In vivo target bio-imaging of cerebral ischemic stroke by real-time labeling of zinc†
Abstract
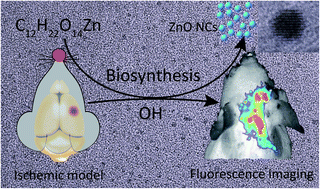
Stroke is a sudden onset disease of the human brain, which has become the second-leading global cause of death after ischemic heart disease. Nowadays, it is especially important to find a rapid, simple, non-toxic targeted imaging reagent. In this contribution we have exploited the potential of zinc gluconate as a novel diagnostic reagent for cerebral ischemic stroke. Our observations demonstrate that zinc ions can readily cross the blood brain barrier in ionic form and assemble under the ischemic and hypoxic condition of stroke supported by inflammation, and in situ biosynthesize fluorescent zinc nanoclusters. This may provide a new strategy for the diagnosis of cerebral ischemic stroke through in vivo fluorescence bioimaging after further investigation.

 Please wait while we load your content...
Please wait while we load your content...