Robust polyelectrolyte microcapsules reinforced with carbon nanotubes†
Karolina Chojnacka-Górka,
Anna Rozpędzik and
Szczepan Zapotoczny *
*
Jagiellonian University, Faculty of Chemistry, Ingardena 3, 30-060 Krakow, Poland. E-mail: zapotocz@chemia.uj.edu.pl
First published on 30th November 2016
Abstract
Carbon nanotubes wrapped with polyanions were incorporated in polyelectrolyte microcapsules across their nanoscale thick walls. The nanotubes were co-precipitated in CaCO3 microparticles that were used as templates for deposition of polyelectrolyte multilayers. Capsules with improved stability and reduced permeability were obtained upon removal of the templates.
Hollow polyelectrolyte capsules with dimensions on the nano- and microscale are very attractive carriers of different sensitive substances and active agents (e.g. drugs, dyes, enzymes, proteins, genes).1,2 Serving as microreactors they are valuable for many catalytic, enzymatic and photochemical reactions, protecting catalysts and enzymes molecules from environmental influences and enabling high reaction selectivity.3 Importantly, their very high surface to volume ratio provides high efficiency of heat and mass transfer.
In 1991 Decher and Hong4 reported that positively and negatively charged polymers can be alternately deposited onto solid substrate by electrostatic self-assembly (Layer-by-Layer, LbL method) forming thin films. This technique was later applied to obtain hollow polymer capsules by deposition of polyelectrolyte multilayers (PEMs) on emulsion droplets5 or colloidal particles serving as cores followed by their decomposition.6 LbL provides control on composition in nanoscale and thus other physicochemical properties of the capsules implying their multifunctionality. Application of LbL was later extended for other charged species like inorganic nanoparticles,7 biomolecules,8 and carbon nanotubes (CNTs)9–11 which can be deposited alternately with polyelectrolytes. Mechanical properties of polyelectrolyte microcapsules directly affect delivery of substances. Strong pressure exerted on the capsules walls during removal of the solid cores12 and cellular uptake13 often leads to deformation and rupturing of capsules. Therefore, there is a need to develop capsules with better mechanical integrity.
Since their discovery by Iijima14 in 1991 CNTs have been broadly investigated due to their unique properties. Axial Young's modulus ranging from 200 to 4000 GPa, an average bending strength of ca. 14 GPa, axial compressive strengths of 100 GPa and tensile strength from 11 to 63 GPa for individual multiwalled CNTs were reported.15 Single walled CNTs reveal properties from a typical semiconductor to a good metal, depending on the diameter and helicity of tubes16 while all multiwalled CNTs (MWCNTs) exhibit high electrical conductivity.17 It was also shown that CNTs exhibit outstanding transport properties of gases,18 water19 and small ions20 but also larger hydrophilic molecules like DNA.21 These exceptional properties make them very attractive for formation of high-strength materials,22 electronics,23 drug delivery and catalysis24 prompting intensive studies of composites with CNTs.
An increasing number of studies have focused on employing LbL method for preparing CNTs-based composites. Fabrication of multilayer polymeric films with CNTs by LbL method has been widely used recently. It was confirmed that there is no phase separation problem and that mechanical properties and conductivity of such composite films were improved.25,26 LbL was also used to assembly CNTs on colloidal particles made of melamine, silica, polystyrene, and poly(methyl methacrylate) forming core/shell composites with mono- or multilayers of CNTs27–29 and to incorporate CNTs into hollow polyelectrolyte microcapsules by depositing CNTs alternately with polymers on colloidal templates.9–11 Sano et al. constructed hollow capsules made of CNTs after etching silica gel templates.30 Caruso et al. and Pastine et al. demonstrate encapsulation of CNTs suspension in microcapsules using an interfacial polymerization method.31 To the best of our knowledge, incorporation of CNTs in polyelectrolyte microcapsules walls by embedding them first into template microparticles has not been reported yet. Such approach enables arrangement of CNTs across the capsules' walls but not coplanarly as typically reported so far. The novel method of incorporation of MWCNTs into polyelectrolyte microcapsules across their walls was developed here. Importantly, significant improvement of mechanical properties and reduction of overall permeability of such capsules reinforced with MWCNTs was demonstrated for the first time.
The method reported here is based on sequential adsorption of oppositely charged polyelectrolytes on the template particle composed of CaCO3 co-precipitated with MWCNTs that were previously wrapped with poly(sodium styrenesulfonate) (PSS). Both low and high molecular weight organic additives influence crystallization of CaCO3.32–34 Thus, PSS can be used to control nucleation and growth of CaCO3 microparticles and hence their structure, morphology and size.35 PSS can be also used to solubilize CNTs in water by either grafting of the polyelectrolyte from the surface36 or wrapping that results in non-covalent association of PSS and CNTs.37
In the first step, MWCNTs with the length 3–6 μm, external diameter equal to 10 nm and internal diameter 4.5 nm were dispersed in water in the presence of PSS under sonication. This treatment provided a negative charge to MWCNTs surface as evidenced by ζ-potential measurements (−45.0 ± 2.5 mV) and led to reduction of MWCNTs length. Sonication is a common method used for cutting CNTs to shorter lengths.38 According to Geng et al.39 sonication-cutting procedure does not affect the inner diameter and rolled-up grapheme sheet structure of the CNTs. Miyata et al. showed that even after 18 h of sonication of MWCNTs dispersion 50% of tubes still consisted of two concentric nanotubes.40 STEM images of MWCNTs wrapped with PSS revealed that 3 h of sonication results in stable (at least several weeks) suspension of well-separated single nanotubes (Fig. S1C†). Small aggregates of MWCNTs were still present in the sample after 0.5 h of sonication (Fig. S1B†) that explain some unwanted precipitation from this suspension after several days of storage. Statistical analysis of the STEM images of MWCNTs after 3 h of sonication revealed that the length of 76% of MWCNTs was in the range 0.1–0.6 μm (Fig. S1D†).
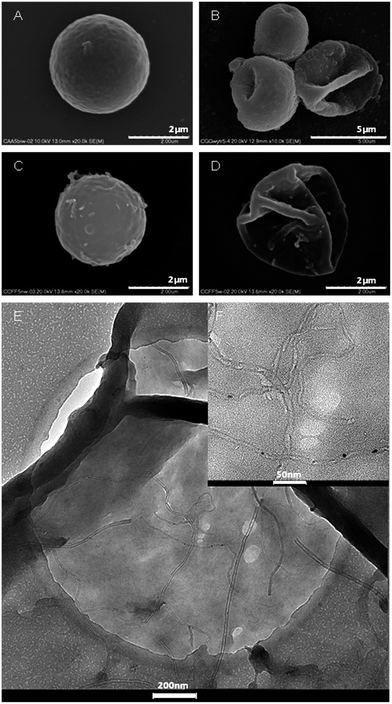
Suspension of MWCNTs was then mixed with Na2CO3 and Ca(NO3)2 solutions and sonicated for 6 min leading to formation of CaCO3 microparticles with embedded MWCNTs (CaCO3-MWCNTs). The morphologies of the precipitated particles were characterized by SEM (Fig. 1). Application in this procedure longer MWCNTs (sonicated 0.5 h) led to formation of particles with irregular shapes. The nucleation of CaCO3 seemed to start at several places along isolated MWCNTs or their bundles leading to formation of chains of particles and flower-like structures (Fig. 1A and B). Short MWCNTs (sonicated 3 h) allowed obtaining particles with spherical shape and smaller size distribution. SEM images show significant differences in the topography of native CaCO3 (Fig. 1E and F) and CaCO3-MWCNTs (Fig. 1C and D) microparticles. In CaCO3-MWCNTs particles nano-sized crystallites are interspersed with nanotubes, fragments of which protrude from the particles interior and/or wrap around them, arranging at different angles to the particles surface. Due to relatively high stiffness of MWCNTs some of them stick out perpendicularly to the surface. This effect was observed even in high vacuum applied during SEM imaging, therefore it can be expected that in aqueous media even larger number of the nanotubes stick out from the particles' surface.
 | ||
| Fig. 1 SEM images of (A, B) CaCO3 particles with long MWCNTs; (C, D) CaCO3 particles with short MWCNTs; (E, F) CaCO3 particles. | ||
Fabricated CaCO3-MWCNTs particles (with average hydrodynamic diameter equal to 3.9 ± 0.15 μm as determined by DLS) were used as templates for preparation of polymeric capsules. The presence of MWCNTs stabilized by anionic polyelectrolyte may contribute also to the negative surface charged of the particles, confirmed by ζ-potential measurement (−27.0 ± 0.1 mV). Therefore, LBL assembly started from the positively charged poly(diallydimethyl ammonium chloride) (PDADMAC) followed by deposition of PSS. The procedure was repeated until 10 layers of the polyelectrolytes were deposited (PSS was the outmost layer). The ζ-potential measurements were performed after each step to confirm formation of polyelectrolyte layers on the microparticles (Fig. S2†).
Fig. 2C presents SEM image of CaCO3-MWCNTs core/shell particles with 5 bilayers of the polyelectrolytes with still visible MWCNTs. To obtain hollow microcapsules CaCO3 cores were dissolved in 0.1 M EDTA (pH = 7). The applied method has been previously developed and should lead to the complete removal of the CaCO3 cores.41 SEM studies (Fig. 2) confirmed effectiveness of the cores' dissolution process and formation of MWCNTs-(PDADMAC/PSS)5 microcapsules with no indication of rupture (Fig. 2D). On the smooth surface of the collapsed capsules fragments MWCNTs can be easily recognized. Additionally, TEM images confirmed the presence of MWCNTs in the capsules walls (Fig. 2E and F and S4†). The content of MWCNTs in the capsules was determined to be ca. 7% based on TGA measurements (Fig. S3†). Capsules templated on native CaCO3 without MWCNTs were also fabricated for comparison using the same procedures for LbL assembly and dissolution of the cores. AFM study (Fig. S5†) allowed to determine the wall thickness of both types of capsules that were similar and equal to 55 ± 5 nm for MWCNTs-(PDADMAC/PSS)5 and 60 ± 8 nm for (PDADMAC/PSS)5. Slightly thinner walls of the capsules reinforced with MWCNTs may indicate more tight arrangement of macromolecules interacting with the embedded nanotubes. SEM images reveal differences in surface topography for both core/shell particles and hollow microcapsules that may be assigned to the presence of MWCNTs in the respective samples (Fig. 2). It seems that the procedure of core removal more significantly affects the capsules without MWCNTs as their surface become more porous and defects appear more frequently (see also Fig. S6C†). The observed defects in the capsules' walls could be a result of osmotic pressure acting on the walls due to high concentration of the core decomposition products inside the capsules. Decreasing the kinetic rate of the core decomposition by reducing the concentration of the chelating agent (EDTA) five times did not bring any significant improvement. Relatively low mechanical stability of (PDADMAC/PSS)5 capsules should be related to low molecular weight of the used PDADMAC (below 100 kDa). Molecular weight of the polyelectrolytes significantly affects their rearrangement in thin films42 and capsules43 implying also entanglements of the chains. Additionally, higher mobility of the shorter chains could lead to shrinkage and rupturing of the capsules at room temperature.39 Analogues capsules consisting of 8 or 10 layers composed of higher molecular weight PDADMAC (200–500 kDa) and the same PSS as the one used here assembled on silica39 or CaCO3 44 did not exhibit significant damages after core decomposition. However, in the current report we intentionally used lower molecular weight PDADMAC in order to easily differentiate stability of native PDADMA/PSS capsules and the ones reinforced with MWCNTs.
FITC labeled dextrans with molecular weights 4 and 70 kDa with approximate hydrodynamics radius 1.9 nm and 6.6 nm, respectively,45 were used as fluorescence probe to study the permeability and integrity of the capsules by fluorescence confocal imaging. Such imaging of MWCNTs-(PDADMAC/PSS)5 verified that the capsules are impermeably to both applied dextrans even after incubation in their solutions for up to 9 weeks (Fig. 3A and B), while they are permeable to small molecules like Rhodamine B (Fig. S7†).
FITC-dextran 70 kDa seems to meet both requirements to be used in testing capsules integrity: it does not permeate the MWCNTs-(PDADMAC/PSS)5 capsules' walls and does not adsorb on the capsules surface terminated with the polyion (Fig. 3B). Therefore, FITC-dextran 70 kDa was used to determine the number of intact (impermeable to FITC-dextran 70 kDa molecules) and broken capsules. Even 7 weeks after formation of the reinforced capsules the percentage of the unbroken ones was determined to be as high as 90%. This number decreased to 81% after 14 weeks and to 75% after 16 weeks, indicating the long-term stability of such capsules.
According to the results of permeability studies performed for similar systems,12,41 (PDADMAC/PSS)5 capsules should be permeable to FITC-dextran 4 kDa and impermeable to 70 kDa. Indeed, fluorescence micrographs of (PDADMAC/PSS)5 showed that the capsules are easily permeable to the lower molecular weight dextran (Fig. 3C). However, the number of capsules permeable to FITC-dextran 70 kDa was found to increase in incubation time (Fig. 3D). The average number of intact capsules after only 160 min was found to be equal to 29%. SEM images confirmed that presence of FITC-dextran 70 kDa in (PDADMAC/PSS)5 capsules' interior was in fact the result of rupturing of the capsules (Fig. S6†) rather than their increasing permeability. Breaking of (PDADMAC/PSS)5 capsules walls was observed also in pure water. 4 weeks after core dissolution and redispersion of hollow capsules in pure water no capsules could be found but only their small fragments (Fig. S8†).
Both, SEM as well as CLSM techniques confirmed that integrity and mechanical long term stability of the obtained capsules were significantly improved by embedding MWCNTs. Due to the very low stability of the native (PDADMAC/PSS)5 capsules it was not possible to compare the mechanical properties of both type of capsules by applying AFM-based46 or osmotic methods.47
Conclusions
We demonstrated here a facile route for incorporation of MWCNTs into polyelectrolyte microcapsules across their walls. It was done by co-precipitation of MWCNTs of tuned lengths within porous calcium carbonate microparticles that served as core templates for preparation of the capsules. Some of the nanotubes sticking out from the microparticles were then built into the polyelectrolyte PDADMAC/PSS multilayers deposited on the templates. We showed that after removal of the templates the formed capsules containing low molecular weight PDADMAC were significantly reinforced by MWCNTs since the native capsules were prone to disintegrate even during dissolution of the cores. Long term stability of the obtained capsules was also significantly improved by embedding MWCNTs even in the amount as small as 7% of their mass. Importantly, permeability to medium-sized molecules (4 kDa) was significantly decreased indicating crucial role of the embedded MWCNTs in tightening of arrangement of macromolecules in the multilayer capsules. The presented here simple approach for incorporation of CNTs into polyelectrolyte capsules may be used to both strengthening of the capsules and tuning their permeability. High robustness of such reinforced capsules may bring them closer to real application where mechanical integrity and long term stability are crucial issues.Acknowledgements
The authors would like to thank the Polish Ministry of Science and Higher Education for the financial support (“Ideas Plus” grant no. IdP2011 000561). Prof. Maria Nowakowska is acknowledged for helpful discussions, Dr Piotr Natkański for TGA measurements and Dr Karol Wolski for AFM measurements. This research was carried out with the equipment purchased thank to the financial support from the European Regional Development Fund in the framework of the Innovation Economy Operational Program (contract no. POIG.02.01.00-12-023/08).Notes and references
- Y. Yan, M. Björnmalm and F. Caruso, Chem. Mater., 2014, 26, 452 CrossRef CAS.
- A. L. Becker, A. P. R. Johnston and F. Caruso, Small, 2010, 6, 1836 CAS.
- J. Gaitzsch, X. Huang and B. Voit, Chem. Rev., 2016, 116, 1053 CrossRef CAS PubMed.
- G. Decher, J. D. Hong and J. Schmitt, Thin Solid Films, 1992, 210, 831 CrossRef.
- (a) K. Szczepanowicz, H. J. Hoel, L. Szyk-Warszyńska, E. Bielańska, A. M. Bouzga, G. Gaudernack, C. Simon and P. Warszyński, Langmuir, 2010, 26, 12592 CrossRef PubMed; (b) J. Szafraniec, M. Janik, J. Odrobińska and S. Zapotoczny, Nanoscale, 2015, 7, 5525 RSC; (c) J. Szafraniec, J. Odrobińska and S. Zapotoczny, RSC Adv., 2016, 6, 31290 RSC.
- E. Donath, G. B. Sukhorukov, F. Caruso and S. A. Davis, Angew. Chem., 1998, 110, 2324 CrossRef.
- F. Caruso and H. Möhwald, Langmuir, 1999, 15, 8276 CrossRef CAS.
- F. Caruso and H. Möhwald, J. Am. Chem. Soc., 1999, 121, 6039 CrossRef CAS.
- J. Shi, Z. Chen, Y. Qin and Z. Guo, J. Phys. Chem. C, 2008, 112, 11617 CAS.
- D. A. Yashchenok, M. V. Lomova, A. M. Pavlov, A. V. Sapelkin, B. S. Shi, G. B. Khomutov, N. A. Kotov, G. B. Sukhorukov, H. Möhwald and A. G. Skirtach, Adv. Funct. Mater., 2010, 20, 3136 CrossRef.
- A. Xiong, X. Lu, Y. Ma, Y. Qin, P. Zhang, J. Shi and Z. Guo, Mater. Lett., 2013, 105, 132 CrossRef CAS.
- W. Dong, J. K. Ferri, T. Adalsteinsson, M. Schönhoff, G. B. Sukhorukov and H. Möhwald, Chem. Mater., 2005, 17, 2603 CrossRef CAS.
- A. M. Javier, O. Kreft, M. Semmling, S. Kempter, A. G. Skirtach, O. T. Bruns, P. Del Pino, M. F. Bedard, J. Raedler, J. Kaes, C. Plank, G. B. Sukhorukov and W. J. Parak, Adv. Mater., 2008, 20, 4281 CrossRef CAS.
- S. Iijima, Nature, 1991, 354, 56 CrossRef CAS.
- M.-F. Yu, O. Lourie, M. J. Dyer, K. Moloni, T. F. Kelly and R. S. Ruoff, Science, 2000, 287, 637 CrossRef CAS PubMed.
- R. Saito, M. Fujita, G. Dresselhaus and M. S. Dresselhaus, Appl. Phys. Lett., 1992, 60, 2204 CrossRef CAS.
- Y. Ando, X. Zhao, H. Shimoyama, G. Sakai and K. Kaneto, Int. J. Inorg. Mater., 1999, 1, 77 CrossRef CAS.
- J. K. Holt, H. G. Park, Y. Wang, M. Stadermann, A. B. Artyukhin, C. P. Griroropoulos, A. Noy and O. Bakajin, Science, 2006, 312, 1034 CrossRef CAS PubMed.
- G. Hummer, J. C. Rasaiah and J. P. Noworyta, Nature, 2001, 414, 188 CrossRef CAS PubMed.
- B. J. Hinds, N. Copra, T. Rantell, R. Andrews, V. Gavalas and L. G. Bachas, Science, 2004, 303, 62 CrossRef CAS PubMed.
- H. Liu, J. He, J. Tang, H. Liu, P. Pang, D. Cao, P. Kristic, S. Joseph, S. Lindsay and C. Nuckollos, Science, 2010, 327, 64 CrossRef CAS PubMed.
- B. Sup Shim, J. Zhu, E. Jan, K. Critchley, S. Ho, P. Podsiadlo, K. Sun and N. A. Kotov, ACS Nano, 2009, 3, 1711 CrossRef PubMed.
- C. Biswas and Y. H. Lee, Adv. Funct. Mater., 2011, 21, 3806 CrossRef CAS.
- M. Zheng, A. Jagota, M. S. Strano, A. P. Santos, P. Barone, S. G. Chou, B. A. Diner, M. S. Dresselhaus, R. S. McLean, G. B. Onoa, G. G. Samsnidze, E. D. Semke, M. Usrey and D. J. Watts, Science, 2003, 302, 1545 CrossRef CAS PubMed.
- P. Podsiadlo, A. K. Kaushik, E. M. Arruda, A. M. Waas, B. Sup Shim, J. Xu, H. Nandivada, B. G. Pumplin, J. Lahann, A. Ramamoorthy and N. A. Kotov, Science, 2007, 318, 80 CrossRef CAS PubMed.
- B. Sup Shim, J. Zhu, E. Jan, K. Critchley, S. Ho, P. Podsiadlo, K. Sun and N. A. Kotov, ACS Nano, 2009, 3, 1711 CrossRef PubMed.
- M. A. Correa-Duarte, A. Ksiorek, W. Kandulski, M. Giersig and L. M. Liz-Marzan, Chem. Mater., 2005, 17, 3268 CrossRef CAS.
- M. A. Correa-Duarte, A. Ksiorek, W. Kandulski, M. Giersig and V. Salgueirino-Maceira, Small, 2006, 2, 220 CrossRef CAS PubMed.
- H. Jin, H. J. Choi, S. H. Yoon, J. M. Seung and S. E. Shim, Chem. Mater., 2005, 17, 4034 CrossRef CAS.
- M. Sano, A. Kamino, J. Okamura and S. Shinkai, Nano Lett., 2002, 2, 531 CrossRef CAS.
- M. M. Caruso, S. R. Schelkopf, A. C. Jackson, A. M. Landry, P. V. Braun and J. S. Moore, J. Mater. Chem., 2009, 19, 6093 RSC.
- J. M. Didymus, P. Oliver, S. Mann, A. L. DeVires, P. V. Hauschka and P. Westbroek, J. Chem. Soc., Faraday Trans., 1993, 89, 2891 RSC.
- T. Kato, T. Suzuki, T. Amamiya, T. Irie, M. Komiyama and H. Yui, Supramol. Sci., 1998, 5, 411 CrossRef CAS.
- M. Długosz, M. Bulwan, G. Kania, M. Nowakowska and S. Zapotoczny, J. Nanopart. Res., 2012, 14, 1313 CrossRef PubMed.
- J. Jada and A. Verraes, Colloids Surf., 2003, 219, 7 CrossRef.
- S. Qin, D. Qin, W. D. Ford, J. E. Herrera, D. E. Resasco, S. M. Bachilo and R. B. Weisman, Macromolecules, 2004, 37, 3965 CrossRef CAS.
- A. Du, K. Yang, T. Zhao, M. Wang and J. Zeng, Polym. Test., 2016, 51, 40 CrossRef CAS.
- R. Fuge, M. Liebscher, C. Schröfl, S. Oswald, A. Leonhardt, B. Büchner and V. Mechtcherine, Diamond Relat. Mater., 2016, 66, 126 CrossRef CAS.
- J. Geng, K. Kim, J. Zhang, A. Escalads, R. Tunuguntla, L. R. Comolli, F. I. Allen, A. V. Shnyrova, K. R. Cho, D. Munoz, Y. M. Wang, C. P. Grigoropoulos, C. M. Ajo-Franklin, V. A. Frolov and A. Noy, Nature, 2014, 514, 612 CrossRef CAS PubMed.
- Y. Miyata, M. Suzuki, M. Fujihara, Y. Asada, R. Kitaura and H. Shinohara, ACS Nano, 2010, 10, 5807 CrossRef PubMed.
- W. Tong, W. Dong, C. Gao and H. Möhwald, J. Phys. Chem. B, 2005, 109, 13159 CrossRef CAS PubMed.
- Z. Sui, D. Salloum and J. B. Schlenoff, Langmuir, 2003, 19, 2491 CrossRef CAS.
- K. Köhler, H. Möhwald and G. B. Sukhorukov, J. Phys. Chem. B, 2006, 110, 24002 CrossRef PubMed.
- Y. Han, J. Bu, J. Zhang, W. Tong and C. Gao, Macromol. Biosci., 2012, 12, 1436 CrossRef CAS PubMed.
- K. Köhler and G. B. Sukhorukov, Adv. Funct. Mater., 2007, 17, 2053 CrossRef.
- K. Köhler, H. Möhwald and G. B. Sukhorukov, J. Phys. Chem. B, 2006, 110, 24002 CrossRef PubMed.
- C. Gao, E. Donath, S. Moya, V. Dudnik and H. Möhwald, Eur. Phys. J. E: Soft Matter Biol. Phys., 2001, 5, 21 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of the experimental part. TEM characterization of CNTs, microscopic and TGA characterization of the capsules and ζ-potential changes during coating of the capsules using polyelectrolytes. See DOI: 10.1039/c6ra21220d |
| This journal is © The Royal Society of Chemistry 2016 |