Synthesis of bicyclic 2-pyridones by regioselective annulations of heterocyclic ketene aminals with anhydrides†
Abstract
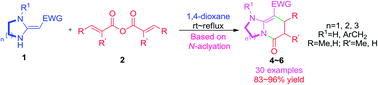
An efficient strategy for the synthesis of substituted bicyclic 2-pyridones is developed. The proposed approach is based on the regioselective N-acylation of heterocyclic ketene aminals (HKAs) with methacrylic anhydride or crotonic anhydride. The tolerance of the developed methodology allows for the construction of fused 2-pyridone ring systems in excellent yields with a variety of functional groups HKAs as synthons under neutral conditions.


 Please wait while we load your content...
Please wait while we load your content...