Open Access Article
Open Access ArticleFunctionalization of fullerene at room temperature: toward new carbon vectors with improved physicochemical properties†
Z.
Beiranvand
a,
A.
Kakanejadifard
a,
I. S.
Donskyi
bc,
A.
Faghani
b,
Z.
Tu
b,
A.
Lippitz
c,
P.
Sasanpour
de,
F.
Maschietto
b,
B.
Paulus
b,
W. E. S.
Unger
c,
R.
Haag
b and
M.
Adeli
*ab
aFaculty of Science, Department of Chemistry, Lorestan University, Khorramabad, Iran
bInstitut für Chemie und Biochemie, Freie Universität Berlin, Takustrasse 3, Berlin 14195, Germany. E-mail: aadeli@fu-berlin.de; mohadeli@yahoo.com
cBAM – Federal Institute for Material Science and Testing, Division of Surface Analysis and Interfacial Chemistry, Unter den Eichen 44-46, 12205 Berlin, Germany
dDepartment of Medical Physics and Biomedical Engineering, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
eSchool of Nanoscience, Institute for Research in Fundamental Sciences (IPM), Tehran, Iran
First published on 23rd November 2016
Abstract
In this work, fullerene has been functionalized with cyanuric chloride at room temperature by a nitrene mediated [2 + 1] cycloaddition reaction. The adduct after functionalization is inherently in the form of azafulleroid and shows broad UV absorption in the wavelength range of 200–800 nm, as well as photothermal conversion and fluorescence with a high quantum yield.
Fullerene (C60) and its functionalized derivatives are interesting candidates for many applications, due to their unique physicochemical properties.1 For example, they can be used as antivirals, anticancer compounds, antioxidants as well as drug delivery agents in biomedical science.2,3 Poor solubility, low processability and other drawbacks that hamper applications of fullerene have been mostly addressed by covalent and noncovalent functionalization.4
In the past few decades a wide range of functionalization approaches has been reported.4,5 Nevertheless, a proper tailoring of the distinct physicochemical properties of fullerene remains challenging.6,7
UV absorption of fullerene is limited to short wavelengths and therefore it does not show a high photothermal conversion. Furthermore, fullerene and its derivatives exhibit very low fluorescence efficiencies, which render them unavailable for different applications such as bioimaging.8 These disadvantages cause fullerenes to be rather inefficient as compared to their one and two dimensional analogues, namely single walled carbon nanotubes and graphene oxide. Due to their defined structure which is a clear advantage over other families of carbon based nanomaterials, fullerene derivatives exhibiting comparable optoelectronic properties could be promising alternatives for different applications including bioimaging9 and photothermal therapy.
Nitrene [2 + 1] cycloaddition reactions and consequently aziridinofullerene-azafulleroid isomerisation is a well-known strategy to obtained nitrogen doped fullerenes with a preserved π-conjugated system.10 This method allows to efficiently functionalize the fullerene cage without affecting the sp2 carbon network, thus leaving the hybridization of the carbon atoms unchanged upon covalent functionalization.11 However, the isomerisation reaction often requires relatively high temperatures, which in turn uncompromise the thermal stability of some of the functional groups involved. Recently, we have shown that 2-azido-4,6-dichloro-1,3,5-triazine is a very efficient nitrene precursor and is able to conjugate to carbon based nanomaterials in a [2 + 1] cycloaddition reaction at room temperature. It was observed that aziridine rings are in the open form and therefore the π-conjugated system of those nanomaterials is preserved upon functionalization.12
In the present work we have successfully extended this strategy for the functionalization of fullerenes. In situ reaction between sodium azide, 2,4,6-trichloro-1,3,5-triazine and fullerene at room temperature results in triazine-functionalized fullerene (Full-Trz) in the azafulleroid form with unusual optoelectronic properties (Scheme 1).
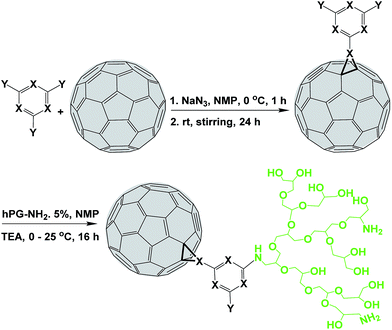 | ||
| Scheme 1 Functionalization of fullerene by an in situ [2 + 1] nitrene cycloaddition reaction at room temperature. Chlorine and nitrogen atoms are symbolized by Y and X, respectively. | ||
The energy path of the cycloaddition reaction shown in Scheme 1 was investigated computationally by means of a CI-NEB13,14 calculation. The latter study revealed that the reaction lacks of an activation energy, hence confirming that the functionalization is energetically favoured and is likely to occur, even at room temperature (Fig. S10†).
The X-ray photoelectron spectroscopy (XPS) spectra of Full-Trz revealed the presence of C, N and Cl atoms, proving the conjugation of triazine groups to fullerene (Fig. S1†). In the C 1s peak spectra, in addition to the peak for carbon atoms of fullerene (CFull) at 284.6 eV, an intense peak at 286.3 eV, which is assigned to the carbon atoms of functional groups (CTrz), provides another evidence for conjugation of triazine to the surface of fullerene (Fig. 1a). The peak area ratio of these two types of carbon atoms (CTrz/CFull) is 0.56, confirming that Full-Trz consists of 56% triazine and 44% fullerene moieties (Table S1†). Such ratio indicates conjugation of 5 triazine units to the surface of fullerene.
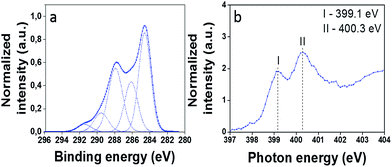 | ||
| Fig. 1 (a) Highly resolved XPS spectra with peak fitting for C 1s Full-Trz, (b) expanded low energy section of N K-edge NEXAFS of Full-Trz. For assignments see Tables S1 and S2.† | ||
The N K-edge NEXAFS spectrum of Full-Trz at the low-energy side (Fig. 1b) reveals two sharp resonances (I, II) that correspond with triazine groups that are attached to fullerene. The low energy resonance (I) is related to the nitrogen atoms in the triazine ring which was found in NEXAFS spectra of triazine in the gas phase.15,16 Resonance (II) also is characteristic for triazine molecules, coupled to carbon materials and was reported in literature.13
In the IR spectra of Full-Trz, two absorbance bands, respectively located at 1181 and 1427 cm−1 are the characteristic peaks of C60 (Fig. S2a†).17 Besides, the absorbance bands at 1510 and 1634 cm−1 are assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds of the triazine and the fullerene fragments, respectively. In the IR spectra, the characteristic bands of the aziridine rings, created by the [2 + 1] cycloaddition reaction, usually appear around 1000 and 1250 cm−1. Those bands are due to symmetric aziridine ring breathing modes and to the sp3-hybridized C–N bonds.18 The absence of such absorbance bands in the IR spectra of Full-Trz could reflect the opening of the aziridine ring and subsequent formation of an azafulleroid structure.
C bonds of the triazine and the fullerene fragments, respectively. In the IR spectra, the characteristic bands of the aziridine rings, created by the [2 + 1] cycloaddition reaction, usually appear around 1000 and 1250 cm−1. Those bands are due to symmetric aziridine ring breathing modes and to the sp3-hybridized C–N bonds.18 The absence of such absorbance bands in the IR spectra of Full-Trz could reflect the opening of the aziridine ring and subsequent formation of an azafulleroid structure.
Furthermore, the number of triazine functional groups conjugated to the surface of fullerene was also calculated by thermogravimetric analysis (TGA). Based on the mass loss at 480 °C, we found that the total mass of triazine groups in Full-Trz is 55%. This indicates that 5 triazines conjugate to one C60 molecule (Fig. S3†). This result is in good agreement with XPS data. The UV-vis spectrum of Full-Trz shows a broad absorption band in the 200–800 nm range (Fig. 2a). The extension of the UV absorption of fullerene from short wavelengths (200–430 nm) to near-IR is due to the substantial changes in its π-conjugated system.
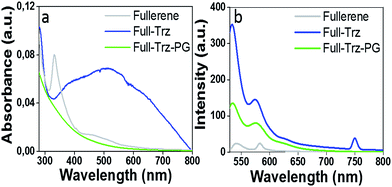 | ||
| Fig. 2 (a) and (b) UV and fluorescence spectra of 0.1 mg ml−1 solution of fullerene (in DMF), Full-Trz (in PBS) and Full-Trz-PG (in PBS), respectively. Spectra are recorded at room temperature. | ||
Usually, covalent functionalization of carbon based nanomaterials by [2 + 1] cycloaddition reaction results in sp3 carbon atoms (at the functionalization site) with consequent disruption of the π-conjugation system.19 As a result, the useful optoelectronic properties of carbon based nanomaterials will be suppressed, which is a hampering factor for their future applications.20 In contrast, the present functionalization improve the intrinsic optochemical properties of fullerene, significantly.
The mechanism we propose, as an explanation for these noticeable observations, implies the opening of the aziridine rings followed by sp3 → sp2 rehybridization of the carbon atoms (Fig. 3).
 | ||
| Fig. 3 Aziridinofullerene-azafulleroid interconversion and rehybridization of the carbon atoms of fullerene at ambient condition. X and Y are nitrogen and chlorine atoms, respectively. | ||
This type of isomerisation, usually referred to as aziridinofullerene-azafulleroid interconversion, has been reported for fullerene derivatives previously.10,11 However, much harder reaction conditions were used.
A similar isomerisation has been observed for single walled carbon nanotubes functionalized by carben21,22 or [2 + 1] nitrene cycloaddition reaction.11 There is a strong correlation between the curvature of tubes and this type of isomerization.21 Therefore, one would expect that this conversion is more feasible for fullerene as a highly curved aromatic system. Accordingly, computational studies were performed to investigate Full-Trz isomerization.
While aziridinofullerene-azafulleroid interconversion requires heating,23,24 the functionalization of fullerene by triazine in 5,6/5 and 5,6/6 positions returns preferentially azafulleroid structures (Fig. 4). Moreover, the relative energy of the frontier orbitals of the pristine and functionalized fullerene was calculated. Interestingly, the HOMO–LUMO separation in the azafulleroid form was found to be very close to the one calculated for the pristine C60 (Table S3†).
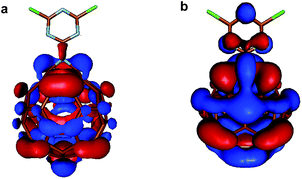 | ||
| Fig. 4 Representation of the HOMO of (a) 5,6/5-Full-Trz and (b) 5,6/6-Full-Trz calculated at the M06-2X/def2-SVP level of theory, using an isosurface value of 0.02 e Å−3. | ||
Given the fact that even a very low number of sp3 carbons would determine a dramatic decrease in the HOMO–LUMO gap of fullerene,25 the computational results strongly uphold the hypothesis of a retained, homogeneous sp2 hybridization of all carbon atoms in Full-Trz.
In the open azafulleroid form, the π-conjugated system of fullerene is regenerated and the π-network extends over the triazine rings (Fig. 4). As a result a broad band appears in the absorption spectra of the Full-Trz.
Optical properties of fullerene derivatives were also investigated computationally (ESI page 9 and 10†). When triazine was arranged in a symmetric shell around fullerene, the UV spectra of Full-Trz was very similar to the experimental data (Fig. S6 and S7†). These results suggest not only a core–shell structure for the functionalized fullerene but also prove the influence of the triazine shell on the optical properties of fullerene.
The near-IR absorption suggests that Full-Trz should exhibit unique optical properties that are observed for single walled carbon nanotubes.
Irradiation of a 5 mg ml−1 dispersion of Full-Trz in PBS increase the temperature of medium to 53 °C after 60 seconds, while this effect was not observed for fullerene when exposed under the same conditions (Fig. 5).
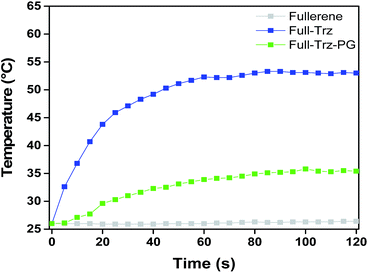 | ||
| Fig. 5 Evaluation of photothermal conversion of fullerene, Full-Trz, and Full-Trz-PG dispersions in PBS (5 mg ml−1) over a period of 2 min under NIR laser irradiation (785 nm, 0.5 W cm−2). | ||
Due to such high photothermal conversion, Full-Trz could be used for variety of applications such as photothermal therapy. Interestingly, Full-Trz shows a high fluorescence emission upon excitation at different wavelengths (Fig. S4†). Therefore, it could be used as an alternative for single-wall carbon nanotubes and in particular for bioimaging.
However, poor water solubility of Full-Trz hampers such applications. Therefore, polyglycerol with few amino functional groups was conjugated to Full-Trz at room temperature, taking advantage of the high reactivity of triazine groups toward nucleophilic reactions (Scheme 1). IR spectrum of fullerene–polyglycerol conjugate (Full-Trz-PG) shows absorbance bands at 3365, 1652 and 1100 cm−1 which are assigned to O–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–O bonds. This result confirms conjugation of polyglycerol to C60 (Fig. S2†). Thermogravimetric analysis (TGA) shows that Full-Trz-PG is consisting of 90% polyglycerol and 10% fullerene moieties5d (Fig. S3†). This compound shows photothermal and fluorescence properties similar to Full-Trz and it is readily soluble in PBS (Fig. 2, 5 and S8†).
C and C–O bonds. This result confirms conjugation of polyglycerol to C60 (Fig. S2†). Thermogravimetric analysis (TGA) shows that Full-Trz-PG is consisting of 90% polyglycerol and 10% fullerene moieties5d (Fig. S3†). This compound shows photothermal and fluorescence properties similar to Full-Trz and it is readily soluble in PBS (Fig. 2, 5 and S8†).
While the fullerene content of Full-Trz-PG is low (10%), its fluorescence is remarkable. Therefore it can be considered as a promising vector with the unique optoelectronic properties for bioimaging (Fig. S9†).
Quantum yields of Full-Trz and Full-Trz-PG are much higher than those reported for fullerene (Table S4†).
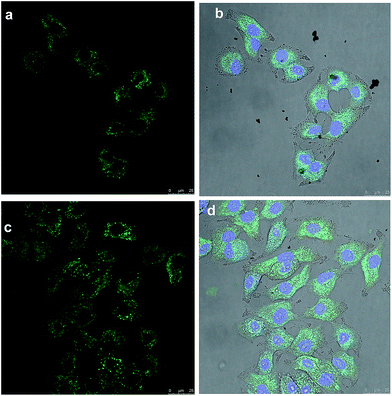
In order to investigate the efficiency of Full-Trz and Full-Trz-PG as fluorescence probes, they were incubated with the A549 cells and then imaged by confocal laser scanning microscopy (CLSM). Fig. 6 show confocal images of A549 cells incubated with these compounds after 4 hours. Uptake of both Full-Trz and Full-Trz-PG can be followed through their fluorescence. However, intensity of fluorescence in the case of Full-Trz-PG is higher, considering 10% Full-Trz content for this compound. Due to the higher solubility of Full-Trz-PG and less aggregation in buffer lower quenching of fluorescence occurs.
Taking advantage of this new functionalization method, fullerene derivatives with the unique optoelectronic and physicochemical properties for a variety of applications could be synthesized. Due to its well-defined structure, Full-Trz is good alternative to nanomaterials with less-defined structures such as single walled carbon nanotubes and quantum dots in bioimaging.
Acknowledgements
The authors would like to thank the SFB 765 for financial support.Notes and references
- (a) E. E. Maroto, M. Izquierdo, S. Reboredo, J. Marco-Martínez, S. Filippone and N. A. Martín, Acc. Chem. Res., 2014, 47, 2660–2670 CrossRef CAS PubMed; (b) M. D. Tzirakis and M. Orfanopoulos, Chem. Rev., 2013, 113, 5262–5321 CrossRef CAS PubMed; (c) S.-E. Zhu and G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7535–7570 RSC; (d) S. S. Babu, H. Möhwald and T. Nakanishi, Chem. Soc. Rev., 2010, 39, 4021–4035 RSC.
- (a) R. Bakry, R. M. Vallant, M. Najam-ul-Haq, M. Rainer, Z. Szabo, C. W. Huck and G. K. Bonn, Int. J. Nanomed., 2007, 2, 639–649 CAS; (b) P. Compain, C. Decroocq, J. Iehl, M. Holler, D. Hazelard, T. M. Barragán, C. O. Mellet and J.-F. Nierengarten, Angew. Chem., Int. Ed., 2010, 49, 5753–5756 CrossRef CAS PubMed; (c) M. Durka, K. Buffet, J. Iehl, M. Holler, J.-F. Nierengarten, J. Taganna, J. Bouckaert and S. P. Vincent, Chem. Commun., 2011, 47, 1321–1323 RSC.
- (a) J. M. Ashcroft, D. A. Tsyboulski, K. B. Hartman, T. Y. Zakharian, J. W. Marks, R. B. Weisman, M. G. Rosenblum and L. J. Wilson, Chem. Commun., 2006, 28, 3004–3006 RSC; (b) T. Y. Zakharian, A. Seryshev, B. Sitharaman, B. E. Gilbert, V. Knight and L. J. Wilson, J. Am. Chem. Soc., 2005, 127, 12508–12509 CrossRef CAS PubMed; (c) R. D. Bolskar, Fullerenes for drug delivery, in Encyclopedia of Nanotechnology, ed. B. Bhushan, Springer, Netherlands, 2012, pp. 898–911 Search PubMed.
- (a) S. Minakata, R. Tsuruoka and M. Komatsu, J. Am. Chem. Soc., 2008, 130, 1536–1537 CrossRef CAS PubMed; (b) Y. Matsuo, K. Kanaizuka, K. Matsuo, Y.-W. Zhong, T. Nakae and E. Nakamura, J. Am. Chem. Soc., 2008, 130, 5016–5017 CrossRef CAS PubMed; (c) C. Yang, J. Y. Kim, S. Cho, J. K. Lee, A. J. Heeger and F. Wudl, J. Am. Chem. Soc., 2008, 130, 6444–6450 CrossRef CAS PubMed.
- (a) C.-L. He, R. Liu, D.-D. Li, S.-E. Zhu and G.-W. Wang, Org. Lett., 2013, 15, 1532–1535 CrossRef CAS PubMed; (b) S. Afreen, K. Muthoosamy, S. Manickam and U. Hashim, Biosens. Bioelectron., 2015, 63, 354–364 CrossRef CAS PubMed; (c) A. Muñoz, D. Sigwalt, B. M. Illescas, J. Luczkowiak, L. Rodríguez-Pérez, I. Nierengarten, M. Holler, J.-S. Remy, K. Buffet, S. P. Vincent, J. Rojo, R. Delgado, J.-F. Nierengarten and N. Martín, Nat. Chem., 2016, 8, 50–57 CrossRef; (d) I. Donskyi, K. Achazi, V. Wycisk, C. Böttcher and M. Adeli, Chem. Commun., 2016, 52, 4373–4376 RSC.
- J. Averdung and J. Mattay, Tetrahedron, 1996, 52, 5407–5420 CrossRef CAS.
- L. Ulmer and J. Mattay, Eur. J. Org. Chem., 2003, 2933–2940 CrossRef CAS.
- J. Jeong, M. Cho, Y. T. Lim, N. W. Song and B. H. Chung, Angew. Chem., Int. Ed., 2009, 48, 5296–5299 CrossRef CAS PubMed.
- J. Jeong, J. Jung, M. Choi, J. W. Kim, S. J. Chung, S. Lim, H. Lee and B. H. Chung, Adv. Mater., 2012, 24, 1999–2003 CrossRef CAS PubMed.
- T. Nakahodo, M. Okada, H. Morita, T. Yoshimura, M. O. Ishitsuka, T. Tsuchiya, Y. Maeda, H. Fujihara, T. Akasaka, X. Gao and S. Nagase, Angew. Chem., Int. Ed., 2008, 47, 1298–1300 CrossRef CAS PubMed.
- H. Hachiya, T. Kakuta, M. Takami and Y. Kabe, J. Organomet. Chem., 2009, 694, 630–636 CrossRef CAS.
- A. Setaro, M. Adeli, M. Glaeske, D. Przyrembel, T. Bisswanger, G. Gordeev, F. Maschietto, A. Faghani, B. Paulus, M. Weinelt, R. Arenal, R. Haag and S. Reich, 2016, Submitted.
- D. Sheppard, R. Terrell and G. Henkelman, J. Chem. Phys., 2008, 128, 134106 CrossRef PubMed.
- G. Henkelman, B. Uberuaga and H. Jónsson, J. Chem. Phys., 2000, 113, 9901–9905 CrossRef CAS.
- E. Apen, A. P. Hitchcock and J. L. Gland, J. Phys. Chem., 1993, 97, 6859–6866 CrossRef CAS.
- G. Vall-llosera, B. Gao, A. Kivimaeki, M. Coreno, J. A. Ruiz, M. de Simone, H. Agren and E. Rachlew, J. Chem. Phys., 2008, 128, 139901 CrossRef.
- K. Saeedfar, L. Y. Heng, T. L. Ling and M. Rezayi, Sensors, 2013, 13, 16851–16866 CrossRef PubMed.
- H. L. Spell, Anal. Chem., 1967, 39, 185–193 CrossRef CAS.
- M. L. Usrey, E. S. Lippmann and M. S. Strano, J. Am. Chem. Soc., 2005, 127, 16129–16135 CrossRef CAS PubMed.
- L. Cognet, D. A. Tuyboulski, J.-D. R. Rocha, C. D. Doyle, J. M. Tour and R. B. Weisman, Science, 2007, 316, 1465–1468 CrossRef CAS PubMed.
- Y.-S. Lee and N. Marzari, Phys. Rev. Lett., 2006, 97, 116801 CrossRef PubMed.
- K. Zhang, Q. Zhang, C. Liu, N. Marzari and F. Stellacci, Adv. Funct. Mater., 2012, 22, 5216–5223 CrossRef CAS.
- M. Cases, M. Duran and M. Solà, J. Mol. Model., 2000, 6, 205–212 CrossRef CAS.
- K. Raghavachari, Chem. Phys. Lett., 1993, 209, 223–228 CrossRef CAS.
- H. Tachikawa, T. Iyama and S. Abe, Phys. Procedia, 2011, 14, 139–142 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra23419d |
| This journal is © The Royal Society of Chemistry 2016 |