectins by means of amidation reaction. Three types of lectins were used for this procedure. After that, CEA and HRP labelled anti-CEA were immobilized onto the arrays respectively to obtain the sandwich structure. The chronoamperometric signal was obtained in the presence of hydroquinone and hydrogen peroxide. The most sensitive results were obtained when wheat-germ agglutinin was utilized as molecular recognition element with linear range of 0.5 ng mL−1 to 7.0 ng mL−1 and LOD of 0.01 ng mL−1.92 On the other hand, glycol-profiling of biomarkers related with the cancerous cells are also providing an important data on both diagnosis and prognosis. In this concept mainly lectin based biosensors associated with the nanomaterials have been considered as key elements.93 The other recent studies could be seen in Table 3.94–96
| Type of analytes | Nanomaterial | Arrays | LOD | Linear range | References |
|---|---|---|---|---|---|
| MRC-5 and QUDB cell lines | CNT | Amine-functionalized vertically aligned carbon nanotubes (VACNTs) conjugated to folic acid molecules | 4 × 10−3 Ω cm−1 | N/A | 96 |
| Vicinal-dithiol-containing proteins (VDPs) | MWCNT | 2-p-Aminophenyl-1, 3, 2-dithiarsenolane (VTA2)-conjugated multiwalled carbon nanotubes (VTA2@MWCNTs) array modified indium tin oxide electrode | 90 cells per mL | 2.7 × 102 to 2.7 × 107 cells per mL | 95 |
| Sequence-specific DNA hybridization events related to TP53 gene | AuNP and MWCNT | AuNP on aligned multi-walled carbon nanotubes | 1.0 × 10−17 M | 1.0 × 10−15 to 1.0 × 10−7 M | 94 |
In conclusion, electrochemical biosensors for cancer biomarkers, electrochemical cytosensors and electrochemical arrays were covered in this review. The addition of nanomaterials in electrochemical biosensors provides electrocatalytic activity and sensitivity. Since more practical and economical methods are needed in cancer diagnosis area, for the future, it is expected to see the increment in production and usage of nanomaterial based electrochemical biosensors. Especially usage of arrays for production pf POC systems has potential to provide necessary fast and practical diagnostic kits for cancer disease.
References
- (a) J. Wang, Biosens. Bioelectron., 2006, 21, 1887–1892 CrossRef CAS PubMed; (b) B. V. Chikkaveeraiah, A. A. Bhirde, N. Y. Morgan, H. S. Eden and X. Chen, ACS Nano, 2012, 6, 6546–6561 CrossRef CAS PubMed; (c) G. Liu, J. Wang, J. Kim, M. R. Jan and G. E. Collins, Anal. Chem., 2004, 76, 7126–7130 CrossRef CAS PubMed; (d) Y. Wan, W. Deng, Y. Su, X. Zhu, C. Peng, H. Hu, H. Peng, S. Song and C. Fan, Biosens. Bioelectron., 2011, 30, 93–99 CrossRef CAS PubMed; (e) S. Zhang, L. Zhang, X. Zhang, P. Yang and J. Caia, Analyst, 2014, 139, 3629–3635 RSC.
- E. Katz, I. Willner and J. Wang, Electroanalysis, 2004, 16, 19–44 CrossRef CAS.
- Z. Zhong, H. Chen, S. Tang, J. Ding, J. Lin and K. L. Tan, Chem. Phys. Lett., 2000, 330, 41–47 CrossRef CAS.
- X. Dai, O. Nekrassova, M. E. Hyde and R. G. Compton, Anal. Chem., 2004, 76, 5924–5929 CrossRef CAS PubMed.
- C. E. Banks, T. J. Davies, G. G. Wildgoose and R. G. Compton, Chem. Commun., 2005, 7, 829–841 RSC.
- Y. Tepeli, B. Demir, S. Timur and U. Anik, RSC Adv., 2015, 5, 53973–53978 RSC.
- R. Viter, I. Iatsunskyi, V. Fedorenko, S. Tumenas, Z. Balevicius, A. Ramanavicius, S. Balme, M. Kempiński, G. Nowaczyk, S. Jurga and M. Bechelany, J. Phys. Chem. C, 2016, 120, 5124–5132 CAS.
- M. Zhang and W. Gorski, Anal. Chem., 2005, 77, 3960–3965 CrossRef CAS PubMed.
- W. Zhang, X. Qiao and J. Chen, Mater. Sci. Eng., B, 2007, 142, 1–15 CrossRef CAS.
- U. Anik, S. Cevik and M. Pumera, Nanoscale Res. Lett., 2010, 5, 846–852 CrossRef CAS PubMed.
- S. Cevik and U. Anik, Sens. Lett., 2010, 8, 667–671 CrossRef CAS.
- S. D. Uzun, F. Kayaci, T. Uyar, S. Timur and L. Toppare, ACS Appl. Mater. Interfaces, 2014, 6, 5235–5243 Search PubMed.
- (a) E. Vernickaite, U. Bubniene, H. Cesiulis, A. Ramanavicius and E. J. Podlaha, J. Electrochem. Soc., 2016, 163, D344–D348 CrossRef CAS; (b) J. A. A. Ho, H.-C. Chang, N.-Y. Shih, L.-C. Wu, Y.-F. Chang, C.-C. Chen and C. Chou, Anal. Chem., 2010, 82, 5944–5950 CrossRef CAS PubMed.
- U. Anik, in Biomedical materials and diagnostic devices, ed. A. Tiwari, M. Ramalingam, H. Kobasyashi and A. P. F. Turner, Scrivener Publishing, Beverly, 2012, ch. 8, pp. 263–277, DOI:10.1002/9781118523025.ch8.
- N. German, A. Kausaite-Minkstimiene, A. Ramanavicius, T. Semashko, R. Mikhailova and A. Ramanaviciene, Electrochim. Acta, 2015, 169, 326–333 CrossRef CAS.
- A. J. Atkinson, W. A. Colburn, V. G. DeGruttola, D. L. DeMets, G. J. Downing, D. F. Hoth, J. A. Oates, C. C. Peck, R. T. Schooley, B. A. Spilker, J. Woodcock and S. L. Zeger, Clin. Pharmacol. Ther., 2001, 69, 89–95 CrossRef PubMed.
- J. F. Rusling, C. V. Kumar, J. S. Gutkind and V. Patel, Analyst, 2010, 135, 2496–2511 RSC.
- B. Jin, P. Wang, H. Mao, B. Hu, H. Zhang, Z. Cheng, Z. Wu, X. Bian, C. Jia, F. Jing, Q. Jin and J. Zhao, Biosens. Bioelectron., 2014, 55, 464–469 CrossRef CAS PubMed.
- Y. R. Yuan, R. Yuan, Y. Q. Chai, Y. Zhuo and X. M. Miao, J. Electroanal. Chem., 2009, 626, 6–13 CrossRef CAS.
- A. Voller, A. Bartlett and D. E. Bidwell, J. Clin. Pathol., 1978, 31, 507–520 CrossRef CAS PubMed.
- M. D. Stanley and J. Goldsmith, Semin. Nucl. Med., 1975, 5, 125–152 CrossRef.
- D. Schmalzing and W. Nashabeh, Electrophoresis, 1997, 18, 2184–2193 CrossRef CAS PubMed.
- R. Aebersold and M. Mann, Nature, 2003, 422, 198–207 CrossRef CAS PubMed.
- A. Gole, C. Dash, C. Soman, S. R. Sainkar, M. Rao and M. Sastry, Bioconjugate Chem., 2001, 12, 684–690 CrossRef CAS PubMed.
- W. J. Parak, T. Pellegrino, C. M. Micheel, D. Gerion, S. C. Williams and A. P. Alivisatos, Nano Lett., 2002, 3, 33–36 CrossRef.
- D. Tang, R. Yuan, Y. Chai, X. Zhong, Y. Liu and J. Dai, Biochem. Eng. J., 2004, 22, 43–49 CrossRef CAS.
- S. Xu and X. Han, Biosens. Bioelectron., 2004, 19, 1117–1120 CrossRef CAS PubMed.
- Y. Liu and H. Jiang, Electroanalysis, 2006, 18, 1007–1013 CrossRef.
- M. Cubukcu, F. N. Ertas and U. Anik, Curr. Anal. Chem., 2012, 8, 351–357 CrossRef CAS.
- U. Anik, M. Cubukcu and Y. Yavuz, Artif. Cells, Nanomed., Biotechnol., 2013, 41, 8–12 CrossRef CAS PubMed.
- S. Aslan, Y. Yavuz and U. Anik, Curr. Anal. Chem., 2014, 10, 435–442 CrossRef CAS.
- X. Yu, B. Munge, V. Patel, G. Jensen, A. Bhirde, J. D. Gong, S. N. Kim, J. Gillespie, J. S. Gutkind, F. Papadimitrakopoulos and J. F. Rusling, J. Am. Chem. Soc., 2006, 128, 11199–11205 CrossRef CAS PubMed.
- S. C. Sultan and U. Anik, Talanta, 2014, 129, 523–528 CrossRef CAS PubMed.
- A. Pandikumar, G. T. S. How, T. P. See, F. S. Omar, S. Jayabal, K. Z. Kamali, N. Yusoff, A. Jamil, R. Ramaraj, S. A. John, H. N. Lim and N. M. Huang, RSC Adv., 2014, 4, 63296–63323 RSC.
- Y. Tepeli and U. Anik, Electrochem. Commun., 2015, 57, 31–34 CrossRef CAS.
- N. Chauhan, R. Rawal, V. Hooda and U. Jain, RSC Adv., 2016, 6, 63624–63633 RSC.
- M. Pumera, A. Ambrosi, A. Bonanni, E. L. K. Chng and H. L. Poh, TrAC, Trends Anal. Chem., 2010, 29, 954–965 CrossRef CAS.
- Z. Chu, Y. Liu, Y. Xu, L. Shi, J. Peng and W. Jin, Electrochim. Acta, 2015, 176, 162–171 CrossRef CAS.
- P. Bollella, G. Fusco, C. Tortolini, G. Sanzò, G. Favero, L. Gorton and R. Antiochia, Biosens. Bioelectron., 2017, 89, 152–166 CrossRef CAS PubMed.
- V. Mani, B. V. Chikkaveeraiah and J. F. Rusling, Expert Opin. Med. Diagn., 2011, 5, 381–391 CrossRef CAS PubMed.
- J. Xie and S. Jon, Theranostics, 2012, 2, 122–124 CrossRef PubMed.
- J. Huang, J. Tian, Y. Zhao and S. Zhao, Sens. Actuators, B, 2015, 206, 570–576 CrossRef CAS.
- K. J. Huang, J. Li, Y. Y. Wu and Y. M. Liu, Bioelectrochemistry, 2013, 90, 18–23 CrossRef CAS PubMed.
- S. Aslan and U. Anik, Electroanalysis, 2014, 26, 1373–1381 CrossRef CAS.
- Y. Zhu, P. Chandra and Y. B. Shim, Anal. Chem., 2013, 85, 1058–1064 CrossRef CAS PubMed.
- Y. Y. Lin, J. Wang, G. Liu, H. Wu, C. M. Wai and Y. Lin, Biosens. Bioelectron., 2008, 23, 1659–1665 CrossRef CAS PubMed.
- J. Lin, C. He, L. Zhang and S. Zhang, Anal. Biochem., 2009, 384, 130–135 CrossRef CAS PubMed.
- W. Liang, W. Yi, Y. Li, Z. Zhang, M. Yang, C. Hu and A. Chen, Mater. Lett., 2010, 64, 2616–2619 CrossRef CAS.
- Q. Wei, T. Li, G. Wang, H. Li, Z. Qian and M. Yang, Biomaterials, 2010, 31, 7332–7339 CrossRef CAS PubMed.
- Z. Zhong, W. Wu, D. Wang, D. Wang, J. Shan, Y. Qing and Z. Zhang, Biosens. Bioelectron., 2010, 25, 2379–2383 CrossRef CAS PubMed.
- Y. Zhuo, Y. Q. Chai, R. Yuan, L. Mao, Y. L. Yuan and J. Han, Biosens. Bioelectron., 2011, 26, 3838–3844 CrossRef CAS PubMed.
- S. Liu, R. Yuan, Y. Chai and H. Su, Sens. Actuators, B, 2011, 156, 388–394 CrossRef CAS.
- R. Li, D. Wu, H. Li, C. Xu, H. Wang, Y. Zhao, Y. Cai, Q. Wei and B. Du, Anal. Biochem., 2011, 414, 196–201 CrossRef CAS PubMed.
- K. Mao, D. Wu, Y. Li, H. Ma, Z. Ni, H. Yu, C. Luo, Q. Wei and B. Du, Anal. Biochem., 2012, 422, 22–27 CrossRef CAS PubMed.
- J. Zhou, J. Zhuang, M. Miró, Z. Gao, G. Chen and D. Tang, Biosens. Bioelectron., 2012, 35, 394–400 CrossRef CAS PubMed.
- J. Han, Y. Zhuo, Y. Q. Chai, Y. L. Yuan and R. Yuan, Biosens. Bioelectron., 2012, 31, 399–405 CrossRef CAS PubMed.
- J. Han, Y. Zhuo, Y. Chai, R. Yuan, W. Zhang and Q. Zhu, Anal. Chim. Acta, 2012, 746, 70–76 CrossRef CAS PubMed.
- L. Zhu, L. Xu, N. Jia, B. Huang, L. Tan, S. Yang and S. Yao, Talanta, 2013, 116, 809–815 CrossRef CAS PubMed.
- A. Ravalli, G. P. dos Santos, M. Ferroni, G. Faglia, H. Yamanaka and G. Marrazza, Sens. Actuators, B, 2013, 179, 194–200 CrossRef CAS.
- H. Wang, Y. Zhang, H. Yu, D. Wu, H. Ma, H. Li, B. Du and Q. Wei, Anal. Biochem., 2013, 434, 123–127 CrossRef CAS PubMed.
- D. Wu, A. Guo, Z. Guo, L. Xie, Q. Wei and B. Du, Biosens. Bioelectron., 2014, 54, 634–639 CrossRef CAS PubMed.
- H. Wang, X. Li, K. Mao, Y. Li, B. Du, Y. Zhang and Q. Wei, Anal. Biochem., 2014, 465, 121–126 CrossRef CAS PubMed.
- F. Yang, J. Han, Y. Zhuo, Z. Yang, Y. Chai and R. Yuan, Biosens. Bioelectron., 2014, 55, 360–365 CrossRef CAS PubMed.
- W. Li, L. Li, S. Ge, X. Song, L. Ge, M. Yan and J. Yu, Biosens. Bioelectron., 2014, 56, 167–173 CrossRef CAS PubMed.
- X. Chen, Q. Zhang, C. Qian, N. Hao, L. Xu and C. Yao, Biosens. Bioelectron., 2015, 64, 485–492 CrossRef CAS PubMed.
- C. Ma, W. Li, Q. Kong, H. Yang, Z. Bian, X. Song, J. Yu and M. Yan, Biosens. Bioelectron., 2015, 63, 7–13 CrossRef CAS PubMed.
- H. Fan, Z. Guo, L. Gao, Y. Zhang, D. Fan, G. Ji, B. Du and Q. Wei, Biosens. Bioelectron., 2015, 64, 51–56 CrossRef CAS PubMed.
- B. Seven, M. Bourourou, K. Elouarzaki, J. F. Constant, C. Gondran, M. Holzinger, S. Cosnier and S. Timur, Electrochem. Commun., 2013, 37, 36–39 CrossRef CAS.
- R. Laocharoensuk, Electroanalysis, 2016, 28, 1716–1729 CrossRef CAS.
- R. Wang, J. Di, J. Ma and Z. Ma, Electrochim. Acta, 2012, 61, 179–184 CrossRef CAS.
- D. Sun, J. Lu, Y. Zhong, Y. Yu, Y. Wang, B. Zhang and Z. Chen, Biosens. Bioelectron., 2016, 75, 301–307 CrossRef CAS PubMed.
- Y. Tepeli, F. B. Barlas, S. Timur and U. Anik, Electrochim. Acta, 2016, 211, 71–76 CrossRef CAS.
- U. A. Kirgoz, H. Tural and F. N. Ertas, Talanta, 2005, 65, 48–53 Search PubMed.
- U. Anik and S. Cevik, Microchim. Acta, 2009, 166, 209–213 CrossRef CAS.
- Z. Wang, S. Chen, C. Hu, D. Cui and N. Jia, Electrochem. Commun., 2013, 29, 4–7 CrossRef CAS.
- F. B. Barlas, D. Ag Seleci, M. Ozkan, B. Demir, M. Seleci, M. Aydin, M. A. Tasdelen, H. M. Zareie, S. Timur, S. Ozcelik and Y. Yagci, J. Mater. Chem. B, 2014, 2, 6412–6421 RSC.
- G. O. Eyrilmez, S. Doran, E. Murtezi, B. Demir, D. O. Demirkol, H. Coskunol, S. Timur and Y. Yagci, Macromol. Biosci., 2015, 15, 1233–1241 CrossRef PubMed.
- W. Cheng, L. Ding, J. Lei, S. Ding and H. Ju, Anal. Chem., 2008, 80, 3867–3872 CrossRef CAS PubMed.
- T. Li, Q. Fan, T. Liu, X. Zhu, J. Zhao and G. Li, Biosens. Bioelectron., 2010, 25, 2686–2689 CrossRef CAS PubMed.
- C. Ding, S. Qian, Z. Wang and B. Qu, Anal. Biochem., 2011, 414, 84–87 CrossRef CAS PubMed.
- H. Wang, T. Wang, Y. X. Ye, Y. X. Zhang, P. H. Yang, H. H. Cai and J. Y. Cai, Chin. J. Anal. Chem., 2012, 40, 184–190 CrossRef CAS.
- S. Xu, J. Liu, T. Wang, H. Li, Y. Miao, Y. Liu and J. Wang, Talanta, 2013, 104, 122–127 CrossRef CAS PubMed.
- G. Yang, J. Cao, L. Li, R. K. Rana and J. J. Zhu, Carbon, 2013, 51, 124–133 CrossRef CAS.
- M. Su, L. Ge, S. Ge, N. Li, J. Yu, M. Yan and J. Huang, Anal. Chim. Acta, 2014, 847, 1–9 CrossRef CAS PubMed.
- S. A. Soper, K. Brown, A. Ellington, B. Frazier, G. Garcia-Manero, V. Gau, S. I. Gutman, D. F. Hayes, B. Korte, J. L. Landers, D. Larson, F. Ligler, A. Majumdar, M. Mascini, D. Nolte, Z. Rosenzweig, J. Wang and D. Wilson, Biosens. Bioelectron., 2006, 21, 1932–1942 CrossRef CAS PubMed.
- E. Petryayeva and W. R. Algar, RSC Adv., 2015, 5, 22256–22282 RSC.
- A. Rasooly, Biosens. Bioelectron., 2006, 21, 1847–1850 CrossRef CAS PubMed.
- C. I. L. Justino, T. A. P. Rocha-Santos and A. C. Duarte, TrAC, Trends Anal. Chem., 2013, 45, 24–36 CrossRef CAS.
- A. T. Woolley, C. Guillemette, C. L. Cheung, D. E. Housman and C. M. Lieber, Nat. Biotechnol., 2000, 18, 760–763 CrossRef CAS PubMed.
- Y. Huang and C. M. Lieber, Pure Appl. Chem., 2004, 76, 2051–2068 CrossRef CAS.
- L. Z. Swisher, A. M. Prior, M. J. Gunaratna, S. Shishido, F. Madiyar, T. A. Nguyen, D. H. Hua and J. Li, Nanomed.-Nanotechnol., 2015, 11, 1695–1704 CrossRef CAS PubMed.
- L. Zhao, C. Li, H. Qi, Q. Gao and C. Zhang, Sens. Actuators, B, 2016, 235, 575–582 CrossRef CAS.
- L. Kluková, T. Bertok, P. Kasák and J. Tkac, Anal. Methods, 2014, 6, 4922–4931 RSC.
- H. Fayazfar, A. Afshar, M. Dolati and A. Dolati, Anal. Chim. Acta, 2014, 836, 34–44 CrossRef CAS PubMed.
- Y. Xu, H. Wu, C. Huang, C. Hao, B. Wu, C. Miao, S. Chen and N. Jia, Biosens. Bioelectron., 2015, 66, 321–326 CrossRef CAS PubMed.
- S. Zanganeh, F. Khodadadei, S. R. Tafti and M. Abdolahad, J. Mater. Sci. Technol., 2016, 32, 617–625 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |
