Room temperature synthesis of manganese oxide quantum dots and their application as a fluorescent probe for the detection of metal ions in aqueous media†
Shaopeng Rongab,
Pengyi Zhang*ab,
Yajie Yanga and
Fang Liua
aState Key Joint Laboratory of Environment Simulation and Pollution Control, School of Environment, Tsinghua University, Beijing 100084, P. R. China. E-mail: zpy@tsinghua.edu.cn
bBeijing Key Laboratory of Indoor Air Quality Evaluation and Control, Beijing 100084, P. R. China
First published on 30th November 2016
Abstract
A rapid and facile route to access manganese oxide quantum dots (MOQDs) has been developed for the first time at room temperature by separating them from a manganese oxide nanosheet colloidal suspension using ultrafiltration. The size and thickness of the as-prepared MOQDs was 3.0–9.0 nm and 1.0–2.5 nm, respectively, which can be controlled by changing the molecular weight cut-off of the membrane. The photoluminescence behavior of the MOQDs is found to be strongly dependent on the excitation wavelength as well as particle size, but insignificant dependence on pH. Moreover, the MOQDs showed excellent long term resistance to photobleaching. The features of excellent photostability, mild pH variation and air resistance, make MOQDs a good candidate as a fluorescent probe for the detection of metal ions in aqueous media. The experiments of selectivity and competition towards various metal ions indicate that the MOQDs exhibit high selectivity for Mn2+ and Fe3+, and the other metal ions have an insignificant effect on the sensing system.
1. Introduction
In recent years, fluorescent quantum dot (QD) materials have generated enormous excitement due to their promising potential in fluorescent probes,1 photovoltaic devices,2 photocatalysis,3 and bioimaging.4 Due to high emission quantum yields and size tunable emission profiles, QDs have proven themselves as one of the most extensively used optical sensing nanomaterials in the detection of nucleic acids, enzymes, proteins, metal ions, and other small molecules, especially due to their long term resistance to photobleaching.5–9 Among many of the QD materials, carbon quantum dots (CQDs) or graphene quantum dots (GQDs) are undoubtedly the hottest research materials, which have attracted enormous attention and research interests. The exceptional properties of CQDs or GQDs have continuously stimulated the rapid development of their synthetic methods. Ever since the first synthesis of CQDs by laser ablation,10 various synthetic methods have been developed for CQD synthesis. Approaches for synthesizing CQDs with tunable size have been generally classified into two groups: top-down and bottom-up methods. The top-down methods include electron beam lithography,11 acidic exfoliation,12,13 electrochemical oxidation,14 microwave-assisted hydrothermal synthesis,15 and so on. CQDs synthesized by top-down methods are derived from the “cleavage” or “broken off” larger carbon materials. Compared with the top-down methods, literature focusing on the bottom-up methods is relatively scarce. Bottom-up methods consist of solution chemistry,16 cyclodehydrogenation of polyphenylene precursors,17 carbonizing some special organic precursors,18 the cage-opening of fullerene,19 and so on. Besides CQDs or GQDs, other quantum dots materials also have been successfully produced. Li's group through liquid–solid-solution (LSS) process successfully synthesized a large variety of nanocrystals with different chemistries and properties, including noble metal (Pd, Ag, Pt, Au, Ir, Ru and Rh), magnetic (MFe2O4, M = Fe, Co, Mn, Zn, Mg), semiconducting (MS and MSe, M = Zn, Cd, Mn, Cu, Pb), rare-earth fluorescent (NaYF4, YF3, LaF3, PrF3, YbF3), dielectric MTiO3 (BaTiO3, PbTiO3, SrTiO3).20 Ternary group QDs are alternative to binary QDs. The synthesis of ternary QDs is difficult due to the necessity of balancing of the reactivity of more than two precursors to form the nuclei of ternary nanocrystals that subsequently grow into QDs with the same composition.21 Malik et al. first synthesized ternary QDs (CuInSe2) quantum dots via injecting trioctylphosphine selenide at 250 °C into a mixture of CuCl and InCl3 in trioctylphosphine oxide.22 Deng et al. synthesized ZnAgInS quantum dots with a best quantum yield of ∼30% by hydrothermal synthesis method.23 Examination of the literatures reveals that there have been numerous articles published on ternary QDs pertaining to their synthesis, properties, and applications in diverse fields of science.Among the many properties of QDs, the most fascinating is its photoluminescence (PL). Based on the PL of QDs, different sensors have been fabricated as a fluorescent probe for the detection of metal ions and other small molecules. Wang et al., firstly reported that GQDs could be used for Fe3+ detection on the basis of the selective fluorescence quenching effect to Fe3+.24 In addition, there were also several successful examples of using CQDs or GQDs as fluorescent sensing materials for the detection of metal ions such as Cu2+ and Hg2+, as well as the discrimination of Fe3+ and Fe2+ in living cells or aqueous media.25,26 Barman et al. successfully synthesized and characterized highly fluorescent blue g-carbon nitride quantum dots, and found that these quantum dots were highly sensitive and selective florescent probes for mercuric ions in aqueous media.27 On the other hand, besides CQDs or GQDs, other quantum dots materials (CdS, PbSe and CdSe, etc.) also have been successfully used as a fluorescent probe for the detection of metal ions.28,29 However, no effort has been made to synthesize manganese oxide quantum dots (MOQDs), which are similar to CQDs or GQDs. Noticeably, the detection of Mn2+ in aqueous media via QDs is also rarely reported in the literature.
Herein, for the first time, we report a rapid and facile route to access MOQDs at room temperature. The PL behavior of MOQDs is detailed studied. In addition, the experiments of selectivity and competition towards various metal ions indicate that the MOQDS own high selectivity for Mn2+ and Fe3+, and the other metal ions have insignificant effect on the sensing system. The as-prepared MOQDs can serve as a fluorescent probe for the detection of Mn2+ and Fe3+.
2. Experimental
2.1 Materials
Tetramethylammonium hydroxide (TMA·OH, 25 wt% in H2O), MnCl2·4H2O and H2O2 (30 wt% in H2O) were commercially available and used without purification. For the detection of various metal ions, KCl, NaCl, MnCl2·4H2O, CuCl2·2H2O, CrCl2·6H2O, Pb(NO3)2, HgCl2, NiCl·6H2O, MgCl2, ZnCl2, CaCl2 and FeCl3·6H2O have been used as various ion sources. All chemicals used were of analytical grade and used with received. Solutions in all experiments were prepared with ultrapure water obtained using an ultrapure water system (18.2 MΩ, Thermo Co., USA).2.2 Preparation of manganese oxide quantum dots (MOQDs)
The synthesis of MOQDs contains two steps which were the preparation of MnO2 nanosheets stage and ultrafiltration treatment stage. MnO2 nanosheets were synthesized through a one-step procedure according to a literature procedure reported previously.30 Briefly, the mixed aqueous solution prepared from 4.4 mL of TMA·OH, 2 mL of 30 wt% H2O2 and 15 mL of ultrapure water was poured into 10 mL of 0.3 M MnCl2·4H2O with stirring, and kept stirring for 12 h in the open air at room temperature. Subsequently, the light brown upper solution contained MnO2 nanosheets was collected by centrifugation at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min. Then, ultrafiltration cell (Model 8200, Millipore, USA) equipped with different molecular weight cut-off membrane (3 kD, 10 kD) was used to separate MOQDs from the MnO2 nanosheets colloidal suspension at room temperature. The membrane use in this study was flat sheet regenerated cellulose (RC) membrane (Merck, Millipore, USA) with 28.7 cm2 effective membrane area and membrane diameter of 63.5 mm. The working pressure was provided by a connected high-pressure tank filled with nitrogen, with a gas pressure regulator. The samples separated with the different molecular weight cut-off membrane hereafter are referred to as MOQDs-3 kD and MOQDs-10 kD, respectively. The sample labeled as MOQDs was separated with the 3 kD molecular weight cut-off membrane for all experiments unless otherwise mentioned in the discussion.
000 rpm for 30 min. Then, ultrafiltration cell (Model 8200, Millipore, USA) equipped with different molecular weight cut-off membrane (3 kD, 10 kD) was used to separate MOQDs from the MnO2 nanosheets colloidal suspension at room temperature. The membrane use in this study was flat sheet regenerated cellulose (RC) membrane (Merck, Millipore, USA) with 28.7 cm2 effective membrane area and membrane diameter of 63.5 mm. The working pressure was provided by a connected high-pressure tank filled with nitrogen, with a gas pressure regulator. The samples separated with the different molecular weight cut-off membrane hereafter are referred to as MOQDs-3 kD and MOQDs-10 kD, respectively. The sample labeled as MOQDs was separated with the 3 kD molecular weight cut-off membrane for all experiments unless otherwise mentioned in the discussion.
2.3 Fluorescence sensing of metal ion
For the detection of various metal ions, 50 μL various amounts of metal ions were added to solution of MOQDs (2 mL). The MOQDs solution was prepared by ultrafiltration cell equipped with 3 kD molecular weight cut-off membrane. The solution was mixed thoroughly at room temperature for 1 min before the spectral measurements. The fluorescence spectra were recorded under excitation at 320 nm for all the detection.2.4 Characterization
Ultraviolet-visible light (UV-vis) absorption spectra were recorded on a Hach DR6000 UV-vis spectrometer (Hach, USA) in the range of 200–800 nm. Fluorescence spectra were collected using a Hitachi F-7000 Fluorometer (Hitachi, Japan). Scanning electron microscopy (SEM) were conducted with a Hitachi S-5500 field emission scanning electron microscope (Hitachi, Japan) operated at 10 kV. Atomic force microscopy (AFM) images of samples were carried out in tapping mode with a Veeco NanoManVS (Veeco Instruments, USA). Transmission electron microscopic (TEM) was recorded on JEM-2011 (JEOL, Japan) operated at an accelerating voltage of 150 kV.3. Results and discussions
3.1 The synthesis and characterization of MOQDs
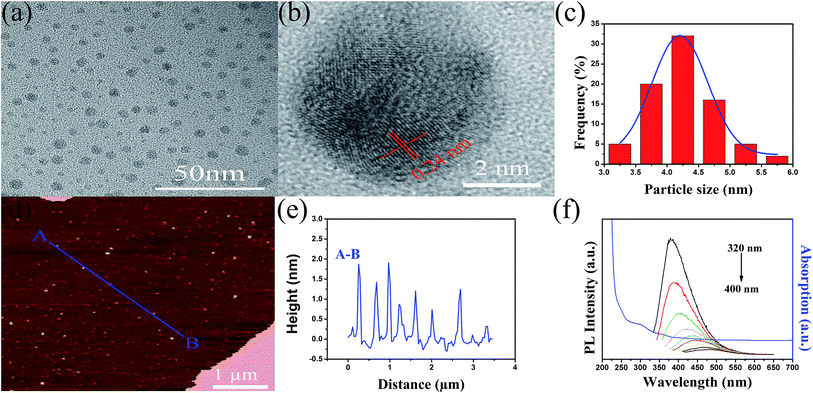
The synthesis of MOQDs contains two steps, i.e. preparation of MnO2 nanosheets and then ultrafiltration treatment. Firstly, MnO2 nanosheets was synthesized by chemical oxidation of Mn2+ ions in the presence of tetramethylammonium cations in an aqueous solution. In the nanosheets suspension, the Tyndall light scattering was observed which confirms the colloidal dispersion of the MnO2 nanosheet suspension (Fig. S1 in ESI†), and no precipitation was observed within a long time. As shown in Fig. 1a, the SEM images of freeze-dried aggregates of the sample revealed abundant two-dimensional nanosheets with lateral dimension of micrometers. These sheet-like structure was also evident from AFM image of the sample deposited by dropping of the colloidal suspension onto mica substrate (Fig. 1c). From the AFM height profile scan along the line A–B demonstrates a fairly flat surface of the sheets with an approximate thickness of 2.0 nm (Fig. 1d). According to the results of SEM and AFM, the morphology characterization indicates that the synthesized MnO2 is layer-structured nanosheets with a high ratio of width to thickness. The nanostructure of the layered MnO2 nanosheets was further confirmed by UV-vis absorption spectra. Fig. 1b shows the UV-vis absorption spectra in the range of 200–800 nm as a function of concentration of the MnO2 nanosheets colloidal suspension. The MnO2 nanosheets colloidal suspension shows a broad absorption peak centered around 383 nm. The absorbance is linearly dependent on the MnO2 nanosheets concentration. According to previous reports, the absorption peak in the range of 300–400 nm was a typical optical characteristic of the nanosheets materials. Omomo et al. have reported similar optical properties for MnO2 nanosheets which were prepared by a multistep process, and the nanosheets showed an absorption peak centered around 374 nm.31 Zhao et al. took graphene oxide as a template to synthesize layered MnO2 nanosheets with the thickness of 14.3 nm, which showed a broad absorption peak at 324 nm.32 The absorption band of the colloidal suspension is attributable to the d–d transition of Mn ions in the MnO6 octahedra of the MnO2 nanosheets.33From the results of AFM (Fig. 1c and d), we found that lateral dimension of the MnO2 nanosheets was distributed in a wide range, and the thickness of different nanosheets is not uniform. There are a number of nano-dots with a smaller lateral scale and thickness in the colloidal suspension. As shown in Fig. 1c, the AFM height profile along the C–D line demonstrates the topographic heights of nano-dots is mostly around 1 nm, which are smaller and thinner than other nanosheets. Thus, ultrafiltration cell equipped with various molecular weight cut-off membrane was used to separate MOQDs from the MnO2 nanosheets colloidal suspension at room temperature. As shown in Fig. 2a by transmission electron microscopy (TEM) images, the separated MOQDs are very tiny dots. High resolution TEM (HRTEM) images further confirmed the particle size and showed that the individual MOQDs possess a crystalline structure, the lattice spacing of 0.24 nm could be easily identified. According to size distribution histograms (Fig. 2c) indicated that the diameters of MOQDs are mainly distributed in the range of 3.0–6.0 nm (average diameter = 4.2 nm), and the size distribution accords well with Gaussian distribution. AFM images (Fig. 2d and e) shows the height of these MOQDs in the range of 1–2 nm, which indicates that most MOQDs consist of 1–3 layers.
3.2 The PL behavior of MOQDs
As mentioned above, the MnO2 nanosheets colloid shows a broad absorption centered around 383 nm. For the MOQDs, the broad absorption peak at 383 nm disappeared, while a new absorption peak around 300 nm was observed (Fig. 2f). In addition to the UV absorption, PL is more interesting behavior exhibited by QDs. Up to now, various kinds of QDs with different PL colors, ranging from visible to near-infrared, have been reported. In order to learn the PL behavior of as-prepared MOQDs, a detailed PL spectra were recorded using different excitation wavelengths. As shown in Fig. 2f, under excitation of 320 nm, a strong emission peak at 380 nm is observed in the PL emission spectrum of MOQDs. Similar to CQDs or GQDs, the emission spectra of the as-prepared MOQDs also show an excitation-dependent feature, which is very common phenomenon found in fluorescent carbon materials. As shown in Fig. 2f, when the excitation wavelength changed from 320 to 400 nm, the PL peak of MOQDs shifts to longer wavelengths and its PL intensity decreases significantly. We think that the excitation dependence properties may result from optical selection of differently sized and/or different surface states. On the one hand, the excitation wavelength dependent phenomenon is due to the co-existence of MOQDs with different sizes in each sample. On the other hand, it may be due to the different surface states in MOQDs. MOQDs consist of Mn–O core, as well as O, H, N and TMA containing functional groups on the surface. These functional groups have various energy levels, which may result in a series of emissive traps. When a certain excitation wavelength illuminates the MOQDs, a surface state emissive trap will dominate the emission. When the excitation wavelength changes, the emission will be dominated by another surface state emissive trap. The emissive traps induced by surface states of the functional groups should be closely related to the emission of MOQDs. Therefore, the uniformity both in the size and the surface state contained in MOQDs may be responsible for the excitation-dependent phenomenon of MOQDs in this case.Besides the excitation wavelength dependence, the size-dependence of PL emission is also a common behavior with QDs.34 When QDs is smaller than their exciton Bohr radius, the quantum confinement effect will occur.35 Briefly, as the QDs particles are smaller, the peak position of the PL will shift to shorter wavelength. In order to explore the size-dependence PL of MOQDs, ultrafiltration cell equipped with two kinds of molecular weight cut-off membrane (3 kD, 10 kD) was used to separate different size MOQDs from the MnO2 nanosheet colloid. From the Fig. 3, the diameters of MOQDs-10 kD are mainly in the range of 4.0–9.0 nm (average diameter = 6.7 nm), and the size distribution accords well with Gaussian distribution. AFM images shows the height of these MOQDs-10 kD is between 1.5 and 2.5 nm. The PL emission spectra and UV-vis absorption spectrum of MOQDs-10 kD are similar to MOQDs as discussed above. Similar to MOQDs-3 kD, the excitation-dependent feature also has been observed in MOQDs-10 kD. However, the peak position of the emission PL changed with the size of the MOQDs. As shown in Fig. 4a, the PL peak position of two different size MOQDs are 380 nm and 395 nm, respectively. The results reveals that the PL peak will shift to red color with the increase of QDs size. Compared with the CQDs or GQDs reported previously,36 they all had an obvious size-dependence, and the PL peak had a big shift with the increase of QDs size. Although the size of the MOQDs increases with molecular weight cut-off membrane, the PL emission peak of the solutions does not change significantly. The reason may because there is no big difference in the size of the MOQDs, the particles size separated by various molecular weight cut-off ultrafiltration membrane are close to each other. In addition, the PL peak position was also dependent on the preparation method. Some literatures have reported that the emission colors of GQDs prepared via different methods were contrary to what would be expected based solely on quantum confinement effects, namely, smaller GQDs had longer emission wavelength, and larger GQDs exhibited shorter one.37 Consequently, the particle size plays an important role in the luminescence of QDs, but it is not the only or absolute ones.
QDs synthetized by various methods probably exhibit distinct PL mechanism, which leads to different dependence of their PL on size, excitation wavelength, pH. Some QDs also showed the pH-dependent phenomenon. Pan et al. have prepared GQDs by hydrothermal method, under alkaline conditions the GQDs emit strong PL. Whereas, under acidic conditions the PL is almost quenched.38 And when pH is switched repeatedly between 13 and 1, the PL intensity varies reversibly. Herein, MOQDs also showed a weak pH-dependence as seen in Fig. 4b. Similar to the GQDs prepared by Pan,38 under alkaline conditions, the MOQDs emit strong PL. However, when the pH was changed to acidic conditions, the PL was not completely quenched. From Fig. 4b, we also could find that the PL intensity increased with the pH changing from 1 to 13. In contrast to GQDs as discussed above, MOQDs is not sensitive to the pH of the solution, which makes it good candidate as a fluorescent probe. Moreover, GQDs reported by Dong et al. showed the strongest PL intensity in weak acidic solutions (pH 4–6) and decreased under alkaline conditions.39 The incoherence in pH-dependent PL behaviors obviously suggests dissimilar PL mechanisms in various QDs, but the exact mechanism remains unclear and need to be explored. In addition, if pH is switched repeatedly between 13 and 1, the PL intensity of MOQDs varies reversibly, which is similar to those of fluorescent carbon nanoparticles. Moreover, it is noticeable that the pH had almost no effect on the PL peak position, but just affected the PL intensities of MOQDs.
Furthermore, we also noticed that MOQDs showed high photostability in aqueous media, the photobleaching phenomenon was not found under continuous long-term excitation test. The photostability of the MOQDs was tested by recording the change of the PL intensity with irradiation time. As shown in Fig. 4c, no obvious reduction in PL intensity was observed under continuous excitation over 60 min, indicating that the MOQDs were resistant to photobleaching. It is worth pointing out that the MOQDs are very stable in aqueous solution, and no obvious precipitation was found in several months.
3.3 Fluorescence sensing of Mn2+ and Fe3+ based on MOQDs
The MOQDs have showed excellent photostability, mild pH variation and air exposure, and all these features make MOQDs a good candidate as a fluorescent probe. Fig. S2 (ESI†) shows that the PL intensity gradually quenched with increasing concentration of Fe3+. Moreover, the obviously changes of PL peak position were not found with the addition of Fe3+, indicating that the concentration of Fe3+ had little effect on the PL peak position. A good linear correlation was observed over the concentration in range of 10–500 μM (Fig. S3 in ESI†), indicating their excellent sensing properties in the detection of Fe3+. The complexity of aqueous medium poses a great importance in selectivity to the metal ions detection. Thus, the selectivity and competition experiments towards various metal ions were also investigated. Fig. 5a shows the PL spectra of MOQDs in the absence and presence of various metal ions, showing that the PL intensities in the presence of 500 μM Fe3+ ions decreased to ∼20% of its initial value, while no obvious decrease of the PL intensity were observed for other metal ions (K+, Na+, Cu2+, Zn2+, Mg2+, Cr2+, Ni2+, Pb2+, Ca2+ and Hg2+) at the same concentration. In addition, when the Fe3+ was subsequently injected into the various metal ions solutions, the PL intensities of all the mixed solutions decreased to ∼20% of its initial value, which indicated that these metal ions had negligible effects on the detection of Fe3+. Thus, these metal ions do not interfere the signal for Fe3+ sensing, suggesting the good selectivity of MOQDs toward Fe3+.Besides the MOQDs show the property to detect Fe3+, the same phenomenon could also be observed for Mn2+. Similar to experiments carried out on the detection of Fe3+, with the increase of Mn2+ concentration, the PL intensity gradually decreased to ∼2% of its initial value (Fig. S4 in ESI†). Also, the PL intensity ratios presented an almost linear dependence on the Mn2+ concentration in the range of 0 to 100 μM (Fig. S5 in ESI†). Compared with adding Fe3+, 100 μM Mn2+ could completely quench the PL of MOQDs, indicating that MOQDs has a higher sensitivity to Mn2+. In order to investigate whether the MOQDs was selective for Mn2+, the effect of various metal ions in aqueous solution was also studied. As shown in Fig. 5b, the PL of MOQDs was effectively quenched by only Mn2+, whereas no obvious quenched of PL was observed by other metal ions. The above results clearly indicated that the MOQDs also showed high selectivity in the detection toward Mn2+.
Up to now, the exact mechanism of PL for QDs remains unclear, the luminescence has been tentatively suggested to arise from excitons of carbon, emissive traps, quantum confinement effect, aromatic structures, oxygen-containing groups, free zigzag sites and edge defects.36,40–42 A widely accepted mechanism for good selectivity against other metal ions needs systematic investigation. However, the possible mechanism could be attributed to the strong interaction between TMA molecule on surface of MOQDs and Fe3+ or Mn2+. During the course of two-dimensional growth of nanosheets, TMA cations can be adsorbed on the surface of MnO2, which effectively inhibit the self-assembly of the MnO2 monolayer and limits the thickness of MnO2. As a result, MnO2 grows along two-dimensional direction to form nanosheet. The MOQDs was separated from MnO2 nanosheets colloidal suspension using ultrafiltration. Therefore, the surface of MOQDs would be covered with TMA molecule. The high selectivity could be attributed to the strong interaction between TMA molecule and Fe3+ or Mn2+. This indicates that TMA was stably confined onto the MOQDs surface and may coordinate with Fe3+ or Mn2+. The greater affinity of Fe3+ or Mn2+ ions toward nitrogen atoms of TMA would form the stable complexes. Although TMA may also bind with other metal ions, it is obvious that there is an optimum potential for electron transfer between MOQDs–TMA and metal ions. It may be the optimum potential for the electron transfer between MOQDs–TMA and Fe3+ or Mn2+ ions. Then the as-formed complexes would facilitate the charge transfer under irradiation, leading to significant fluorescence quenching.
4. Conclusions
In summary, we successfully obtained MOQDs for the first time at room temperature by simply separating from manganese oxide nanosheets colloid with ultrafiltration. Similar to QDs synthetized by various methods, the PL behavior of MOQDs was strongly dependent on the excitation wavelength and particle's size, but independent of pH. Furthermore, the features of excellent photostability, mild pH variation and air resistance, made MOQDs a good candidate as a fluorescent probe for the detection of metal ions in aqueous media. Results indicated that the MOQDs showed high selectivity for Mn2+ and Fe3+, and other metal ions have little interference. However, the clear mechanism for good selectivity against other metal ions was still unknown at the present stage, the essential reasons needed to be further explored. This strategy may provide a rapid and facile route to prepared MOQDs, which have a promising applicability for the detection of metal ions in aqueous media.Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No. 21677083, 21521064, 21411140032) and Tsinghua University Initiative Scientific Research Program (20131089251).Notes and references
- Q. Qu, A. W. Zhu, X. L. Shao, G. Y. Shi and Y. Tian, Chem. Commun., 2012, 48, 5473–5475 RSC.
- P. Mirtchev, E. J. Henderson, N. Soheilnia, C. M. Yip and G. A. Ozin, J. Mater. Chem., 2012, 22, 1265–1269 RSC.
- H. T. Li, X. D. He, Z. H. Kang, H. Huang, Y. Liu, J. L. Liu, S. Y. Lian, C. H. A. Tsang, X. B. Yang and S. T. Lee, Angew. Chem., Int. Ed., 2010, 49, 4430–4434 CrossRef CAS PubMed.
- L. Cao, S. T. Yang, X. Wang, P. J. G. Luo, J. H. Liu, S. Sahu, Y. M. Liu and Y. P. Sun, Theranostics, 2012, 2, 295–301 CrossRef CAS PubMed.
- J. Wang, G. Liu and A. Merkoçi, J. Am. Chem. Soc., 2003, 125, 3214–3215 CrossRef CAS PubMed.
- R. Freeman and I. Willner, Nano Lett., 2009, 9, 322–326 CrossRef CAS PubMed.
- J. H. Choi, K. H. Chen and M. S. Strano, J. Am. Chem. Soc., 2006, 128, 15584–15585 CrossRef CAS PubMed.
- R. Gill, L. Bahshi, R. Freeman and I. Willner, Angew. Chem., Int. Ed., 2008, 47, 1676–1679 CrossRef CAS PubMed.
- Y. Wang and L. Chen, Nanomedicine, 2011, 7, 385–402 CAS.
- Y. P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. F. Wang, P. J. G. Luo, H. Yang, M. E. Kose, B. L. Chen, L. M. Veca and S. Y. Xie, J. Am. Chem. Soc., 2006, 128, 7756–7757 CrossRef CAS.
- L. A. Ponomarenko, F. Schedin, M. I. Katsnelson, R. Yang, E. W. Hill, K. S. Novoselov and A. K. Geim, Science, 2008, 320, 356–358 CrossRef CAS PubMed.
- Y. Dong, C. Chen, X. Zheng, L. Gao, Z. Cui, H. Yang, C. Guo, Y. Chi and C. M. Li, J. Mater. Chem., 2012, 22, 8764–8766 RSC.
- J. Peng, W. Gao, B. K. Gupta, Z. Liu, R. Romero-Aburto, L. Ge, L. Song, L. B. Alemany, X. Zhan, G. Gao, S. A. Vithayathil, B. A. Kaipparettu, A. A. Marti, T. Hayashi, J.-J. Zhu and P. M. Ajayan, Nano Lett., 2012, 12, 844–849 CrossRef CAS PubMed.
- Y. Li, Y. Hu, Y. Zhao, G. Shi, L. Deng, Y. Hou and L. Qu, Adv. Mater., 2011, 23, 776–780 CrossRef CAS.
- L. L. Li, J. Ji, R. Fei, C. Z. Wang, Q. Lu, J. R. Zhang, L. P. Jiang and J. J. Zhu, Adv. Funct. Mater., 2012, 22, 2971–2979 CrossRef CAS.
- X. Yan, X. Cui and L. S. Li, J. Am. Chem. Soc., 2010, 132, 5944–5945 CrossRef CAS PubMed.
- X. Yan, X. Cui, B. Li and L. S. Li, Nano Lett., 2010, 10, 1869–1873 CrossRef CAS PubMed.
- L. Tang, R. Ji, X. Cao, J. Lin, H. Jiang, X. Li, K. S. Teng, C. M. Luk, S. Zeng, J. Hao and S. P. Lau, ACS Nano, 2012, 6, 5102–5110 CrossRef CAS PubMed.
- D. V. Kosynkin, A. L. Higginbotham, A. Sinitskii, J. R. Lomeda, A. Dimiev, B. K. Price and J. M. Tour, Nature, 2009, 458, 872–876 CrossRef CAS PubMed.
- X. Wang, J. Zhuang, Q. Peng and Y. D. Li, Nature, 2005, 437, 121–124 CrossRef CAS PubMed.
- A. Das and P. T. Snee, ChemPhysChem, 2016, 17, 598–617 CrossRef CAS PubMed.
- M. A. Malik, P. O'Brien and N. Revaprasadu, Adv. Mater., 1999, 11, 1441–1444 CrossRef CAS.
- D. Deng, J. Gao, L. Qu, S. Achilefu and Y. Gu, Phys. Chem. Chem. Phys., 2013, 15, 5078–5083 RSC.
- D. Wang, L. Wang, X. Y. Dong, Z. Shi and J. Jin, Carbon, 2012, 50, 2147–2154 CrossRef CAS.
- S. Liu, J. Q. Tian, L. Wang, Y. W. Zhang, X. Y. Qin, Y. L. Luo, A. M. Asiri, A. O. Al-Youbi and X. P. Sun, Adv. Mater., 2012, 24, 2037–2041 CrossRef CAS PubMed.
- Q. Mei, C. Jiang, G. Guan, K. Zhang, B. Liu, R. Liu and Z. Zhang, Chem. Commun., 2012, 48, 7468–7470 RSC.
- S. Barman and M. Sadhukhan, J. Mater. Chem., 2012, 22, 21832–21837 RSC.
- T. W. Sung and Y. L. Lo, Sens. Actuators, B, 2012, 165, 119–125 CrossRef CAS.
- C. L. Wu and Y. B. Zhao, Anal. Bioanal. Chem., 2007, 388, 717–722 CrossRef CAS PubMed.
- K. Kai, Y. Yoshida, H. Kageyama, G. Saito, T. Ishigaki, Y. Furukawa and J. Kawamata, J. Am. Chem. Soc., 2008, 130, 15938–15943 CrossRef CAS PubMed.
- Y. Omomo, T. Sasaki, L. Wang and M. Watanabe, J. Am. Chem. Soc., 2003, 125, 3568–3575 CrossRef CAS PubMed.
- G. X. Zhao, J. X. Li, L. Jiang, H. L. Dong, X. K. Wang and W. P. Hu, Chem. Sci., 2012, 3, 433–437 RSC.
- L. Wang, Y. Omomo, N. Sakai, K. Fukuda, I. Nakai, Y. Ebina, K. Takada, M. Watanabe and T. Sasaki, Chem. Mater., 2003, 15, 2873–2878 CrossRef CAS.
- R. E. Bailey and S. Nie, J. Am. Chem. Soc., 2003, 125, 7100–7106 CrossRef CAS PubMed.
- X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538–544 CrossRef CAS PubMed.
- H. Li, X. He, Z. Kang, H. Huang, Y. Liu, J. Liu, S. Lian, C. H. A. Tsang, X. Yang and S. T. Lee, Angew. Chem., Int. Ed., 2010, 49, 4430–4434 CrossRef CAS PubMed.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS PubMed.
- D. Y. Pan, J. C. Zhang, Z. Li and M. H. Wu, Adv. Mater., 2010, 22, 734–738 CrossRef CAS PubMed.
- Y. Dong, C. Chen, X. Zheng, L. Gao, Z. Cui, H. Yang, C. Guo, Y. Chi and C. M. Li, J. Mater. Chem., 2012, 22, 8764–8766 RSC.
- J. Zhou, C. Booker, R. Li, X. Zhou, T. K. Sham, X. Sun and Z. Ding, J. Am. Chem. Soc., 2007, 129, 744–745 CrossRef CAS PubMed.
- J. Lu, J. X. Yang, J. Z. Wang, A. L. Lim, S. Wang and K. P. Loh, ACS Nano, 2009, 3, 2367–2375 CrossRef CAS PubMed.
- H. X. Zhao, L. Q. Liu, Z. D. Liu, Y. Wang, X. J. Zhao and C. Z. Huang, Chem. Commun., 2010, 46, 8812–8814 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S5. See DOI: 10.1039/c6ra23604a |
| This journal is © The Royal Society of Chemistry 2016 |