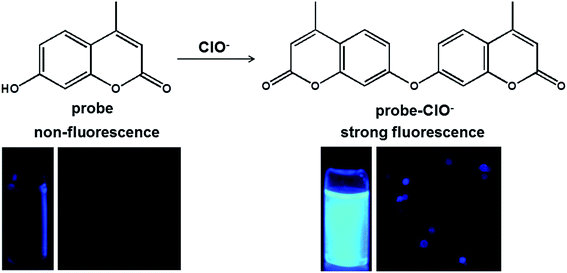
A highly selective and sensitive fluorescence probe for rapid detection of hypochlorite in tap water and cancer cells†
Lanfang Panga,
Yanmei Zhou*ab,
Wenli Gaoa,
Haohan Songa,
Xiao Wanga and
Yong Wanga
aInstitute of Environmental and Analytical Sciences, College of Chemistry and Chemical Engineering, Henan University, Kaifeng, Henan 475004, P. R. China. E-mail: zhouyanmei@henu.edu.cn; Fax: +86-371-23881589; Tel: +86-371-22868833-3422
bState Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024, China
First published on 1st November 2016
Abstract
The 7-hydroxy-4-methylcoumarin as an “off–on” fluorescent probe exhibited excellent selectivity and sensitivity towards hypochlorite over other reactive oxygen species and relevant ions. Upon treatment with hypochlorite, the fluorescence intensity of the probe gradually increased 86-fold. A good linear increase of the fluorescence intensity could be observed with increasing hypochlorite concentration over a wide linear range of 0–150 μM, and a detection limit as low as 67 nM was obtained. Moreover, the probe could also be successfully applied for the detection of hypochlorite in tap water and living cells, which provided great potential for the quantitative detection of hypochlorite in water samples and cancer cells.
Introduction
As a type of biologically important reactive oxygen species, hypochlorous acid (HClO) and its conjugate base hypochlorite (ClO−) play an essential role in diverse normal biochemical functions and abnormal pathological processes.1–3 Endogenous hypochlorous acid (HClO/ClO−) is mainly converted from peroxidation of chloride ions (Cl−) by the catalysis of enzyme myeloperoxidase in leukocytes including macrophages, monocytes and neutrophils,4,5 and it is a potent antimicrobial agent which protects the immune system of the mammals during microbial invasion.6,7 More and more established evidences show that the abnormal production of ClO− may lead to tissue damage and diseases,8–10 such as lung injury,11 inflammatory,12 atherosclerosis,13 rheumatoid arthritis14 and even cancer.15,16 Moreover, ClO− is widely used in our daily life as a disinfectant agent or bleaching agent. For example, in disinfection of drinking water, cool-water treatment and household bleach, ClO− is used at a concentration in the range of 10−5 to 10−2 M.17,18 The excessive concentration of ClO− solutions are a potential health hazard to humans and animals.19 Because of the biological and environmental importance of ClO−, the development of efficient techniques for detecting ClO− in biological samples and water samples has become an important issue.20,21 Among the various detection methods that have been reported in the past decades, fluorescent probes are considered to be the most convenient and efficient approach for both chemical sensing and biological imaging owing to their rapid response, high sensitivity as well as technical simplicity.22,23Recently, several fluorescent probes for ClO− by the modification of common fluorophores such as rhodamine,24,25 fluorescein,26,27 BODIPY28,29 coumarin,30,31 1,8-naphthalimide32,33 have been reported in the literature. However, most of the modification of common fluorophores require labor-intensive syntheses, complicated operation and analyze. Thus, it's interesting to design a simple and practicable ClO− probe with high sensitivity and selectivity.
Among the various organic fluorophores, the coumarin was used as the mother molecule due to its widely accepted superiority (e.g., high fluorescence quantum yield, large stokes shift, high photostability, and good cell permeability, etc.)34–37 In this work, we used the commercially available 7-hydroxy-4-methylcoumarin as the fluorescent probe for the ClO− detection. The addition of ClO− induced the probe from no fluorescence to a strongly blue fluorescence, and the selectivity of the probe to ClO− was hardly affected in the presence of the other relevant analytes. Furthermore, the probe could be successfully applied for the detection of ClO− in tap water and living cells.
Experimental
Apparatus
The UV-Vis absorption spectra were recorded on a U-4100 spectrophotometer. An Edinburgh FS5 spectrofluorometer was used for fluorescence measurements. The MS spectra were performed on Bruker ESQUIRE HPLC-MS AB 4000Q. An Olympus Zeiss 710 laser scanning confocal microscopy was used for fluorescence image of cells. The pH value was measured using Jingke PHS-3D digital pH-meter.Materials
7-Hydroxy-4-methylcoumarin and reactive oxygen species were purchased from Aladdin. The solvents were used as received without further purification. Distilled water was used throughout.General spectra measurements
A 10 mM of stock solution of the probe was prepared in C2H5OH. Stock solutions of the relevant analytes (i.e. ClO−, H2O2, ·OH, O2−, TBHP, TBO·, NO·, K+, Na+, Mg2+, Ca2+, Zn2+, Cl−, I−, MnO4−, HPO42−, NO3−, SO42−) were prepared with a concentration of 10 mM for UV-Vis absorption and fluorescence spectra experiments. In a typical experiment, test solutions were prepared by placing 40 μL of the probe stock solution into a solution of 4 mL C2H5OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v/v = 1/1) solution. The fluorescence measurements were carried out after the addition of analytes for 30 min at room temperature (λex = 370 nm, slit width: 2 nm/2 nm).
H2O (v/v = 1/1) solution. The fluorescence measurements were carried out after the addition of analytes for 30 min at room temperature (λex = 370 nm, slit width: 2 nm/2 nm).
Cells culture
The A549 cells were obtained from Pharmaceutical Institute of Henan University. A549 cells were seeded in glass bottom culture dishes and grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 2.5% fetal bovine serum (FBS) and 15% horse serum at 37 °C with 5% CO2 atmosphere until harvesting for the experiment. When harvesting, the DMEM was drawn out from the culture dishes, and the dishes were rinsed three times with 10 mM phosphate buffer saline (PBS) and then treated with 4 mL trypsinase solution which contain 0.25% EDTA for 3 min in the incubator.Results and discussion
The selectivity of probe for hypochlorite
The high selectivity toward a kind of analyte over other substances is an important feature in the field of molecular sensing. The fluorescence spectra and UV-Vis absorption spectra of the probe (10 μM) were explored in C2H5OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v/v = 1/1) in the presence of various reactive oxygen species and other relevant cations and anions. As shown in Fig. 1 and S1,† the addition of other relevant analytes (150 μM) such as H2O2, ·OH, O2−, TBHP, TBO·, NO·, K+, Na+, Mg2+, Ca2+, Zn2+, Cl−, I−, MnO4−, HPO42−, NO3− and SO42− did not cause obvious change in the emission spectra, and the fluorescence emission intensity at 450 nm enhanced dramatically only in the presence of ClO− (150 μM). In addition, the competition experiments in Fig. 1 also indicated that the selectivity of probe to ClO− was hardly affected in the presence of the other relevant analytes.
H2O (v/v = 1/1) in the presence of various reactive oxygen species and other relevant cations and anions. As shown in Fig. 1 and S1,† the addition of other relevant analytes (150 μM) such as H2O2, ·OH, O2−, TBHP, TBO·, NO·, K+, Na+, Mg2+, Ca2+, Zn2+, Cl−, I−, MnO4−, HPO42−, NO3− and SO42− did not cause obvious change in the emission spectra, and the fluorescence emission intensity at 450 nm enhanced dramatically only in the presence of ClO− (150 μM). In addition, the competition experiments in Fig. 1 also indicated that the selectivity of probe to ClO− was hardly affected in the presence of the other relevant analytes.
Similarly, the UV-Vis spectra also revealed the selectivity of probe for ClO−. Upon the addition of the ClO−, the absorption peak at 323 nm decreased and a new peak at 375 nm appeared (Fig. 2), implying the increase of intramolecular conjugated structure in the probe. However, the UV-Vis absorption spectra didn't change significantly in the presence of other analytes, which clearly confirmed that the probe selectively responded to ClO−.
 | ||
Fig. 2 The UV-Vis absorption spectra of the probe (10 μM) with ClO− (150 μM) and other various analytes (150 μM) in C2H5OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v/v = 1/1) solution. H2O (v/v = 1/1) solution. | ||
Fluorescence and UV-Vis spectra of detecting hypochlorite
A detailed fluorescence spectra titration for ClO− was carried out in C2H5OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v/v = 1/1) solution. As shown in Fig. 3, the probe (10 μM) has a very weak fluorescence (ΦF = 0.005) under a 365 nm UV lamp that can hardly be observed with the naked eye. The addition of ClO− induced a variation in the solution from no fluorescence to a strongly blue fluorescence (ΦF = 0.44), and the fluorescence intensity at 450 nm increased up to 86-fold upon increasing the concentration of ClO− (0–150 μM). As envisioned, a good linear increase of fluorescence intensity could be observed with increasing concentration of ClO− over a wide linear range of 0–150 μM (Fig. 4), indicating the suitability for quantitative determination of ClO− in water samples. The regression equation is Y = 24
H2O (v/v = 1/1) solution. As shown in Fig. 3, the probe (10 μM) has a very weak fluorescence (ΦF = 0.005) under a 365 nm UV lamp that can hardly be observed with the naked eye. The addition of ClO− induced a variation in the solution from no fluorescence to a strongly blue fluorescence (ΦF = 0.44), and the fluorescence intensity at 450 nm increased up to 86-fold upon increasing the concentration of ClO− (0–150 μM). As envisioned, a good linear increase of fluorescence intensity could be observed with increasing concentration of ClO− over a wide linear range of 0–150 μM (Fig. 4), indicating the suitability for quantitative determination of ClO− in water samples. The regression equation is Y = 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 924.5X − 159
924.5X − 159![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 883 (R = 0.9897). Then, we obtained a low detection limit of 67 nM based on 3 × δblank/k (where δblank is the standard deviation of the blank solution and k is the slope of the calibration plot). The relative fluorescence quantum yields of probe-ClO− were determined to be 0.44 with quinine sulfate dehydrate in 0.1 M H2SO4 as a standard, which was much higher than the relative fluorescence quantum yields of the probe (ΦF = 0.005). The corrected emission spectra were measured for the quinine sulfate dehydrate standard (λex = 360 nm; A (absorbance) < 0.01; ΦF = 0.56), and calculated using the following equation.38
883 (R = 0.9897). Then, we obtained a low detection limit of 67 nM based on 3 × δblank/k (where δblank is the standard deviation of the blank solution and k is the slope of the calibration plot). The relative fluorescence quantum yields of probe-ClO− were determined to be 0.44 with quinine sulfate dehydrate in 0.1 M H2SO4 as a standard, which was much higher than the relative fluorescence quantum yields of the probe (ΦF = 0.005). The corrected emission spectra were measured for the quinine sulfate dehydrate standard (λex = 360 nm; A (absorbance) < 0.01; ΦF = 0.56), and calculated using the following equation.38where subscripts x and s refer to the unknown and the standard, Φ stands for quantum yield, F represents integrated area under the emission curve, A is the absorbance intensity at the excitation wavelength, λex exhibits the excitation wavelength, n is index of refraction of the solution.
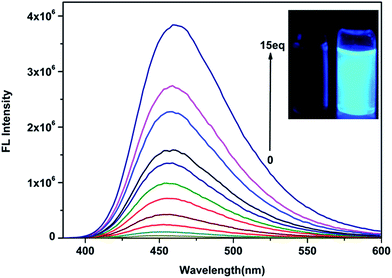 | ||
Fig. 3 Fluorescence spectra of the probe (10 μM) in the presence of various concentrations of ClO− (0–150 μM) in C2H5OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v/v = 1/1) solution (λex = 370 nm, slit width: 2 nm/2 nm). H2O (v/v = 1/1) solution (λex = 370 nm, slit width: 2 nm/2 nm). | ||
 | ||
Fig. 4 The change in the fluorescence intensity of the probe (10 μM, λex = 370 nm) against varied concentrations of ClO− from 0 to 150 μM in C2H5OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v/v = 1/1) solution. H2O (v/v = 1/1) solution. | ||
The changes in the UV-Vis absorption spectra of the probe (10 μM) in the absence or presence of ClO− (0–150 μM) in C2H5OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v/v = 1/1) solution were exhibited in Fig. 5. With increasing ClO− concentration (0–150 μM), the absorption peaks of the probe at 325 nm and 201 nm gradually decreased, and a new absorption peak developed at 375 nm increased gradually.
H2O (v/v = 1/1) solution were exhibited in Fig. 5. With increasing ClO− concentration (0–150 μM), the absorption peaks of the probe at 325 nm and 201 nm gradually decreased, and a new absorption peak developed at 375 nm increased gradually.
 | ||
Fig. 5 The UV-Vis absorbance spectra of the probe (10 μM) in the presence of various concentrations of ClO− (0–150 μM) in C2H5OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v/v = 1/1) solution (λex = 370 nm, slit width: 2 nm/2 nm). H2O (v/v = 1/1) solution (λex = 370 nm, slit width: 2 nm/2 nm). | ||
Conditional experiments
We firstly investigated the influence of system solvent and fraction of water on the interaction of the probe with ClO−. In Fig. S2 and S5,† it was obvious that the combination of C2H5OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v/v = 1/1) proved to be highly efficient for the sensing process. Then we investigated the effect of pH values on the fluorescence intensity changes of the probe in the absence and presence of ClO−. Fig. S6† showed the relationship between pH value and the fluorescence intensity at 450 nm. As it can be seen, the probe exhibited an obvious response around pH 6.0. So, in the UV-Vis absorption and fluorescence spectra experiments, C2H5OH
H2O (v/v = 1/1) proved to be highly efficient for the sensing process. Then we investigated the effect of pH values on the fluorescence intensity changes of the probe in the absence and presence of ClO−. Fig. S6† showed the relationship between pH value and the fluorescence intensity at 450 nm. As it can be seen, the probe exhibited an obvious response around pH 6.0. So, in the UV-Vis absorption and fluorescence spectra experiments, C2H5OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v/v = 1/1) solution was selected as the optimum system to investigate the spectral response of the probe to ClO−.
H2O (v/v = 1/1) solution was selected as the optimum system to investigate the spectral response of the probe to ClO−.
To acquire a better understanding of the reaction, the time dependent fluorescent response of the probe to ClO− were monitored at 450 nm. Fig. 6 showed the fluorescence intensity reached its maximum value at about 60 s, demonstrating that the probe reacted rapidly with ClO− and could be used to monitor ClO− in real time.
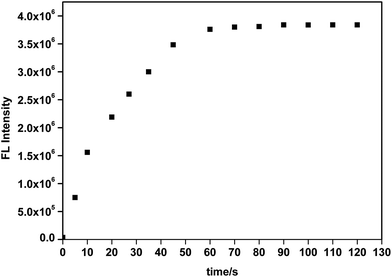 | ||
| Fig. 6 Time-dependent fluorescence intensity of the probe (10 μM) at 450 nm in the presence of 150 μM ClO−. | ||
Proposed mechanism
The proposed mechanism of the probe with ClO− was shown in Scheme 1. In the presence of the ClO−, it could promote the probe undertake intermolecular dehydration reaction of hydroxyl groups or phenolic hydroxyl groups.39 We speculated that the intermolecular dehydration reaction of phenolic hydroxyl groups increases the conjugated structure of the probe, leading to the fluorescence signal enhanced dramatically, and accompanied with a red shift in UV-Vis absorption spectra.To investigate this mechanism, ESI mass spectrometry provided evidence for the reaction mixture of probe with ClO−. As shown in Fig. S3,† the peak at m/z = 174.8 corresponded to the probe. And the peak at m/z = 351.5 corresponded to the reaction product probe-ClO−. All these results indicated that the ClO− promoted the probe undertake intermolecular dehydration reaction of phenolic hydroxyl groups.
Applications of the probe
Among various applications of the probe, to detect and quantify ClO− in water samples and biological samples are crucial and rather practical.To employ the probe for the rapid detection and quantification of ClO− in water samples, the probe (10 μM) was used to detect ClO− in tap water from the campus of Henan University. As shown in Fig. S4,† the fluorescence emission spectra were recorded before and after addition of different quantity of tap water to the solution of probe. And the fluorescence intensity at 450 nm was gradually increased with increasing the quantity of tap water. In addition, we depicted a calibration curve of fluorescence intensity vs. concentration of ClO− (0–150 μM) in Fig. 4. According to the linear regression equation, the concentration of ClO− in the tap water was 3.11 × 10−4 M, which met the standards of the required concentrations in tap water.40 These results indicated that the probe could quantitative analysis of ClO− levels in tap water or other environmental water samples.
To further employ the probe for the determination of ClO− in a biological sample, the fluorescence microscopy experiments were carried out in lung adenocarcinoma A549 cells. As shown in Fig. 7a, the living A549 cells exhibited almost no fluorescence signal with the incubation of the probe (10 μM) in control group for 30 min at 37 °C (λex = 405 nm). However, the control group after treatment with different concentrations of ClO− (225, 300, 400 μM) for 30 min at 37 °C, the bright blue fluorescence was observed in the A549 cells (Fig. 7b–d), and the fluorescent signal was gradually enhanced with increasing ClO− concentration (0–400 μM). Fig. 8 showed a good linear increase of the fluorescence intensity with increasing concentration of ClO− (0–400 μM), the regression equation is Y = 49.0820X + 8.71825 (R = 0.9653), indicating the suitability for quantitative determination of ClO− in cancer cells.
 | ||
| Fig. 8 Fluorescence intensity of the probe (10 μM) with various concentrations of ClO− (0, 225 μM, 300 μM, 400 μM) in A549 cells. | ||
Conclusions
In summary, the commercially available 7-hydroxy-4-methylcoumarin as the probe for the discriminative detection of ClO− has a wide linear range and low detection limit. The probe was successfully applied for the quantitative detection of ClO− in tap water. And, the confocal fluorescence microscopy imaging of the A549 cells was also carried out successfully, which provided a potential for the quantitative detection of hypochlorite in cancer cells.Acknowledgements
The authors are grateful for the State Key Laboratory of Fine Chemicals (KF1514), National Natural Science Foundation of China (21576071), the International Science and Technology Cooperation Project of Henan Province (152102410023).Notes and references
- H. Xiao, K. Xin, H. Dou, G. Yin, Y. Quan and R. Wang, Chem. Commun., 2015, 51, 1442–1445 RSC.
- T. Cheng, J. Zhao, Z. Wang, J. An, Y. Xu, X. Qian and G. Liu, Dyes Pigm., 2016, 126, 218–223 CrossRef CAS.
- E. Wang, H. Qiao, Y. Zhou, L. Pang, F. Yu, J. Zhang and T. Ma, RSC Adv., 2015, 5, 73040–73045 RSC.
- Y. Koide, Y. Urano, K. Hanaoka, T. Terai and T. Nagano, J. Am. Chem. Soc., 2011, 133, 5680–5682 CrossRef CAS PubMed.
- M. Sun, H. Yu, H. Zhu, F. Ma, S. Zhang, D. Huang and S. Wang, Anal. Chem., 2014, 86, 671–677 CrossRef CAS PubMed.
- T. Guo, L. Cui, J. Shen, R. Wang, W. Zhu, Y. Xu and X. Qian, Chem. Commun., 2013, 49, 1862–1864 RSC.
- S. T. Manjare, J. Kim, Y. Lee and D. G. Churchill, Org. Lett., 2014, 16, 520–523 CrossRef CAS PubMed.
- G. Li, Q. Lin, L. Sun, C. Feng, P. Zhang, B. Yu, Y. Chen, Y. Wen, H. Wang, L. Ji and H. Chao, Biomaterials, 2015, 53, 285–295 CrossRef CAS PubMed.
- J. Zha, B. Fu, C. Qin, L. Zeng and X. Hu, RSC Adv., 2014, 4, 43110–43113 RSC.
- X. Wang, F. Song and X. Peng, Dyes Pigm., 2016, 125, 89–94 CrossRef CAS.
- S. Hammerschmidt, N. Büchler and H. Wahn, Chest, 2002, 121, 573–581 CrossRef CAS PubMed.
- S. A. Weitzman and L. I. Gordon, Blood, 1990, 76, 655–663 CAS.
- A. Daugherty, J. L. Dunn, D. L. Rateri and J. W. Heinecke, J. Clin. Invest., 1994, 94, 437–444 CrossRef CAS PubMed.
- S. M. Wu and S. V. Pizzo, Arch. Biochem. Biophys., 2001, 391, 119–126 CrossRef CAS PubMed.
- M. R. Ramsey and N. E. Sharpless, Nat. Cell Biol., 2006, 8, 1213–1215 CrossRef CAS PubMed.
- Y. Yuan, X. Huang, S. Liu, J. Yang, R. Duan and X. Hu, RSC Adv., 2016, 6, 3393–3398 RSC.
- S. Goswami, A. Manna, S. Paul, C. K. Quah and H. K. Fun, Chem. Commun., 2013, 49, 11656–11658 RSC.
- X. Wang, L. Zhou, F. Qiang, F. Wang, R. Wang and C. Zhao, Anal. Chim. Acta, 2016, 911, 114–120 CrossRef CAS PubMed.
- S. Goswami, S. Paul and A. Manna, Dalton Trans., 2013, 42, 10097–10101 RSC.
- S. R. Liu and S. P. Wu, Org. Lett., 2013, 15, 878–881 CrossRef CAS PubMed.
- W. C. Chen, P. Venkatesan and S. P. Wu, Anal. Chim. Acta, 2015, 882, 68–75 CrossRef CAS PubMed.
- Y. Zhang, L. Guan, H. Yu, Y. Yan, L. Du, Y. Liu, M. Sun, D. Huang and S. Wang, Anal. Chem., 2016, 88, 4426–4431 CrossRef CAS PubMed.
- Y. Wu, J. Wang, F. Zeng, S. Huang, J. Huang, H. Xie, C. Yu and S. Wu, ACS Appl. Mater. Interfaces, 2016, 8, 1511–1519 CAS.
- J. T. Hou, K. Li, J. Yang, K. K. Yu, Y. X. Liao, Y. Z. Ran, Y. H. Liu, X. D. Zhou and X. Q. Yu, Chem. Commun., 2015, 51, 6781–6784 RSC.
- L. Long, D. Zhang, X. Li, J. Zhang, C. Zhang and L. Zhou, Anal. Chim. Acta, 2013, 775, 100–105 CrossRef CAS PubMed.
- W. Yin, H. Zhu and R. Wang, Dyes Pigm., 2014, 107, 127–132 CrossRef CAS.
- X. Jin, Y. Jia, W. Chen, P. Chui and Z. Yang, Sens. Actuators, B, 2016, 232, 300–305 CrossRef CAS.
- P. Venkatesan and S. P. Wu, Analyst, 2015, 140, 1349–1355 RSC.
- J. Fan, H. Mu, H. Zhu, J. Du, N. Jiang, J. Wang and X. Peng, Ind. Eng. Chem. Res., 2015, 54, 8842–8846 CrossRef CAS.
- S.-Y. Yu, C.-Y. Hsu, W.-C. Chen, L.-F. Wei and S.-P. Wu, Sens. Actuators, B, 2014, 196, 203–207 CrossRef CAS.
- L. Long, Y. Wu, L. Wang, A. Gong, F. Hu and C. Zhang, Chem. Commun., 2015, 51, 10435–10438 RSC.
- K. Xiong, F. Huo, C. Yin, J. Chao, Y. Zhang and M. Xu, Sens. Actuators, B, 2015, 221, 1508–1514 CrossRef CAS.
- J. Li, P. Li, F. Huo, C. Yin, T. Liu, J. Chao and Y. Zhang, Dyes Pigm., 2016, 130, 209–215 CrossRef CAS.
- S. Maiti, N. Park, J. H. Han, H. M. Jeon, J. H. Lee, S. Bhuniya, C. Kang and J. S. Kim, J. Am. Chem. Soc., 2013, 135, 4567–4572 CrossRef CAS PubMed.
- L. He, Q. Xu, Y. Liu, H. Wei, Y. Tang and W. Lin, ACS Appl. Mater. Interfaces, 2015, 7, 12809–12813 CAS.
- W. Xu, C. L. Teoh, J. Peng, D. Su, L. Yuan and Y. T. Chang, Biomaterials, 2015, 56, 1–9 CrossRef CAS PubMed.
- G. Li, D. Zhu, Q. Liu, L. Xue and H. Jiang, Org. Lett., 2013, 15, 2002–2005 CrossRef CAS PubMed.
- H. Zhu, J. Fan, J. Wang, H. Mu and X. Peng, J. Am. Chem. Soc., 2014, 136, 12820–12823 CrossRef CAS PubMed.
- J. Li, C. Yin, F. Huo, K. Xiong, J. Chao and Y. Zhang, Sens. Actuators, B, 2016, 231, 547–551 CrossRef CAS.
- X. Lou, Y. Zhang, Q. Li, J. Qin and Z. Li, Chem. Commun., 2011, 47, 3189–3191 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: The selectivity of the probe; the solvent dependence; ESI-MS of the probe-ClO−; the fluorescence detection of ClO− in tap water; comparison with other reported hypochlorite probes. See DOI: 10.1039/c6ra23548d |
| This journal is © The Royal Society of Chemistry 2016 |