Synthesis of nanohybrid alcogels of SiO2 and Ni–Cr/Mg–Cr–LDH: study of their rheological and dip coating properties
Pinky Saikiaab,
Arvind Gautambc and
R. L. Goswamee*ab
aAdvanced Materials Group, Materials Science and Technology Division, North-East Institute of Science and Technology (CSIR-NEIST), Jorhat-785006, Assam, India. E-mail: goswamirl@rrljorhat.res.in; rajibgoswamee@yahoo.com; Tel: +91-376-2370121-2523
bAcademy of Scientific and Innovative Research (AcSIR), CSIR-NEIST Campus, India
cPolymer Petroleum and Coal Chemistry Group, Materials Science and Technology Division, North-East Institute of Science & Technology (CSIR-NEIST), Jorhat-785006, Assam, India
First published on 22nd November 2016
Abstract
Silica supported Ni–Cr and Mg–Cr–LDH nanohybrid alcogels were synthesized by a ‘soft chemical’ non-aqueous sol–gel route using a mixture of metal acetylacetonate and tetraethylorthosilicate (TEOS) precursors. Faster hydrolysis of the alkoxide than the metal acetylacetonates precursors gives an inner SiO2 core around which crystalline LDH sheets are dispersed to give one gel network in an organic medium. These were characterized by XRD, FT-IR, TGA-DTA, particle-size distribution, zeta-potential, SEM, TEM, EDXA, BET surface area and Rheological analysis. The steady shear and dynamic oscillatory measurements showed that the apparent viscosity decreased with shear strain and the complex viscosity decreased with angular frequency indicating the shear thinning behaviour of these nanohybrids. At the linear viscoelastic region (LVE) the storage modulus (G′) was greater than the loss modulus (G′′) indicating the presence of elastic or gel like behaviour. With the rise of the amount of SiO2 in the nanohybrids the surface area and the negative zeta potentials were found to increase. This helped the dispersibility of the alcogels as well as the formation of thin films over solid surfaces by dip-coating. These findings are expected to be useful in designing crack free catalytic barriers for inorganic membrane applications having transition metal mixed oxide/hydroxide nano-sheets as the active catalyst.
1. Introduction
Layered double hydroxides (LDHs), also called anionic clays, are represented by the general formula [M1−x2+Mx3+(OH)2]x+[Ax/n]n−·mH2O and they belong to two-dimensional layered materials. The different M2+ and M3+ metal cations uniformly distribute in an orderly or random arrangement in the brucite-like sheets, and various charge-compensating anions (An−) are present in the interlayer space.1–4 LDHs have lots of applications in various fields such as catalysis, optics, medical sciences, inorganic–organic nanocomposites and anion exchangers due to their ability to intercalate a diverse range of interlayer anions.5–8 Compared to other clay minerals such as smectites, kaolins, pyrophyllite, and talc, LDHs possess distinct advantages by bearing positive charges on their surface due to the existence of metal ions like Cr3+, Al3+, Fe3+ and negative charges along the interlayers which are very sensitive and can be used in the formulation of many rheologically important fluids.9,10 In the past, LDH nanocomposites dispersed with polyaniline (PANI), polypyrrole (PPy), polystyrene (PS), polyacrylamide, polypropylene, silica, titania, zeolite, clay or other polarisable or semiconducting particles in a continuous phase like water, silicone oil or corn oil were synthesized.10–20 These inorganic materials have an anisotropic morphology and a low cost. Now-a-days, research on hybrid materials containing layered inorganic materials such as LDH sheet, graphene, transition metal dichalcogenides are some of the most attractive fields of research.10,21–24 LDH synthesized by conventional co-precipitation method exhibit poor dispersibility due to the aggregation of LDH sheets.5,10 To avoid the aggregation of LDH sheets the laying of LDH on the nano-supports is a promising strategy.5 Many researchers have synthesized the hierarchical core–shell LDH materials using SiO2 as core component.5,10,25–30 They have reported the routes like co-precipitation followed by layer-by-layer deposition, sol–gel hydrolysis of alkoxides to get core–shell type LDHs.25–32 LDH based core–shell structured nanocomposites have lots of applications such as protein separations,27 supercapacitors,30 drug release32 and flame retardant.32–35Rheological studies are very much helpful to understand the nature of the particle–particle interactions in such core shell based dispersions. The rheological studies of polymer/LDH nanocomposites, SiO2–LDH core–shell composites, clay/LDH dispersions were investigated by many researchers.10–20 X. Ji et al. reported the enhanced electrorheological properties of SiO2@Ni–Al LDH core–shell composites.10 They reported the electrorheological properties of core–shell structured SiO2@Ni–Al layered double hydroxide composites prepared by a combination of sol–gel non-aqueous SiO2 hydrolysis and hydrothermal aqueous LDH synthesis method. The method adopted had several steps of precipitation, separation, purification and dispersion to obtain SiO2@LDH nanocomposites. Apart from that there are several other reports where preparation of viscous gels of otherwise non-dispersible LDH nanosheets is made. In such gels LDH functions helps as a viscosity builder component in a composite of binary or ternary components. One example is the study of flow behaviour of LDH–Na-montmorillonite dispersions made by Li et al.11 Similarly, Z. Hu et al. reported the rheological properties of LDH/Polyacrylamide nanocomposites15 and S. Chakraborty et al. reported the PMMA/Ni–Al LDH composites.19 B. Baruah et al. reported the effect of particle size of clay on the viscosity build up property of Mg–Al LDH–Na-montmorillonite composites.20 In all of these reports LDH was prepared by aqueous hydrolysis method where a step of hydrothermal crystallisation is invariably involved.36 As hydrothermal treatment during LDH synthesis changes the physicochemical properties like morphology, particle size and crystallinity the colloidal nature of the particles are lost to a good extent.
Unlike in the reports cited above, the approach described in the present paper does not involve any step of aqueous hydrolysis followed by a hydrothermal treatment. Moreover, by the present method synthesis of hybrid nanocomposites of SiO2@LDH core shells at varying LDH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) silica ratios could be achieved. We have reported here a sol–gel method of synthesis of SiO2@Ni–Cr–LDH and Mg–Cr–LDH core shells by using metal acetylacetonates instead of alkoxides as precursors for LDH component while tetraethylorthosilicate (TEOS) was used as precursor for the silica component.37–39 The use of metal acetylacetonates to obtain the LDH component give the advantages of a slow and controlled hydrolysis whereas SiO2 component obtained from the faster hydrolysis of alkoxide TEOS gives the inner centers or cores for LDH particles to assemble around.39–42 Accordingly, at first TEOS was hydrolysed in acidic pH to form –Si–O–Si– oligomer then it was introduced to metal acetylacetonates and further hydrolysis carried out. Study of the rheological properties of the prepared materials helped to understand the shear-thinning behaviour of the alcogels which is an essential set of data to optimise the process of coating of such alcogels on ceramic surfaces to obtain a thin-film of mixed metal hydroxides.43 Such coatings of mixed metal nano hydroxides or their calcined derivative over suitable ceramic preforms are expected to be highly useful for design of nano sheet based devices for environmental and organic reactions both as catalyst and as separation barrier. Because of the organic solvent base these compositions on controlled drying would produce crack free thin film barriers having membrane properties.
silica ratios could be achieved. We have reported here a sol–gel method of synthesis of SiO2@Ni–Cr–LDH and Mg–Cr–LDH core shells by using metal acetylacetonates instead of alkoxides as precursors for LDH component while tetraethylorthosilicate (TEOS) was used as precursor for the silica component.37–39 The use of metal acetylacetonates to obtain the LDH component give the advantages of a slow and controlled hydrolysis whereas SiO2 component obtained from the faster hydrolysis of alkoxide TEOS gives the inner centers or cores for LDH particles to assemble around.39–42 Accordingly, at first TEOS was hydrolysed in acidic pH to form –Si–O–Si– oligomer then it was introduced to metal acetylacetonates and further hydrolysis carried out. Study of the rheological properties of the prepared materials helped to understand the shear-thinning behaviour of the alcogels which is an essential set of data to optimise the process of coating of such alcogels on ceramic surfaces to obtain a thin-film of mixed metal hydroxides.43 Such coatings of mixed metal nano hydroxides or their calcined derivative over suitable ceramic preforms are expected to be highly useful for design of nano sheet based devices for environmental and organic reactions both as catalyst and as separation barrier. Because of the organic solvent base these compositions on controlled drying would produce crack free thin film barriers having membrane properties.
Thus, the novelty of the present work lies on both the synthesis of the hybrid nano alcogels on the basis of different rates of hydrolysis of metal acetylacetonate and alkoxide as well as on the prospects of the alcogels to derive nano devices. Such studies on SiO2@LDH core shells has not been reported yet.
2. Experimental
2.1. Materials
Metal acetylacetonates such as Ni(acac)2, Cr(acac)3, Mg(acac)2 were purchased from Sigma Aldrich (98% pure), TEOS (tetraethyl orthosilicate from Acros Scientific, purity 98%), ethanol and acetone (from Merck chemicals, purity 98%), ammonia were used. Ethanol and acetone were distilled, dried and purified before using.442.2. Synthesis of unsupported and SiO2@Ni–Cr–LDH and Mg–Cr–LDH by soft chemical sol–gel method
Unsupported and silica@LDH at different ratios synthesized by the soft chemical sol–gel method37–39 involved the following steps-Step-1: 0.02 mol nickel acetylacetonate (Ni(acac)2)/magnesium acetylacetonate (Mg(acac)2) was dissolved in 80 cm3 of distilled ethanol at pH 6.
Step-2: 0.007 mol chromium acetylacetonate (Cr(acac)3) was dissolved in 80 cm3 ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone (1
acetone (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture.
1) mixture.
These two solutions were mixed at 80 °C by stirring for 2–3 hours. The pH of the mixture was maintained at 8–9 by adding few drops of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 NH3
2 NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O mixture and finally refluxed for 6–7 hours to get Ni–Cr–LDH and Mg–Cr–LDH. The product obtained was then filtered and dried in air oven at 40 °C.
H2O mixture and finally refluxed for 6–7 hours to get Ni–Cr–LDH and Mg–Cr–LDH. The product obtained was then filtered and dried in air oven at 40 °C.
For the synthesis of SiO2@Ni–Cr–LDH/Mg–Cr–LDH, TEOS was used as a source of silica. 4.43 cm3 TEOS, 0.72 cm3 of 0.2 M aqueous HCl and 4.85 cm3 distilled ethanol were taken and aged the mixture for 45 min in a magnetic stirrer at room temperature. The solutions of step-1 and step-2 were mixed at 80 °C and refluxed for 2 hours. Added hydrolysed TEOS to the mixed acetylacetonate solution and raised the pH upto 8–9 by adding 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 NH3
2 NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O mixture stirred continuously for another 6–7 hours to get silica supported LDH. A portion of the product was separated from the obtained free flowing gels by filtration, washed in hot water and dried at 40 °C in air oven for further characterizations like XRD, TGA-DTA, particle-size analysis, zeta-potential study, FT-IR, & BET surface area analysis.
H2O mixture stirred continuously for another 6–7 hours to get silica supported LDH. A portion of the product was separated from the obtained free flowing gels by filtration, washed in hot water and dried at 40 °C in air oven for further characterizations like XRD, TGA-DTA, particle-size analysis, zeta-potential study, FT-IR, & BET surface area analysis.
Silica supported LDH were prepared by varying silica to LDH ratios also. The schematic diagram for this synthesis is shown in Fig. 1.
2.3. Characterizations
Structure identification of inorganic phases formed were carried out in a powder X-ray diffractometer (Model Rigaku Ultima IV) using CuKα radiation of a wavelength of 1.54056 Å at 40 mA and 40 kV X-ray generator current setting with a step size of 0.2° 2θ min−1.Fourier transform infrared (FTIR) spectra of the prepared samples were recorded in spectrophotometer (Perkin-Elmer 2000 System) in 4000–400 cm−1 range at a spectral resolution of 4 cm−1 using KBr pellets.
Field Emission Scanning Electron Microscopy (FESEM) analysis was carried out in a Carl Zeiss-Sigma VP equipment, with an accelerating voltage of 20 kV. Before the analysis the gels were dried at 40 °C in air oven for 1 week to avoid the moisture absorption. Finally, the sample surfaces were gold coated in high vacuum. Transmission electron microscopy (TEM) images were recorded on a JEOL JEM-2011 electron microscope operated at an accelerating voltage of 200 kV. The chemical composition was identified by using an energy-dispersive X-ray spectroscopy (EDX) detector on the scanning electron microscope Model Carl Zeiss Sigma VP.
The thermogravimetric measurements were carried out in a simultaneous TG-DTA analyzer (model Q-600, M/S TA Instruments) using α-Al2O3 as reference. Samples weighing about 5.0 mg were heated from 30 to 700 °C at a rate of 10 °C min−1 in an argon atmosphere in a non-isothermal condition.
Specific surface area of the samples were recorded via nitrogen gas adsorption at 77 K applying Brunauer–Emmett–Teller (BET) calculations using Autosorb-iQ Station 1 (Quantachrome, USA). Prior to performing the experiment the samples were degassed at 100 °C for 1.5 h.
The zeta potential of the nanohybrid alcogels were measured with the Laser Doppler Velocimetry technique at 25 °C under a 10 Mw He–Ne laser (M/S Malvern Instruments Zetasizer Nano Z5). The particle size distribution of these nanohybrids were carried by DLS (Dynamic Light Scattering) technique in zeta sizer (M/S Malvern Instruments Zetasizer Nano Z5). To carry out these studies the samples were filtered, washed with hot water and dried in air oven at 40 °C. The dried mass were ground gently in an agate mortar and redispersed in aqueous phase by shaking in an ultrasonic processor (M/S Sonics) with a 13 mm Ti probe for 30 minutes under 20 kHz frequency and 25% amplitude of vibration.
Rheological properties of the nanohybrid alcogels were investigated by both rotational and oscillatory rheometer. The steady shear measurements were carried out by rotational rheometer Rheolab QC (Anton Paar) with a measuring cup C-CC27/SS/QC and measuring system CC27/P6 at 15 °C. The preliminary studies such as variation of viscosity and shear stress with shear strain and the flow modeling of these alcogels were investigated by it. The temperature of 15 °C was maintained to minimise the concentration change of dispersions by evaporation of organic solvents from the surface. The steady shear measurements were carried out in the shear rate ranging from 100–1000 s−1.
For finer study involving dynamic oscillatory measurement a modular compact rheometer MCR 302 (Anton Paar) with a rough parallel plate geometry of 25 mm diameter and 1 mm gap was used at 15 °C. The sample was submitted to the parallel-plate and the amplitude of oscillation was increased up to 500% apparent shear strain maintaining the angular frequency at 1 rad s−1 for the amplitude sweep test. For the frequency sweep test the shear strain was kept at 5%. The oscillatory rheometer gave data of viscoelastic properties G′ (storage modulus) and G′′ (loss modulus).
Finally some of the well characterized nanohybrid alcogels were coated over cordierite 2MgO·2Al2O3·5SiO2 honey-comb ceramic monolith by a dip coater (KSVD from M/S KSV Instrument, Finland) at 20 mm min−1 speed withdrawal and dipping rate. The honeycombs had 360 cells per square inch and a 32 mm diameter.
3. Results and discussion
3.1. XRD analysis
The presence of LDH phase was analysed by XRD (Fig. 2(A) and (B)) analysis. XRD patterns of samples without SiO2 component showed the peaks related to hkl reflections at (003), (006), (012) originating from LDH sheet in both Ni–Cr–LDH and Mg–Cr–LDH. Even with the increase of the SiO2 component in the nanohybrids the XRD pattern invariably showed sharp 00l reflections of the LDH phase indicating the existence of SiO2 part in a poorly crystalline form. The position of 00l peaks remained almost constant for each of the phases Ni–Cr and Mg–Cr indicating absence of intercalation of any new ions in the interlayer positions with the increase of silica content. In case of SiO2@Ni–Cr–LDH the peaks due to SiO2 were obtained at 18°, 22.98°, 25.94° 2θ with hkl reflections at (131), (101), (331) and (300) respectively. It was also observed that with the increase of SiO2 content the weaker peaks due to SiO2 became more and more intense from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 SiO2
1 SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LDH ratios. Whereas, in case of SiO2@Mg–Cr–LDH peaks were obtained with hkl reflections (201), (331) and (220) respectively for the SiO2 phase.45,46 Also, it was observed that in case of SiO2 bearing LDH nanohybrids additional peaks of LDH phase becomes visible, such enhancement of XRD reflections indicate a possible delaminated orientation of LDH sheets around the silica surface forming a core–shell like arrangement.
LDH ratios. Whereas, in case of SiO2@Mg–Cr–LDH peaks were obtained with hkl reflections (201), (331) and (220) respectively for the SiO2 phase.45,46 Also, it was observed that in case of SiO2 bearing LDH nanohybrids additional peaks of LDH phase becomes visible, such enhancement of XRD reflections indicate a possible delaminated orientation of LDH sheets around the silica surface forming a core–shell like arrangement.
 | ||
| Fig. 2 XRD pattern of SiO2 supported Ni–Cr–LDH (A) and SiO2 supported Mg–Cr–LDH (B) at different SiO2 to LDH ratios. | ||
3.2. FT-IR analysis
FT-IR analysis (Fig. 3(A) and (B)) showed the presence of –OH stretching vibrations at around 3480–3362 cm−1 and 3450–3392 cm−1 in case of SiO2@Ni–Cr–LDH and Mg–Cr–LDH nanohybrids due to the presence of interlayer hydroxyls and H2O molecules respectively. The peaks at around 1650–1610 cm−1 and 1633–1548 cm−1 were formed due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of residual hydrolysed acetylacetonate group present in both the SiO2 supported and unsupported Ni–Cr–LDH and Mg–Cr–LDH nanohybrids. The peaks at around 1022–1213 cm−1 and 1049–1219 cm−1 were due to C–O stretching vibrations in both of these nanohybrids. The peaks due to C–H stretching and C–H bending vibrations were obtained at around 2984–2966 cm−1 and 1365–1408 cm−1 whereas for C–C stretching vibrations were obtained at 1950–2332 cm−1 due to the presence of acetylacetone in the interlayer. The peaks at around 956–771 cm−1 and 579–447 cm−1 were due to the M–O and Si–O stretching vibrations respectively.
O stretching vibrations of residual hydrolysed acetylacetonate group present in both the SiO2 supported and unsupported Ni–Cr–LDH and Mg–Cr–LDH nanohybrids. The peaks at around 1022–1213 cm−1 and 1049–1219 cm−1 were due to C–O stretching vibrations in both of these nanohybrids. The peaks due to C–H stretching and C–H bending vibrations were obtained at around 2984–2966 cm−1 and 1365–1408 cm−1 whereas for C–C stretching vibrations were obtained at 1950–2332 cm−1 due to the presence of acetylacetone in the interlayer. The peaks at around 956–771 cm−1 and 579–447 cm−1 were due to the M–O and Si–O stretching vibrations respectively.
 | ||
| Fig. 3 FT-IR pattern of SiO2 supported Ni–Cr–LDH (A) and SiO2 supported Mg–Cr–LDH (B) nanohybrids at different SiO2 to LDH ratios. | ||
3.3. BET surface area analysis
BET surface area analysis showed that with the increase of SiO2 component from 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 SiO2
1 SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LDH ratios in the oven dried samples the surface area also increased. In case of SiO2 supported Ni–Cr–LDH the surface area increased from 13.4 m2 g−1 to 68.6 m2 g−1 and from 23.6 m2 g−1 to 103.6 m2 g−1 in case of SiO2 supported Mg–Cr–LDH (Table 1). On the other hand, SiO2@Mg–Cr–LDH core shells exhibit higher surface area as compared to SiO2@Ni–Cr–LDH core shell nanohybrids. The increase of surface area indicates their good prospects for application in different catalytic applications.5,27
LDH ratios in the oven dried samples the surface area also increased. In case of SiO2 supported Ni–Cr–LDH the surface area increased from 13.4 m2 g−1 to 68.6 m2 g−1 and from 23.6 m2 g−1 to 103.6 m2 g−1 in case of SiO2 supported Mg–Cr–LDH (Table 1). On the other hand, SiO2@Mg–Cr–LDH core shells exhibit higher surface area as compared to SiO2@Ni–Cr–LDH core shell nanohybrids. The increase of surface area indicates their good prospects for application in different catalytic applications.5,27
| Nanohybrids | Surface area (m2 g−1) |
|---|---|
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni–Cr–LDH (0 Ni–Cr–LDH (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
13.42 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni–Cr–LDH (1 Ni–Cr–LDH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
15.14 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni–Cr–LDH (2 Ni–Cr–LDH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
33.82 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni–Cr–LDH (3 Ni–Cr–LDH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
68.64 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg–Cr–LDH (0 Mg–Cr–LDH (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
23.57 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg–Cr–LDH (1 Mg–Cr–LDH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
26.99 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg–Cr–LDH (2 Mg–Cr–LDH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
48.02 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg–Cr–LDH (3 Mg–Cr–LDH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
103.59 |
3.4. TGA-DTA analysis
The thermal stability of the SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LDH nanohybrids were analysed by TGA-DTA analysis. Fig. 4A–D show the TGA and DTG patterns of SiO2 supported Ni–Cr and Mg–Cr–LDH nanohybrids. It was observed that these nanohybrids showed different thermal changes upto 700 °C. Basically they undergo three step thermal degradations at temperatures around 100–160 °C, 200–300 °C and 300–700 °C respectively. The weight loss at around 100–160 °C correspond to surface solvent loss, whereas the weight loss in the temperature range 200 to 300 °C correspond to loss of pore and interlayer solvent molecules. Since, the amount of residual solvent molecule present in such a system largely depends upon various uncontrollable factors like precipitation during hydrolysis, interlayer surface exposure, oven drying time, humidity they result in the presence of varied amounts of residual solvent molecules in these nanohybrids. Therefore, a trend in the weight loss percentages in these temperature ranges with variation of composition was not observed. However, above 300 °C it was observed that with the decrease of LDH content in the system the weight loss in the temperature range 300–700 °C gradually decreased due to decrease of dehydroxylation. In case of SiO2
LDH nanohybrids were analysed by TGA-DTA analysis. Fig. 4A–D show the TGA and DTG patterns of SiO2 supported Ni–Cr and Mg–Cr–LDH nanohybrids. It was observed that these nanohybrids showed different thermal changes upto 700 °C. Basically they undergo three step thermal degradations at temperatures around 100–160 °C, 200–300 °C and 300–700 °C respectively. The weight loss at around 100–160 °C correspond to surface solvent loss, whereas the weight loss in the temperature range 200 to 300 °C correspond to loss of pore and interlayer solvent molecules. Since, the amount of residual solvent molecule present in such a system largely depends upon various uncontrollable factors like precipitation during hydrolysis, interlayer surface exposure, oven drying time, humidity they result in the presence of varied amounts of residual solvent molecules in these nanohybrids. Therefore, a trend in the weight loss percentages in these temperature ranges with variation of composition was not observed. However, above 300 °C it was observed that with the decrease of LDH content in the system the weight loss in the temperature range 300–700 °C gradually decreased due to decrease of dehydroxylation. In case of SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni–Cr–LDH nanohybrids in this temperature range the weight loss trend is 18.96%, 14.1%, 13.34% and 11.38% weight/weight as the SiO2
Ni–Cr–LDH nanohybrids in this temperature range the weight loss trend is 18.96%, 14.1%, 13.34% and 11.38% weight/weight as the SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LDH ratio changes gradually from 0
LDH ratio changes gradually from 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 respectively; whereas in case of SiO2
1 respectively; whereas in case of SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg–Cr–LDH the same trend is in the range 13.94%, 11.4%, 5.56% and 3.77% weight/weight respectively. At this stage the mixed metal hydroxides phase transforms to mixed metal oxides where metal ions are distributed in the same nanosheet.47 From the DTG patterns it was further observed that, with the increase of SiO2
Mg–Cr–LDH the same trend is in the range 13.94%, 11.4%, 5.56% and 3.77% weight/weight respectively. At this stage the mixed metal hydroxides phase transforms to mixed metal oxides where metal ions are distributed in the same nanosheet.47 From the DTG patterns it was further observed that, with the increase of SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LDH ratios from 0
LDH ratios from 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 their maximum decomposition temperature also decreased.
1 their maximum decomposition temperature also decreased.
 | ||
| Fig. 4 TGA and DTG curves for SiO2 supported Ni–Cr–LDH (A & B) and SiO2 supported Mg–Cr–LDH (C & D) at different SiO2 to LDH ratios. | ||
3.5. Particle size analysis
The particle size distribution analysis (Fig. 5) of the oven dried nanohybrids showed that a high amount of the particles were in the 100–1000 nm region indicating the attainment of higher particle size by the nanohybrid alcogels an agglomerated state during the process of normal washing with hot water and drying at 40 °C. During such drying the alcogels are converted to xerogels with the increase of their particle size, therefore a critical point drying is favourable especially when drying of their thin films are considered. | ||
Fig. 5 Particle size distribution curves of SiO2 supported Mg–Cr–LDH (A) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and Ni–Cr–LDH (B) 1 1 and Ni–Cr–LDH (B) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 SiO2 to LDH ratios. 1 SiO2 to LDH ratios. | ||
3.6. Zeta potential study
The dispersibility of the SiO2@Ni–Cr–LDH and Mg–Cr–LDH core shell nanohybrids solids in aqueous medium were studied by zeta potential analysis. As shown in Table 2 the negative zeta potential of these nanohybrids after dispersion in aqueous medium increases with the increase of silica. Since, the isoelectric point of SiO2 varies in the range 2 to 4 whereas for LDH it is 12 (ref. 5 and 46) therefore, with the rise of SiO2 component in the nanohybrids the magnitude of negative zeta potential increased which helped in the dispersibility of the system as well as the possibility for the formation of thin films over solid surfaces by dip-coating or spin coating.48| Nanohybrids | Zeta-potential values (mV) | pH |
|---|---|---|
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg–Cr–LDH (0 Mg–Cr–LDH (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
18.8 | 8.8 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg–Cr–LDH (1 Mg–Cr–LDH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
−16.3 | 8.7 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg–Cr–LDH (2 Mg–Cr–LDH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
−16.6 | 8.8 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg–Cr–LDH (3 Mg–Cr–LDH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
−19.5 | 9.0 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni–Cr–LDH (0 Ni–Cr–LDH (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
14.9 | 8.8 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni–Cr–LDH (1 Ni–Cr–LDH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
−16.8 | 8.7 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni–Cr–LDH (2 Ni–Cr–LDH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
−17.7 | 8.8 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni–Cr–LDH (3 Ni–Cr–LDH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
−21.5 | 9.0 |
3.7. SEM, TEM and EDX analysis
The structural morphology and elemental compositions were studied by SEM, TEM (Fig. 6A–E and 7A–E) and EDX (Fig. 7F and G) analysis. FESEM images showed the thin platelets of Ni–Cr and Mg–Cr–LDH where the LDH sheets were arranged in nano sheets and stacked over one another (Fig. 6A and D). Similarly, TEM images (Fig. 6B and C) showed the aggregates of LDH layers of Ni–Cr–LDH with 0.243 nm lattice fringes that can be attributed to the 012 plane of Ni–Cr–LDH. TEM image (Fig. 6E) of Mg–Cr–LDH also showed the stacking of LDH layers with 57.3 nm dimension of one hexagonal plate. | ||
| Fig. 6 SEM and TEM images of Ni–Cr–LDH (A & B); lattice fringes of Ni–Cr–LDH (C), SEM (D) and TEM images of Mg–Cr–LDH showing the stacking of layers (E). | ||
Fig. 7A–E shows SEM and TEM images of SiO2 supported Ni–Cr and Mg–Cr–LDH nanohybrids where poorly crystalline SiO2 formed a continuous matrix on which nano-sheets of Ni–Cr and Mg–Cr–LDH were distributed. SEM and TEM images showed the hexagonal platelets of Ni–Cr–LDH which were stacked over one another and LDH layers were dispersed over SiO2 spheres in both of these nanohybrids. Fig. 7E shows the lattice fringes with dimensions of 0.243 nm and 0.144 nm indicating the presence of 012 and 331 plane of LDH and SiO2 respectively. The presence of Ni–Cr and Mg–Cr–LDH as well as the SiO2 in the composites were further confirmed by selected area EDX analysis. From EDXA the weight percentage of Ni and Cr in case of SiO2 supported Ni–Cr–LDH were found as 13.67% and 6.84% whereas, the weight percentage of Mg and Cr in case SiO2 supported Mg–Cr–LDH were found as 15.73% and 7.88% (Fig. 7F and G). The elemental mapping patterns (Fig. 7H–L) showed the spatial distribution of O, Si, Mg, Cr and Ni as per their synthesis ratio as well as Mg, Cr and Ni centers were uniformly distributed around the Si centers. The presence of C and O in EDX patterns also indicated the presence of surface adsorbed acetylacetonate and alcohol molecules formed during the hydrolysis of metal acetylacetonates and alkoxide.
3.8. Rheological study
| Shear stress (τ) = a + b × shear rate (γ) | (1) |
 | ||
| Fig. 8 Shear rate vs. shear stress curves (A & C) and shear rate vs. viscosity (B & D) of SiO2 supported Ni–Cr–LDH and SiO2 supported Mg–Cr–LDH at different SiO2 to LDH ratios. | ||
The Correlation ratio (R) was found in the range from 0.9933–0.9987 in case of SiO2/Ni–Cr nanohybrids and 0.9883–0.9983 in case of SiO2/Mg–Cr–LDH nanohybrids (Table 3). In all the rheograms it was found that the systems were basically non-Newtonian with gel strength increasing as the SiO2 content rise. The alcogel of the LDH alone did not show much gelation despite the increasing viscosity as well as the increase of shear rate but the alcogel of SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LDH composites showed increasing gelation at low shear rate as the silica content increased. At high SiO2
LDH composites showed increasing gelation at low shear rate as the silica content increased. At high SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LDH ratio of 3
LDH ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 the thixotropy was found highest due to local spatial rearrangement of micro structure units in the structured gel. This was due to the breakdown of the silica network. It is responsible for the increasing gelation with increasing SiO2 content. This behaviour helped for holding the LDH component dispersed.
1 the thixotropy was found highest due to local spatial rearrangement of micro structure units in the structured gel. This was due to the breakdown of the silica network. It is responsible for the increasing gelation with increasing SiO2 content. This behaviour helped for holding the LDH component dispersed.
| Nanohybrids | a (yield stress in Pa) | b (shear viscosity in Pa s) | R (correlation ratio) |
|---|---|---|---|
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni–Cr–LDH (0 Ni–Cr–LDH (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
4.412 | 0.009 | 0.9933 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni–Cr–LDH (1 Ni–Cr–LDH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
4.349 | 0.011 | 0.9975 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni–Cr–LDH (2 Ni–Cr–LDH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.385 | 0.007 | 0.9987 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni–Cr–LDH (3 Ni–Cr–LDH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.243 | 0.007 | 0.9987 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg–Cr–LDH (0 Mg–Cr–LDH (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.162 | 0.013 | 0.9883 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg–Cr–LDH (1 Mg–Cr–LDH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.043 | 0.003 | 0.9916 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg–Cr–LDH (2 Mg–Cr–LDH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.043 | 0.003 | 0.9916 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg–Cr–LDH (3 Mg–Cr–LDH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
5.338 | 0.017 | 0.9983 |
 | ||
| Fig. 9 Amplitude sweep for SiO2 supported Ni–Cr–LDH (A) and Mg–Cr–LDH (B) at different SiO2 to LDH ratios at 1 rad s−1 angular frequency. | ||
 | ||
| Fig. 10 Frequency sweep for SiO2 supported Ni–Cr–LDH at different SiO2 to LDH ratios at 5% shear strain. | ||
| Nanohybrids | Cross-over point (Pa) |
|---|---|
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni–Cr–LDH (0 Ni–Cr–LDH (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
5.91 × 101 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni–Cr–LDH (1 Ni–Cr–LDH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
3.06 × 101 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni–Cr–LDH (2 Ni–Cr–LDH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1.093 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni–Cr–LDH (3 Ni–Cr–LDH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
3.66 × 102 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg–Cr–LDH (0 Mg–Cr–LDH (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
6.38 × 102 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg–Cr–LDH (1 Mg–Cr–LDH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
5.02 × 101 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg–Cr–LDH (2 Mg–Cr–LDH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1.98 × 103 |
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg–Cr–LDH (3 Mg–Cr–LDH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1.59 × 103 |
The frequency sweep test for these nanohybrids was done in the linear viscoelastic region (Fig. 9(B) and 10(B)) by keeping shear strain constant at 5%. G′ was found to be greater than G′′ in both of these nanohybrids indicating the elastic behaviour. The G′ value for the SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LDH ratio at 3
LDH ratio at 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in SiO2 supported Mg–Cr–LDH and Ni–Cr–LDH was found to be higher than 0
1 in SiO2 supported Mg–Cr–LDH and Ni–Cr–LDH was found to be higher than 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 SiO2
1 SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LDH ratios indicating its higher gel-like or solid-like viscoelastic behaviour. It was further observed that upto high angular frequency region G′ remained greater than G′′ in both of these nanohybrids indicating the occurrence of viscoelastic gel like structure with higher stability.
LDH ratios indicating its higher gel-like or solid-like viscoelastic behaviour. It was further observed that upto high angular frequency region G′ remained greater than G′′ in both of these nanohybrids indicating the occurrence of viscoelastic gel like structure with higher stability.
Complex viscosity (η*) is an important parameter which describes the flow resistance of the materials in the structured state, originating as viscous or elastic flow resistance to the oscillating movement of the materials.50,51 The mathematical expression for complex viscosity (η*) is given below
 | (2) |
| ω = 2πf | (3) |
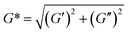 , ω is the angular frequency in rad s−1 and f is the frequency in Hertz.
, ω is the angular frequency in rad s−1 and f is the frequency in Hertz.
The frequency dependence of complex viscosity (η*) of SiO2 supported Ni–Cr–LDH and Mg–Cr–LDH curves showed the gradual decrease of complex viscosity with the increase of angular frequency (Fig. 11(A) and (B)) showing the shear thinning behaviour. The complex viscosity (η*) was found to be dependent on SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LDH ratios. The values of complex viscosity was greater for the 3
LDH ratios. The values of complex viscosity was greater for the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 SiO2
1 SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LDH ratio. As the SiO2 ratios increased from 0
LDH ratio. As the SiO2 ratios increased from 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 there was a greater decrease of complex viscosity and hence showing a good shear-thinning behaviour which is a prerequisite for dip coating.
1 there was a greater decrease of complex viscosity and hence showing a good shear-thinning behaviour which is a prerequisite for dip coating.
3.9. Coating of SiO2 supported Ni–Cr and Mg–Cr–LDH over honeycomb monolithic substrates
The commercial application of ceramic honeycomb monolithic substrates in the field of environmental catalysis is well known. Simultaneously, the catalytic uses and prospects of LDH based materials especially their calcined product the mixed metal nano oxides are also well known. However, one of the serious impediments in integrating the advantages of ceramic honeycomb monoliths and LDHs is the difficulty in wash or dip coating the latter over honeycomb channels. Unlike swelling clays e.g. montmorillonite the LDH does not undergo easy dispersions which prevents in laying LDH based coats over honeycomb channels. Since, SiO2@Ni–Cr–LDH and Mg–Cr–LDHs core–shells was found to give good stable dispersions therefore both of them in the ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 SiO2
1 SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LDH were dip coated over honey comb monolith (Fig. 12). Such coated honeycombs could be used for various gas–solid catalytic applications and are widely used in automotive sector.52,53
LDH were dip coated over honey comb monolith (Fig. 12). Such coated honeycombs could be used for various gas–solid catalytic applications and are widely used in automotive sector.52,53
 | ||
Fig. 12 Coated honey-comb monolith with (A) SiO2 supported Ni–Cr–LDH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) & (B) SiO2 supported Mg–Cr–LDH (3 1) & (B) SiO2 supported Mg–Cr–LDH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
4. Conclusions
The SiO2@Ni–Cr–LDH and Mg–Cr–LDH core–shell type nanohybrid alcogels were successfully synthesized by ‘soft chemical’ sol–gel method by using a mixed system of silicon alkoxide and metal acetylacetonate as precursors. The synthesis techniques adopted helped in dispersing otherwise difficult to exfoliate and disperse LDH nanosheets in a continuous network of silica particles. The synthesis was basically carried out in alcohol rich organic medium which bears the specific prospect of drying the gels by using specialised critical point drying techniques at low temperatures where solvents would be removed without much disturbing the microstructure of solid network. Such alcogels after normal low temperature drying when characterized by XRD, DTA-TGA, IR, zeta-potential study, particle size analysis, SEM, TEM and EDX analysis confirm the formation of nanohybrids having LDH sheets assembled around silica centers. BET surface area analysis of oven dried samples itself showed rise in surface area with the rise of silica content indicating good prospects of these materials in the field of catalysts. The flow behaviour study showed that the alcogels have Bingham fluid behaviour with a favourable shear thinning property. It was further observed that with the increase of SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LDH ratios from 0
LDH ratios from 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 their elastic or gel like behaviour increased with G′ (storage modulus) remaining greater than G′′ (loss modulus). Also, it was observed that there is a gradual decrease of complex viscosity with the increase of angular frequency showing a good shear-thinning behaviour. Simultaneously, with the increase of SiO2 component the dispersibility of these nanohybrids also kept on increasing due to which it could be easily coated over solid surface which was also observed from zeta potential analysis showing the higher negative zeta potential of SiO2@LDH nanohybrids with 3
1 their elastic or gel like behaviour increased with G′ (storage modulus) remaining greater than G′′ (loss modulus). Also, it was observed that there is a gradual decrease of complex viscosity with the increase of angular frequency showing a good shear-thinning behaviour. Simultaneously, with the increase of SiO2 component the dispersibility of these nanohybrids also kept on increasing due to which it could be easily coated over solid surface which was also observed from zeta potential analysis showing the higher negative zeta potential of SiO2@LDH nanohybrids with 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 SiO2
1 SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LDH ratio. Thus, it can be concluded that the reported route can be a facile route for synthesis of asymmetric mesoporous membranes over solid ceramic supports. Further studies are going on the environmental catalytic application of such systems.54
LDH ratio. Thus, it can be concluded that the reported route can be a facile route for synthesis of asymmetric mesoporous membranes over solid ceramic supports. Further studies are going on the environmental catalytic application of such systems.54
Acknowledgements
Authors are grateful to Director, CSIR-NEIST, Jorhat for allowing to publish the paper. Authors are also grateful CSIR for funding the work under CSIR Net-Work Project CSC-0104 and CSC-0408 for providing the facility of SEM analysis. Authors are also grateful to DST-ARCI Hyderabad, India for providing the ceramic honeycomb monoliths as a part of ongoing scientific collaboration.References
- V. Rives, Layered double hydroxides: present and future, Nova Science Publishers, 2001 Search PubMed.
- X. Duan and D. G. Evans, Layered double hydroxides, Springer Verlag, 2006 Search PubMed.
- F. Cavani, F. Trifiro and A. Vaccari, Catal. Today, 1991, 11, 173–301 CrossRef CAS.
- T. Hibino and W. Jones, J. Mater. Chem., 2001, 11, 1321–1323 RSC.
- C. Chen, R. Felton, J. C. Buffet and D. O'Hare, Chem. Commun., 2015, 51, 3462 RSC.
- C. Li, M. Wei, D. G. Evans and X. Duan, Small, 2014, 10, 4469–4486 CrossRef CAS PubMed.
- S. V. Prasana, P. V. Kamath and C. Shivakumara, Mater. Res. Bull., 2007, 42, 1028–1039 CrossRef.
- M. Meyn, K. Beneke and G. Lagaly, Inorg. Chem., 1990, 29, 5201–5207 CrossRef CAS.
- J. T. Kloprogge, S. Komarneni and J. E. Amonette, Clays Clay Miner., 1990, 47, 529–554 Search PubMed.
- X. Ji, W. Zhang, L. Shan, Y. Tian and J. Liu, Sci. Rep., 2015, 5, 18367 CrossRef CAS PubMed.
- S. P. Li and K. Liu, Colloids Surf., A, 2007, 304, 14–17 CrossRef CAS.
- P. Fu, K. Xu, H. Song, G. Chen, J. Yang and Y. Niu, J. Mater. Chem., 2010, 20, 3869–3876 RSC.
- Y. Gao, J. Wu, Z. Zhang, R. Jin, X. Zhang, X. Yan, A. Umar, Z. Guob and Q. Wang, J. Mater. Chem. A, 2013, 1, 9928 CAS.
- Z. Hu and G. Chen, RSC Adv., 2013, 3, 12021–12025 RSC.
- Z. Hu and G. Chen, J. Mater. Chem. A, 2014, 2, 13593 CAS.
- Li. Yan, B. Haoyu and S. Shuanglong, Chin. J. Chem., 2011, 2, 1101–1106 Search PubMed.
- S. Virkar and A. Chaskar, Adv. Mater. Sci. Appl., 2014, 3, 150–156, DOI:10.5963/amsa0303006.
- M. Mishra and R. L. Goswamee, US Pat. No 8951491, 2015.
- S. Chakraborty, M. Kumar, K. Suresh and G. Pugazhenthi, Powder Technol., 2014, 256, 196–203 CrossRef CAS.
- B. Baruah, M. Mishra, C. R. Bhattacharjee, M. C. Nihalani, S. K. Mishra, S. D. Baruah, P. Phukan and R. L. Goswamee, App. Clay Sci., 2013, 80–81, 169–175 CrossRef CAS.
- D. Gamota and F. Filisko, J. Rheol., 1991, 35, 1411–1425 CrossRef CAS.
- F. E. Filisko and L. H. Radzilowski, J. Rheol., 1990, 34, 539–552 CrossRef.
- W. L. Zhang, B. J. Park and H. J. Choi, Chem. Commun., 2010, 46, 5596–5598 RSC.
- J. Yin, Y. Shui, Y. Dong and X. Zhao, Nanotechnology, 2014, 25, 045702 CrossRef PubMed.
- S. D. Jiang, Z. M. Bai, G. Tang, L. Song, A. A. Stec, T. R. Hull, Y. Hu and W. Z. Hu, ACS Appl. Mater. Interfaces, 2014, 6, 14076–14086 CAS.
- S. D. Jiang, Z. M. Bai, G. Tang, L. Song, A. A. Stec, T. R. Hull, Y. Hu and W. Z. Hu, ACS Appl. Mater. Interfaces, 2015, 7, 8506–8514 CAS.
- M. Shao, F. Ning, J. Zhao, M. Wei, D. G. Evans and X. Duan, J. Am. Chem. Soc., 2012, 134, 1071–1077 CrossRef CAS PubMed.
- O. M. Sadek, S. M. Reda and R. K. Al-Bilali, Adv. Nanopart., 2013, 2, 165–175 CrossRef.
- C. Chen, P. Wang, T. T. Lim, L. Liu, S. Liuc and R. Xu, J. Mater. Chem. A, 2013, 1, 3877 CAS.
- M. Shao, F. Ning, Y. Zhao, J. Zhao, M. Wei, D. G. Evans and X. Duan, Chem. Mater., 2012, 24, 1192–1197 CrossRef CAS.
- A. Maria, V. Borau, C. Jimenez, M. Jose, M. R. Ruiz and F. J. Urbano, J. Solid State Chem., 2002, 168, 156–161, DOI:10.1006/jssc.2002.9655.
- X. Bi, T. Fan and H. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 20498–20509 CAS.
- Y. Gao, J. Wu, Q. Wang, C. A. Wilkie and D. O'Hare, J. Mater. Chem. A, 2014, 2, 10996 CAS.
- Q. Wang, X. Zhang, J. Zhu, Z. Guob and D. O'Hare, Chem. Commun., 2012, 48, 7450–7452 RSC.
- Q. Wang, J. P. Undrell, Y. Gao, G. Cai, G. Buffet, C. A. Wilkie and D. O'Hare, Macromolecules, 2013, 46, 6145–6150 CrossRef CAS.
- J. S. Valente, G. R. Gattorno, M. V. Orta and E. T. Garcia, Mater. Chem. Phys., 2012, 133, 2089–2099 CrossRef.
- M. Mishra, M. R. Das and R. L. Goswamee, J. Sol-Gel Sci. Technol., 2010, 54, 57–61 CrossRef CAS.
- F. Prinetto, G. Ghiotti, P. Graffin and D. Tichit, Microporous Mesoporous Mater., 2000, 39, 229–247 CrossRef CAS.
- J. Livage, New J. Chem., 2001, 25, 1 RSC.
- M. Guglielmi and G. Carturan, J. Non-Cryst. Solids, 1988, 100, 16–30 CrossRef CAS.
- L. G. Hubert-Pfalzgraf, Appl. Organomet. Chem., 1992, 6, 627–643 CrossRef CAS.
- A. L. Willis, Z. Chen, J. He, Y. Zhu, N. J. Turro and S. O'Brien, J. Nanomater., 2007, 1–7, DOI:10.1155/2007/14858.
- H. Nasri, S. Khemakhem and R. B. Amar, Period. Polytech., Chem. Eng., 2014, 58(2), 171–178, DOI:10.3311/ppch.7388.
- D. D. Perrin and W. L. F. Armarego, Purification of Labaratory Chemicals, Pergamon, Oxford, 1980 Search PubMed.
- J. L. Schlenker, J. B. Higgins and E. W. Valyocsik, Zeolites, 1990, 10, 293–296 CrossRef CAS.
- M. Sato, Mineral. J., 1964, 4, 215–225 CrossRef.
- F. Li, J. Liu, D. G. Evans and X. Duan, Chem. Mater., 2004, 16, 1597–1602 CrossRef CAS.
- B. Li, Y. Hu, Z. Chen and W. Fan, Mater. Lett., 2007, 61, 2761–2764 CrossRef CAS.
- M. A. Meyers and K. K. Chawla, Mechanical behavior of materials, Prentice-Hall, Upper Saddle River, NJ, 1999, pp. 98–103 Search PubMed.
- T. G. Mezger, The Rheology Handbook, 2nd edn, 2006 Search PubMed.
- R. B. Bird and A. J. Giacomin, Rheol. Acta, 2012, 51, 481–486, DOI:10.1007/s00397-012-0621-2.
- J. L. Williams, Catal. Today, 2001, 69, 3–9 CrossRef CAS.
- A. F. Pérez-Cadenas, M. M. P. Zieverink, F. Kapteijn and J. A. Moulijn, Catal. Today, 2005, 105, 623–628 CrossRef.
- P. Saikia, A. Borah, N. ’G. B. Allou and R. L. Goswamee, Indian pat. applied, Application No. 201611022222 dated 29/06/2016.
| This journal is © The Royal Society of Chemistry 2016 |



