(L)-Prolinamide imidazolium hexafluorophosphate ionic liquid as an efficient reusable organocatalyst for direct asymmetric aldol reaction in solvent-free condition†
Geeta Devi Yadav and
Surendra Singh*
Department of Chemistry, University of Delhi, Delhi, India – 110007. E-mail: ssingh1@chemistry.du.ac.in
First published on 17th October 2016
Abstract
We have designed a new hydrophobic ionic liquid derived from bromoester of trans-4-hydroxy-(L)-prolinamide and N-methylimidazole. (L)-Prolinamide imidazolium hexafluorophosphate ionic liquid (2 mol%) found to be an excellent organocatalyst for direct asymmetric aldol reaction between 4-nitrobenzaldehyde and cyclohexanone using acetic acid (2 mol%) as an additive at −15 °C in solvent-free condition, the aldol product was afforded in excellent yield with diastereomeric ratio (anti/syn; 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and 94% ee of anti-aldol product. Ionic liquids can catalyze the direct aldol reaction between benzaldehyde derivatives and cycloalkanones and the best dr (anti/syn; 99.9/0.01) and 99% ee was obtained for aldol product of 2-trifluoromethyl benzaldehyde and cyclohexanone. (L)-Prolinamide imidazolium hexafluorophosphate ionic liquid was reused up to 6 continuous cycles without decrease in the conversion of the product with 92% ee and found to be superior than its counterpart trans-4-hydroxy-(L)-prolinamide. Continuous cycle experiments do not require isolation of the catalyst after each cycle. The results of reusability of the ionic liquid catalyst were found to be better than most of other reported reusable catalysts.
3) and 94% ee of anti-aldol product. Ionic liquids can catalyze the direct aldol reaction between benzaldehyde derivatives and cycloalkanones and the best dr (anti/syn; 99.9/0.01) and 99% ee was obtained for aldol product of 2-trifluoromethyl benzaldehyde and cyclohexanone. (L)-Prolinamide imidazolium hexafluorophosphate ionic liquid was reused up to 6 continuous cycles without decrease in the conversion of the product with 92% ee and found to be superior than its counterpart trans-4-hydroxy-(L)-prolinamide. Continuous cycle experiments do not require isolation of the catalyst after each cycle. The results of reusability of the ionic liquid catalyst were found to be better than most of other reported reusable catalysts.
Introduction
The aldol reaction is recognized as one of the most powerful carbon–carbon bond-forming reactions in modern organic synthesis. It provides an atom-economic approach to β-hydroxyl carbonyls, which make up a large family of chiral intermediates for the synthesis of biologically active substances and natural products.1 Since the early reports in the 1970s that the L-proline catalysed intramolecular aldol reactions2a,b later the pioneering work by List et al. in 2000.2c,d After these reports numerous proline-derived organocatalysts such as proline analogues,3 acyclic amino acids,4 different types of prolinamides,5–13 prolinethioamides,14 sulfonamides,15 chiral amines,16 organic salts17 were exploited as organocatalysts for direct asymmetric aldol reaction. The organocatalyst is usually used for direct asymmetric aldol reaction in a substantial quantity, in some cases up to 30 mol%, its recovery and reuse is vital for reduce the cost and to facilitate the separation of the product from the catalyst. Several research groups are attentive to develop a recoverable organocatalysts for direct asymmetric aldol reaction.18 Immobilization of modified proline derivatives are desirable due to their non-commercial viability and their high reactivity.In our ongoing research on development of organocatalyst for asymmetric Diels–Alder reaction and direct asymmetric aldol reaction.19 We recently reported the trans-4-hydroxy-(L)-prolinamide (1) as organocatalyst for aldol reaction between aromatic aldehydes and cycloalkanes under solvent free conditions.20 Herein, we wish to report synthesis of imidazolium ionic liquids from bromoester of trans-4-hydroxy-(L)-prolinamide and N-methylimidazole and their catalytic activity in direct asymmetric aldol reaction (Fig. 1).
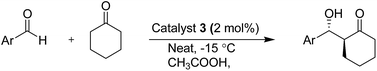
Results and discussion
Prolinamide imidazolium ionic liquids 2 and 3 were synthesized according to Scheme 1. First, trans-4-hydroxy-(S)-proline 4 was protected as N-boc protecting group in quantitative yield. N-Boc-trans-4-hydroxy-(S)-proline 5 was treated with (S)-1-phenylethylamine, in presence of triethylamine and ethylchloroformate, gave corresponding N-boc-prolinamide 6 in 81% yield. The esterification of compound 6 with 5-bromovaleric acid in the presence of DCC and DMAP in dichloromethane gave the product 7 in a 94% yield. The compound 8 was synthesised by the reaction of N-methyl imidazole and compound 7, which affords N-boc protected imidazolium bromide 8 in 97% yield. The bromide anion of the ionic liquid 8 was exchanged by KPF6 to hexafluorophosphate anion in 86% yield. Deprotection of N-boc group in compounds 8 and 9 by TFA in dichloromethane at room temperature gave ionic liquid (IL) 2 and 3.In our initial investigation, the direct asymmetric aldol reaction between 4-nitrobenzaldehyde 10 and cyclohexanone using ionic liquid 3 (10 mol%) and acetic acid (10 mol%) as additives at room temperature gave >99% conversion and 29% enantiomeric excess (ee) of product anti 11 with poor diastereoselectivity (Table 1, entry 1). Decreasing of the reaction temperature to −15 °C, improved the diastereomeric ratio (dr; anti/syn; 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) and ee (96%) of the product anti-11 (Table 1, entry 2). We also varied the catalyst loading and even though at low catalyst loading (2 mol%) with additive acetic acid (2 mol%), product anti-11 was obtained in excellent yield with 94% ee and dr (anti/syn; 97
6) and ee (96%) of the product anti-11 (Table 1, entry 2). We also varied the catalyst loading and even though at low catalyst loading (2 mol%) with additive acetic acid (2 mol%), product anti-11 was obtained in excellent yield with 94% ee and dr (anti/syn; 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) after 24 h (Table 1, entry 3 and 4). We also compared the catalytic result with organocatalyst 1 with ionic liquid 3 (IL 3) under identical conditions and the organocatalyst 1 was found to be more reactive but better ee for product anti-11 was observed with IL 3 (Table 1, entry 5). IL 2 gave the aldol product in poor yield (39%) with 76% ee of anti-11 (Table 1, entry 6).
3) after 24 h (Table 1, entry 3 and 4). We also compared the catalytic result with organocatalyst 1 with ionic liquid 3 (IL 3) under identical conditions and the organocatalyst 1 was found to be more reactive but better ee for product anti-11 was observed with IL 3 (Table 1, entry 5). IL 2 gave the aldol product in poor yield (39%) with 76% ee of anti-11 (Table 1, entry 6).
| Entry | Organocatalyst | Catalyst loading (mol%) | Time (h) | Yieldb (%) | dr (anti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) syn)c syn)c |
ee (%) (anti)d |
|---|---|---|---|---|---|---|
| a Organocatalyst (2–10 mol%), CH3COOH (2–10 mol%), cyclohexanone (3 mmol) and 4-nitrobenzaldehyde (1 mmol) were stirred at −15 °C for specified time.b Conversion was determined by HPLC using response factor for 4-nitrobenzaldehyde 10 and product 11.c Diastereomeric ratio (dr) (anti/syn) and enantiomeric excess (ee) were determined by HPLC using Chiralpak AD-H column.d The absolute configuration was determined by comparing the specific rotation of product anti-11 with literature values and found to be (1′S,2R).e Reaction carried out at room temperature. | ||||||
| 1 | 3 | 10 | 6 | >99 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 41 |
29e |
| 2 | 3 | 10 | 12 | >99 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
96 |
| 3 | 3 | 5 | 12 | >99 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
91 |
| 4 | 3 | 2 | 24 | 99 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
94 |
| 5 | 1 | 2 | 8 | >99 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
92 |
| 6 | 2 | 2 | 24 | 39 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
76 |
We also compared our organocatalyst IL 3 with other reusable organocatalysts reported in literature for the direct asymmetric aldol reaction between 4-nitrobenzaldehyde and cyclohexanone are shown in ESI Table 1.† The results show that IL 3 was found to be better catalyst in terms of catalyst loading and turnover frequency than the most of the reported reusable catalysts.
We have investigated the asymmetric direct aldol reaction between substituted benzaldehydes and cyclohexanone catalyzed by IL 3 (2 mol%) and additive CH3COOH (2 mol%) at −15 °C in solvent-free conditions. All the benzaldehyde derivatives gave the anti diastereomer as major product. Benzaldehyde afforded aldol product in a 60% yield with diastereomeric ratio of anti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) syn (91/9) with 98% ee of anti diastereomer and bulkier naphthaldehyde improved the anti
syn (91/9) with 98% ee of anti diastereomer and bulkier naphthaldehyde improved the anti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) syn ratio but ee was slightly decreased (Table 2, entry 1 and 2). Electron withdrawing group (EWG) such as nitro on benzaldehyde improved the reactivity and 2-nitrobenzaldehyde gave 97% ee (Table 2, entry 3). 4-Halogenated benzaldehydes were found to be less reactive with excellent ee and dr. The order of reactivity of 4-halogenated benzaldehydes (F < Cl < Br) was increasing when electronegativity of halogen is decreasing due to enhancement of electrophilicity of carbonyl carbon of halobenzaldehydes (Table 2, entries 5, 7, and 9). 2-Halobenzaldehydes gave the corresponding aldol products in 88–90% yields and 91–97% ee (Table 2, entries 4, 6, and 8). Electron donating group (EDG) like methoxy on benzaldehyde, found to be less reactive compared to derivatives of EWG. The EWG's enhance the electrophilicity of carbonyl carbons in aldehydes which facilitate the reaction, while EDG's lessen the electrophilicity of carbonyl carbons. 4-Methoxybenzaldehyde was found to be least active and afforded only 5% of the product but by increasing the catalyst loading was increased to 5 mol% and 25% yield of the product was obtained (Table 2, entries 11 and 12). In case of 2-(trifluoromethyl)benzaldehyde, exclusively afforded anti product in 99% ee (Table 2, entry 13).
syn ratio but ee was slightly decreased (Table 2, entry 1 and 2). Electron withdrawing group (EWG) such as nitro on benzaldehyde improved the reactivity and 2-nitrobenzaldehyde gave 97% ee (Table 2, entry 3). 4-Halogenated benzaldehydes were found to be less reactive with excellent ee and dr. The order of reactivity of 4-halogenated benzaldehydes (F < Cl < Br) was increasing when electronegativity of halogen is decreasing due to enhancement of electrophilicity of carbonyl carbon of halobenzaldehydes (Table 2, entries 5, 7, and 9). 2-Halobenzaldehydes gave the corresponding aldol products in 88–90% yields and 91–97% ee (Table 2, entries 4, 6, and 8). Electron donating group (EDG) like methoxy on benzaldehyde, found to be less reactive compared to derivatives of EWG. The EWG's enhance the electrophilicity of carbonyl carbons in aldehydes which facilitate the reaction, while EDG's lessen the electrophilicity of carbonyl carbons. 4-Methoxybenzaldehyde was found to be least active and afforded only 5% of the product but by increasing the catalyst loading was increased to 5 mol% and 25% yield of the product was obtained (Table 2, entries 11 and 12). In case of 2-(trifluoromethyl)benzaldehyde, exclusively afforded anti product in 99% ee (Table 2, entry 13).
| Entry | Ar | Time [h] | Yieldb (%) | dr (anti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) syn)c syn)c |
ee (%) (anti)d |
|---|---|---|---|---|---|
| a IL 3 (0.02 mmol, 2 mol%), CH3COOH (0.02 mmol, 2 mol%), cyclohexanone (3 mmol) and substituted benzaldehyde (1 mmol) were stirred at −15 °C for specified time.b Isolated yield after purification by column chromatography.c Diastereomeric ratios (dr) were determined by 1H-NMR of crude products.d Enantiomeric excess (ee) was determined by HPLC using Chiralpak AD-H and Chiralcel OD-H columns.e 5 mol% catalyst was used. | |||||
| 1 | Phenyl | 24 | 60 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
98 |
| 2 | 1-Naphthyl | 48 | 25 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
93 |
| 3 | 2-NO2C6H4 | 17 | 87 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
97 |
| 4 | 2-FC6H4 | 18 | 88 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
91 |
| 5 | 4-FC6H4 | 24 | 49 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
93 |
| 6 | 2-ClC6H4 | 18 | 90 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
97 |
| 7 | 4-ClC6H4 | 26 | 74 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
97 |
| 8 | 2-BrC6H4 | 18 | 90 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
97 |
| 9 | 4-BrC6H4 | 26 | 84 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
98 |
| 10 | 2-OMeC6H4 | 40 | 23 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
91 |
| 11 | 4-OMeC6H4 | 48 | 5 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
ND |
| 12 | 4-OMeC6H4 | 48 | 25 | 99.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2 |
52e |
| 13 | 2-CF3C6H4 | 17 | 69 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
99 |
| 14 | 4-CF3C6H4 | 17 | 87 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
91 |
We have also studied the direct asymmetric aldol reaction between 2- and 4-substituted benzaldehydes with cyclopetanone catalyzed by IL 3 (2 mol%) at −15 °C in solvent-free conditions (Table 3). The reactivity of benzaldehydes having EWG's was faster than the EDG's. In case of 4-substituted benzaldehydes anti aldol products were obtained as major diastereomers while syn diastereomers were obtained with 2-substituted benzaldehydes except 2-nitrobenzaldehyde (Table 3, entries 1–8). The better ee was obtained for anti diastereomers of the aldol products than syn diastereomers. The reactivity of 4-methoxy was found to be poor and but by increasing the catalyst loading to 5 mol% increased the yield (Table 3, entries 8 and 9).
| Entry | Ar | Time (h) | Yieldb (%) | dr (anti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) syn)c syn)c |
ee (%) (anti/syn)d |
|---|---|---|---|---|---|
| a IL 3 (0.02 mmol, 2 mol%), CH3COOH (0.02 mmol, 2 mol%), cyclopentanone (3 mmol) and 4-nitrobenzaldehyde (1 mmol) were stirred at −15 °C for specified time.b Isolated yield after purification by column chromatography.c Diastereomeric ratios were determined by 1H-NMR of crude products.d The ee was determined by HPLC using Chiralpak AD-H, and Chiralcel OD-H columns.e Reaction carried out using 5 mol% of the catalyst. | |||||
| 1 | 2-NO2C6H4 | 30 | 71 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
99/28 |
| 2 | 4-NO2C6H4 | 30 | 66 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
66/40 |
| 3 | 2-FC6H4 | 40 | 76 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57 57 |
81/26 |
| 4 | 4-FC6H4 | 40 | 69 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
82/20 |
| 5 | 2-ClC6H4 | 38 | 37 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
99/45 |
| 6 | 4-ClC6H4 | 38 | 72 | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 46 |
84/26 |
| 7 | 2-OMeC6H4 | 48 | 33 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 79 79 |
97/33 |
| 8 | 4-OMeC6H4 | 48 | 7 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
70/20 |
| 9 | 4-OMeC6H4 | 44 | 64 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 56 56 |
81/8e |
The reusability performance of the IL 3 (2 mol%) was carried out for the asymmetric aldol reaction of 4-nitrobenzaldehyde 10 (1 mmol) and cyclohexanone (3 mmol) using acetic acid (2 mol%) as additive at −15 °C (Table 4). The reaction was monitored on TLC and HPLC, after complete conversion of 4-nitrobenzaldehyde 10, 4-nitrobenzaldehyde 10 (1 mmol) and cyclohexanone (3 mmol) were added to the reaction mixture without any additive and catalyst. This procedure was repeated for 6 continuous cycles and afforded aldol products in overall >99% conversion of the product with anti/syn 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 with 92% ee of product 11-anti and total turnover number (TON) of the catalyst was found to be more than 346 (Table 4, entry 7 and Fig. 2). The advantages of continuous cycle for the reuse of the catalyst are: (i) IL 3 and catalytic product of the aldol reaction are polar so it's difficult to separate catalyst from the product by using the different solvents therefore use of continuous cycle is beneficial, (ii) it enhances economic viability of the process because it does not require separation of the catalyst after completion of each cycle which save the cost of the manpower and solvents and (iii) the product is stable during the reaction even though we are stirring the reaction up to 7 cycles. Similar strategy was used by List and co-workers in cyanosilylation of aldehydes.20 We also compared the catalysis of IL 3 in low loading (0.4 mol%) in identical reaction conditions and afforded product in >99% yield and anti/syn 95
4 with 92% ee of product 11-anti and total turnover number (TON) of the catalyst was found to be more than 346 (Table 4, entry 7 and Fig. 2). The advantages of continuous cycle for the reuse of the catalyst are: (i) IL 3 and catalytic product of the aldol reaction are polar so it's difficult to separate catalyst from the product by using the different solvents therefore use of continuous cycle is beneficial, (ii) it enhances economic viability of the process because it does not require separation of the catalyst after completion of each cycle which save the cost of the manpower and solvents and (iii) the product is stable during the reaction even though we are stirring the reaction up to 7 cycles. Similar strategy was used by List and co-workers in cyanosilylation of aldehydes.20 We also compared the catalysis of IL 3 in low loading (0.4 mol%) in identical reaction conditions and afforded product in >99% yield and anti/syn 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 with 85% ee of product 11-anti after 4.5 days and these results are found to be poor in terms of ee than the continuous use of the IL 3. We also conducted the aldol reaction using catalyst 1 (2 mol%) under identical conditions to compare the reusability in continuous cycle. Catalyst 1 afforded the desired product anti-11 in 97% yield and 96% ee but reaction took 65 h for completion of a 4th continuous cycle. The overall TON and TOF was found to be better for the catalyst 3 compared to the catalyst 1. The hydrophobic nature of the ionic liquid unit of the catalyst 3 maintains the catalytic activity at dilution of the catalyst concentration in continuous cycle.
5 with 85% ee of product 11-anti after 4.5 days and these results are found to be poor in terms of ee than the continuous use of the IL 3. We also conducted the aldol reaction using catalyst 1 (2 mol%) under identical conditions to compare the reusability in continuous cycle. Catalyst 1 afforded the desired product anti-11 in 97% yield and 96% ee but reaction took 65 h for completion of a 4th continuous cycle. The overall TON and TOF was found to be better for the catalyst 3 compared to the catalyst 1. The hydrophobic nature of the ionic liquid unit of the catalyst 3 maintains the catalytic activity at dilution of the catalyst concentration in continuous cycle.
| Cycle | Time (h) | Conversionb (%) | dr (anti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) syn)c syn)c |
ee (%) (anti)c,d |
|---|---|---|---|---|
| a Catalyst 3 (0.02 mmol, 2 mol%), CH3COOH (0.02 mmol, 2 mol%), cyclopentanone (3 mmol) and 4-nitrobenzaldehyde (1 mmol) were stirred at −15 °C for specified time.b Conversion was determined by HPLC using response factor for 4-nitrobenzaldehyde 10 and product 11.c Diastereomeric ratio (dr) and enantiomeric excess (ee) were determined by HPLC using Chiralpak AD-H column.d The absolute configuration was determined by comparing optical rotation of product 11-anti with literature and found to be (1′S,2R).e Results in parenthesis are for catalyst 1 under identical reaction conditions.f Turn over frequency (TOF) for catalyst 3 = 2.22 h−1 and for catalyst 1 = 1.34 h−1; turn over number (TON) for catalyst 3 = 346 and for catalyst 1 = 194.5. | ||||
| 1 | 20 (8)e | >99 (>99) | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (93 3 (93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
88 (92) |
| 2 | 20 (24) | >99 (96) | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (95 9 (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
88 (93) |
| 3 | 20 (48) | >99 (96) | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (96 5 (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
93 (96) |
| 4 | 24 (65)f | >99 (97) | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (96 4 (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
92 (96) |
| 5 | 24 | >99 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
92 |
| 6 | 24 | >99 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
92 |
| 7 | 24f | >99 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
92 |
Experimental section
General
Benzaldehyde derivatives, cyclohexanone and cyclopentanone were purchased from commercial source and used as such. Proton and carbon nuclear magnetic resonance spectra (1H and 13C NMR, respectively) were recorded on 400 MHz (operating frequencies: 1H, 400.13 MHz; 13C, 100.61 MHz) Jeol-FT-NMR spectrometers at ambient temperature. The chemical shifts (δ) for all compounds are listed in parts per million (ppm) downfield from tetramethylsilane using the NMR solvent as an internal reference. The reference values used for deuterated chloroform (CDCl3) were 7.26 and 77.00 ppm for 1H and 13C NMR spectra, respectively. HRMS analysis was carried out using QSTAR XL Pro system microTOF-Q-II. The reaction was monitored by thin layer chromatography was carried out using Merck Kieselgel 60 F254 silica gel plates. Compounds were purified by column chromatography separations were performed using silica gel 230–400 mesh. The enantiomeric excess was determined on Shimadzu LC-2010HT using Chiralcel OD-H, and Chiralpak AD-H columns. Infrared spectra were recorded on a Perkin-Elmer FT-IR spectrometer. Optical rotations were taken using Rudolph digipol polarimeter. (2S,4R)-1-(tert-Butoxycarbonyl)-4-hydroxypyrrolidine-2-carboxylic acid (5) and (2S,4R)-tert-butyl-4-hydroxy-2-((S)-1-phenylethylcarbamoyl)pyrrolidine-1-carboxylate (6) were synthesised according to our earlier report.19a All the unknown compounds were characterized by 1H, 13C NMR and HRMS. Aldol products were characterized by 1H-NMR and compared with our earlier report.19a Copy of 1H-NMR, 13C-NMR and HPLC chromatogram are given in ESI.†General procedure for asymmetric aldol reaction
The organocatalyst (IL 3) (10.8 mg, 0.02 mmol), cyclohexanone (0.310 mL, 3 mmol) and acetic acid (1.1 μL, 0.02 mmol) was stirred for 20 min at −15 °C, then 4-nitrobenzaldehyde 10 (151 mg, 1 mmol) was added. The reaction mixture was stirred for a specified reaction time period at same temperature. The crude aldol product was purified by flash column chromatography on silica gel (hexane/ethyl acetate (3/1)). The diastereomeric ratio was determined by 1H-NMR of the crude product. The ee of the aldol product was determined by HPLC using chiral column (Chiralpak AD-H and OD-H) using hexane/2-propanol as mobile phase.Conclusions
In summary, we designed a new, efficient, and reusable organocatalyst for the direct asymmetric aldol reaction. IL 3 was synthesised in 55% of overall yield in 6 steps. Only 2 mol% of hydrophobic IL 3 was efficiently catalysed the reaction at −15 °C in solvent-free conditions. The scope and limitations of the catalyst was investigated for the reaction between benzaldehyde derivatives and cycloalkanes, gave corresponding aldol products in 25–90% yields with up to 99% ee of anti diastereomer. Poor yield of the aldol product was obtained with methoxy-benzaldehyde which can be improved by increasing the catalyst loading. The isolation of the product from the catalyst is difficult by precipitation for this reaction. We have used alternative method for resuse of organocatalyst (IL 3) and the catalyst was successfully reused up to 6 continuous cycles with excellent yields of the aldol product with 92% ee and shown better activity in continuous cycles compared to the trans-4-hydroxy-(L)-prolinamide (1).Acknowledgements
SS acknowledges the financial assistance from Science and Engineering Research Board (SERB), Department of Science and technology (DST), India, under scheme Fast Track Young Scientist (SB/FT/CS-020/2012). We are also thankful to University Science Instrumentation Center (USIC), University of Delhi, India, for analytical data. Authors are thankful to DU-DST mass facility at USIC. GDY is thankful to CSIR for providing Senior Research Fellowship (SRF).References
- (a) B. M. Kim, S. F. Williams and S. Masamune, in Comprehensive Organic Synthesis, ed. B. M. Trost, I. Fleming and C. H. Heathcock, Pergamon, Oxford, 1991, pp. 1–10 Search PubMed; (b) B. M. Trost, Science, 1991, 254, 1471–1477 CAS; (c) B. M. Trost, Angew. Chem., Int. Ed., 1995, 34, 259–281 CrossRef CAS; (d) C. Palomo, M. Oiarbide and J. M. Garciak, Chem. Soc. Rev., 2004, 33, 65–75 RSC; (e) X. Y. Xu, Y. Z. Wang, L. F. Cun and L. Z. Gong, Tetrahedron: Asymmetry, 2007, 18, 237–242 CrossRef CAS.
- (a) U. Eder, G. Sauer and R. Wiechert, Angew. Chem., Int. Ed. Engl., 1971, 10, 496–497 CrossRef CAS; (b) Z. G. Hajos and D. R. Parrish, J. Org. Chem., 1974, 39, 1615–1621 CrossRef CAS; (c) B. List, R. A. Lerner and C. F. Barbas III, J. Am. Chem. Soc., 2000, 122, 2395–2396 CrossRef CAS; (d) W. Notz and B. List, J. Am. Chem. Soc., 2000, 122, 7386–7387 CrossRef CAS.
- Proline analogues: (a) J. M. Huang, X. T. Zhang and D. W. Armstrong, Angew. Chem., Int. Ed., 2007, 46, 9073–9077 CrossRef CAS PubMed; (b) S. Chandrasekhar, B. Tiwari, B. B. Parida and C. R. Reddy, Tetrahedron: Asymmetry, 2008, 19, 495–499 CrossRef CAS; (c) D. Font, S. Sayalero, A. Bastero, C. Jimeno and M. A. Pericás, Org. Lett., 2008, 10, 337–340 CrossRef CAS PubMed; (d) F. Giacalone, M. Gruttadauria, P. Agrigento, P. L. Meo and R. Noto, Eur. J. Org. Chem., 2010, 5696–5704 CrossRef CAS; (e) A. Armstrong, Y. Bhonoah and A. J. P. White, J. Org. Chem., 2009, 74, 5041–5048 CrossRef CAS PubMed; (f) E. Montroni, S. P. Sanap, M. Lombardo, A. Quintavalla, C. Trombini and D. D. Dhavale, Adv. Synth. Catal., 2011, 353, 3234–3240 CrossRef CAS.
- Amino acid: (a) A. Bassan, W. Zou, E. Reyes, F. Himo and A. Córdova, Angew. Chem., Int. Ed., 2005, 44, 7028–7032 CrossRef CAS PubMed; (b) A. Córdova, W. Zou, I. Ibrahem, E. Reyes, M. Engqvist and W. W. Liao, Chem. Commun., 2005, 3586–3588 RSC; (c) M. Amedjkouh, Tetrahedron: Asymmetry, 2007, 18, 390–395 CrossRef CAS; (d) C. L. Wu, X. Q. Long, S. Li and X. K. Fu, Tetrahedron: Asymmetry, 2012, 23, 315–328 CrossRef CAS.
- N-Arylprolinamides: (a) Y. Q. Fu, Z. C. Li, L. N. Ding, J. C. Tao, S. H. Zhang and M. S. Tang, Tetrahedron: Asymmetry, 2006, 17, 3351–3357 CrossRef CAS; (b) S. Sathapornvajana and T. Vilaivan, Tetrahedron, 2007, 63, 10253–10259 CrossRef CAS; (c) Y. Q. Fu, Z. C. Li, L. N. Ding, J. C. Tao, S. H. Zhang and M. S. Tang, Tetrahedron: Asymmetry, 2006, 17, 3351–3357 CrossRef CAS; (d) J. N. Morrthy and S. Saha, Eur. J. Org. Chem., 2009, 739–748 Search PubMed; (e) Z.-H. Tzeng, H.-Y. Chen, R. J. Reddy, C.-T. Huang and K. Chen, Tetrahedron, 2009, 65, 2879–2888 CrossRef CAS; (f) S. P. Zhang, X. K. Fu and S. D. Fu, Tetrahedron Lett., 2009, 50, 1173–1176 CrossRef CAS; (g) J. W. Xu, X. K. Fu, C. L. Wu and X. Y. Hu, Tetrahedron: Asymmetry, 2011, 22, 840–850 CrossRef CAS; (h) T. Mino, A. Omura, Y. Uda, K. Wakui, Y. Haga, M. Sakamoto and T. Fugita, Tetrahedron: Asymmetry, 2011, 22, 2024–2028 CrossRef CAS; (i) P. B. Thorat, S. V. Goswami, B. C. Khade and S. R. Bhusare, Tetrahedron Lett., 2012, 53, 6083–6086 CrossRef CAS; (j) R. L. Sutar and N. N. Joshi, Tetrahedron: Asymmetry, 2013, 24, 43–49 CrossRef CAS; (k) X. R. Huang, Q. Liu, J. Wang, J. A. Xiao and H. Yang, Tetrahedron: Asymmetry, 2014, 25, 1590–1598 CrossRef CAS.
- Prolinamides derived from carbohydrates: (a) J. Agarwal and R. K. Peddinti, Tetrahedron: Asymmetry, 2010, 21, 1906–1909 CrossRef CAS; (b) J. Agarwal and R. K. Peddinti, Eur. J. Org. Chem., 2012, 6390–6406 CrossRef CAS; (c) C. Shen, F. Shen, G. Zhou, H. Xia, X. Chen, X. Liu and P. Zhang, Catal. Commun., 2012, 26, 6–10 CrossRef CAS; (d) D. Miura, T. Fujimoto, A. Tsutsui and T. Machinami, Synlett, 2013, 1501–1504 CAS.
- BINAM-prolinamide: (a) G. Guillena, M. C. Hita and C. Najera, Tetrahedron: Asymmetry, 2006, 17, 1493–1497 CrossRef CAS; (b) G. Guillena, M. C. Hita and C. Najera, Tetrahedron: Asymmetry, 2006, 17, 729–733 CrossRef CAS; (c) S. Guizzetti, M. Benaglia, L. Raimondi and G. Celentano, Org. Lett., 2007, 9, 1247–1250 CrossRef CAS PubMed; (d) S. F. Viózquez, A. B. Caballero, G. Guillena, C. Nájera and E. G. Bengoa, Org. Biomol. Chem., 2012, 10, 4029–4035 RSC.
- Prolinamide with β-amino alcohol: (a) M. R. Vishnumaya, S. K. Ginotra and V. K. Singh, Org. Lett., 2006, 8, 4097–4099 CrossRef PubMed; (b) V. Maya, M. Raj and V. K. Singh, Org. Lett., 2007, 9, 2593–2595 CrossRef CAS PubMed; (c) Y. Okuyama, H. Nakano, Y. Watanabe, M. Makabe, M. Takeshita, K. Uwai, C. Kabuto and E. Kwon, Tetrahedron Lett., 2009, 50, 193–197 CrossRef CAS; (d) R. M. Vishnumaya and V. K. Singh, J. Org. Chem., 2009, 74, 4289–4297 CrossRef PubMed; (e) X. H. Chen, J. Yu and L. Z. Gong, Chem. Commun., 2010, 6437–6448 RSC.
- Prolinamide of cholic acid methylester: G. L. Puleoa and A. Iuliano, Tetrahedron: Asymmetry, 2007, 18, 2894–2900 CrossRef.
- Aminoimidazole containing prolinamides: G. K. Tang, U. Gun and H. J. Altenbach, Tetrahedron, 2012, 68, 10230–10235 CrossRef CAS.
- (L)-Prolinamides of chiral and achiral diamines: (a) S. Samanta, J. Liu, R. Dodda and C. G. Zhao, Org. Lett., 2005, 7, 5321–5323 CrossRef CAS PubMed; (b) R. Pedrosa, J. M. Andres, R. Manzano and P. Rodriguez, Eur. J. Org. Chem., 2010, 5310–5319 CrossRef CAS; (c) J. P. Delaney and L. C. Henderson, Adv. Synth. Catal., 2012, 354, 197–204 CrossRef CAS.
- Prolinamide of camphor: Z. H. Tzeng, H. Y. Chen, C. T. Huang and K. Chen, Tetrahedron Lett., 2008, 49, 4134–4137 CrossRef CAS.
- Dipeptides and pseudopeptides: (a) M. Lei, L. X. Shi, G. Li, S. L. Chen, W. H. Fang, Z. M. Ge, T. M. Cheng and R. T. Li, Tetrahedron, 2007, 63, 7892–7898 CrossRef CAS; (b) F. B. Chen, S. Huang, H. Zhang, F. Y. Liu and Y. G. Peng, Tetrahedron, 2008, 64, 9585–9591 CrossRef CAS; (c) S. Chandrasekhar, K. Johny and C. R. Reddy, Tetrahedron: Asymmetry, 2009, 20, 1742–1745 CrossRef CAS; (d) A. J. Pearson and S. Panda, Org. Lett., 2011, 13, 5548–5551 CrossRef CAS PubMed; (e) J. G. Hernandez and E. Juaristi, J. Org. Chem., 2011, 76, 1464–1467 CrossRef CAS PubMed; (f) E. Machuca, Y. Rojas and E. Juaristi, Asian J. Org. Chem., 2015, 4, 46–51 CrossRef CAS; (g) I. Triandafillidi, A. Bisticha, E. Voutyritsa, G. Galiatsatou and C. G. Kokotos, Tetrahedron, 2015, 71, 932–940 CrossRef CAS; (h) E. Machuca and E. Juaristi, Tetrahedron Lett., 2015, 56, 1144–1148 CrossRef CAS.
- Recent examples of aldol reactions catalyzed by prolinethioamides, see: (a) D. Gryko and R. Lipinski, Adv. Synth. Catal., 2005, 347, 1948–1952 CrossRef CAS; (b) D. Almasi, D. A. Alonso and C. Najera, Adv. Synth. Catal., 2008, 350, 2467–2472 CrossRef CAS; (c) D. Almasi, D. A. Alonso, A. N. Balaguer and C. Najera, Adv. Synth. Catal., 2009, 351, 1123–1131 CrossRef CAS; (d) B. Wang, X. W. Liu, L. Y. Liu, W. X. Chang and J. Li, Eur. J. Org. Chem., 2010, 5951–5954 CrossRef CAS; (e) S. Fotaras, C. G. Kokotos, E. Tsandi and G. Kokotos, Eur. J. Org. Chem., 2011, 1310–1317 CrossRef CAS; (f) S. Fotaras, C. G. Kokotos and G. Kokotos, Org. Biomol. Chem., 2012, 10, 5613–5619 RSC; (g) P. Revelou, C. G. Kokotos and P. M. Minakakis, Tetrahedron, 2012, 68, 8732–8738 CrossRef CAS; (h) C. D. Maycock and M. R. Ventura, Tetrahedron: Asymmetry, 2012, 23, 1262–1271 CrossRef CAS; (i) C. G. Kokotos, J. Org. Chem., 2012, 77, 1131–1135 CrossRef CAS PubMed.
- Recent examples of aldol reactions catalyzed by sulfonamides, see: (a) K. Mei, S. Zhang, S. He, P. Li, M. Jin, F. Xue, G. Luo, H. Zhang, L. Song, W. Duan and W. Wang, Tetrahedron Lett., 2008, 49, 2681–2684 CrossRef CAS; (b) E. Tsandi, C. G. Kokotos, S. Kousidou, V. Ragoussis and G. Kokotos, Tetrahedron, 2009, 65, 1444–1449 CrossRef CAS; (c) S. P. Zhang, X. K. Fu, S. D. Fu and J. F. Pan, Catal. Commun., 2009, 10, 401–405 CrossRef CAS; (d) S. J. Aratake, T. Itoh, T. Okano, N. Nagae, T. Sumiya, M. Shoji and Y. Hayashi, Chem.–Eur. J., 2007, 13, 10246–10256 CrossRef CAS PubMed; (e) S. Gandhi and V. K. Singh, J. Org. Chem., 2008, 73, 9411–9416 CrossRef CAS PubMed; (f) H. Yang, S. Mahapatra, P. H. Cheong and R. G. Carter, J. Org. Chem., 2010, 75, 7279–7290 CrossRef CAS PubMed; (g) H. Yang and R. G. Carter, Org. Lett., 2008, 10, 4649–4652 CrossRef CAS PubMed; (h) N. Hara, R. Tamura, Y. Funahashi and S. Nakamura, Org. Lett., 2011, 13, 1662–1665 CrossRef CAS PubMed; (i) L. S. Zu, H. X. Xie, H. Li, J. Wang and W. Wang, Org. Lett., 2008, 10, 1211–1214 CrossRef CAS PubMed; (j) H. R. Cabrera, G. Huelgas, J. M. H. Perez, P. J. Walsh, R. Somanathan and C. A. D. Parrodi, Tetrahedron: Asymmetry, 2015, 26, 163–172 CrossRef; (k) J. R. Chen, H. H. Lu, X. Y. Li, L. Cheng, J. Wan and W. Xiao, Org. Lett., 2005, 7, 4543–4545 CrossRef CAS PubMed; (l) M. T. Robak, M. A. Herbage and J. A. Ellman, Tetrahedron, 2011, 67, 4412–4416 CrossRef CAS.
- Chiral amines, see: (a) S. Z. Luo, H. Xu, J. Y. Li, L. Zhang and J. P. Cheng, J. Am. Chem. Soc., 2007, 129, 3074–3075 CrossRef CAS PubMed; (b) B. L. Zheng, Q. Z. Liu, C. S. Guo, X. L. Wang and L. He, Org. Biomol. Chem., 2007, 5, 2913–2915 RSC; (c) S. Z. Luo, H. Xu, L. Zhang, J. Y. Li and J. P. Cheng, Org. Lett., 2008, 10, 653–656 CrossRef CAS PubMed; (d) X. H. Yu and W. Wang, Org. Biomol. Chem., 2008, 6, 2037–2046 RSC; (e) L. Li, L. W. Xu, Y. D. Ju and G. Q. Lai, Synth. Commun., 2009, 39, 764–774 CrossRef CAS; (f) J. Y. Li, S. Z. Luo and J. P. Cheng, J. Org. Chem., 2009, 74, 1747–1750 CrossRef CAS PubMed; (g) C. S. Da, L. P. Che, Q. P. Guo, F. C. Wu, X. Ma and Y. N. Jia, J. Org. Chem., 2009, 74, 2541–2546 CrossRef CAS PubMed; (h) S. Z. Luo, J. Y. Li, L. Zhang, H. Xu and J. P. Cheng, Chem.–Eur. J., 2008, 14, 1273–1281 CrossRef CAS PubMed; (i) J. Agarwal and R. K. Peddinti, J. Org. Chem., 2011, 76, 3502–3505 CrossRef CAS PubMed; (j) B. Xu, L. Li and S. Gou, Tetrahedron: Asymmetry, 2013, 24, 1556–1561 CrossRef CAS; (k) J.-H. Lin, C.-P. Zhang and J.-C. Xiao, Green Chem., 2009, 11, 1750–1753 RSC.
- Organic salts: (a) M. Lombardo, S. Easwar, F. Pasi and C. Trombini, Adv. Synth. Catal., 2009, 351, 276–282 CrossRef CAS; (b) V. Bisai and V. K. Singh, Synlett, 2009, 933–936 Search PubMed; (c) A. Karmakar, T. Maji, S. Wittmann and O. Reiser, Chem.–Eur. J., 2011, 17, 11024–11029 CrossRef CAS PubMed; (d) T. Ichibakase and M. Nakajima, Org. Lett., 2011, 13, 1579–1581 CrossRef CAS PubMed; (e) E. Montroni, M. Lombardo, A. Quintavalla, C. Trombini, M. Gruttadauria and F. Giacalone, ChemCatChem, 2012, 4, 1000–1006 CrossRef CAS; (f) E. G. Doyaguez and A. F. Mayoralas, Tetrahedron, 2012, 68, 7345–7354 CrossRef CAS; (g) L. A. A. Momani and A. Lataifeh, Inorg. Chim. Acta, 2013, 394, 176–183 CrossRef.
- Recyclable proline based organocatalysts: (a) W. Miaoa and T. H. Chan, Adv. Synth. Catal., 2006, 348, 1711–1718 CrossRef; (b) Y. Wu, Y. Zhang, M. Yu, G. Zhao and S. Wang, Org. Lett., 2006, 8, 4417–4420 CrossRef CAS PubMed; (c) D. Front, C. Jimeno and M. A. Pericas, Org. Lett., 2006, 8, 4653–4655 CrossRef PubMed; (d) Y. Hayashi, T. Sumiya, J. Takahashi, H. Gotoh, T. Urushima and M. Shoji, Angew. Chem., Int. Ed., 2006, 45, 958–961 CrossRef CAS PubMed; (e) F. Giacalone, M. Gruttadauria, A. M. Marculescu and R. Noto, Tetrahedron Lett., 2007, 48, 255–259 CrossRef CAS; (f) S. Luo, X. Mi, L. Zhang, S. Liu, H. Xu and J. P. Cheng, Tetrahedron, 2007, 63, 1923–1930 CrossRef CAS; (g) G. Guillena, M. C. Hita, C. Najera and S. F. Viozquez, J. Org. Chem., 2008, 73, 5933–5943 CrossRef CAS PubMed; (h) J. Gao, J. Liu, D. Jiang, B. Xiao and Q. Yang, J. Mol. Catal. A: Chem., 2009, 313, 79–87 CrossRef CAS; (i) Y. Yang, Y. H. He, Z. Guan and W. D. Huang, Adv. Synth. Catal., 2010, 352, 2579–2587 CrossRef CAS; (j) S. S. Khan, J. Shah and J. Liebscher, Tetrahedron, 2010, 66, 5082–5088 CrossRef CAS; (k) T. Miura, K. Imai, M. Ina, N. Tada, N. Imai and A. Itoh, Org. Lett., 2010, 12, 1620–1623 CrossRef CAS PubMed; (l) C. L. Wu, X. K. Fu and S. Li, Tetrahedron, 2011, 67, 4283–4290 CrossRef CAS; (m) T. Miura, K. Imai, H. Kasuga, M. Ina, N. Tada, N. Imai and A. Itoh, Tetrahedron, 2011, 67, 6340–6346 CrossRef CAS; (n) A. M. Castaneda, B. Poladura, H. R. guez-Solla, C. Concellon and V. Amo, Org. Lett., 2011, 13, 3032–3035 CrossRef PubMed; (o) J. G. Hernandez and E. Juaristi, Tetrahedron, 2011, 67, 6953–6959 CrossRef CAS; (p) V. Gauchot and A. R. Schmitzer, J. Org. Chem., 2012, 77, 4917–4923 CrossRef CAS PubMed; (q) X. Zhang, W. Zhao, C. Qu, L. Yang and Y. Cui, Tetrahedron: Asymmetry, 2012, 23, 468–473 CrossRef CAS; (r) H. L. Yang, S. W. Li, X. Y. Wang, F. W. Zhang, X. Zhong, Z. P. Dong and J. T. Ma, J. Mol. Catal. A: Chem., 2012, 363–364, 404–410 CrossRef CAS; (s) J. G. Hernandez, V. Garcia-Lopez and E. Juaristi, Tetrahedron, 2012, 68, 92–97 CrossRef CAS; (t) A. B. Caballero, G. Guillena, C. Nájera, E. Faggi, R. M. Sebástian and A. Vallribera, Tetrahedron, 2013, 69, 1307–1315 CrossRef; (u) C. Qu, W. Zhao, L. Zhang and Y. Cui, Chirality, 2014, 26, 209–213 CrossRef CAS PubMed; (v) D. E. Siyutkin, A. S. Kucherenko and S. G. Zlotin, Tetrahedron, 2010, 66, 513–518 CrossRef CAS; (w) A. Bañón-Caballero, G. Guillena and C. Nàjera, Green Chem., 2010, 12, 1599–1606 RSC; (x) A. Monge-Marcet, X. Cattoën, D. A. Alonso, C. Nàjera, M. W. C. Man and R. Pleixats, Green Chem., 2012, 14, 1601–1610 RSC; (y) P. Llanes, S. Sayalero, C. Rodriguez-Escrich and M. A. Pericàs, Green Chem., 2016, 18, 3507–3512 RSC.
- (a) G. D. Yadav and S. Singh, Tetrahedron: Asymmetry, 2015, 26, 1156–1166 CrossRef CAS; (b) M. S. Chauhan, P. Kumar and S. Singh, RSC Adv., 2015, 5, 52636–52641 RSC; (c) G. D. Yadav and S. Singh, Tetrahedron: Asymmetry, 2016, 27, 123–129 CrossRef CAS; (d) G. D. Yadav and S. Singh, Tetrahedron: Asymmetry, 2016, 27, 463–466 CrossRef CAS.
- Z. Zhang, H. Y. Bae, J. Guin, C. Rabalakos, M. V. Gemmeren, M. Leutzsch, M. Klussmann and B. List, Nat. Commun., 2016, 7, 12478 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra23652a |
| This journal is © The Royal Society of Chemistry 2016 |