Synthesis of europium-doped ZnS nano-crystalline thin films with strong blue photoluminescence†
M. J. Rivera-Medinaa,
J. Hernández-Torresb,
J. L. Boldú-Olaizolac,
J. Barreto-Renteríac,
J. M. Hernández-Alcántarac,
V. Jancikd and
J. C. Alonso-Huitrón*a
aInstituto de Investigaciones en Materiales, Universidad Nacional Autónoma de México, A.P.70-360, Coyoacán 04510, Ciudad de México, Mexico. E-mail: alonso@unam.mx
bCentro de Investigación en Micro y Nanotecnología, Universidad Veracruzana, Boca del Río 94294, Veracruz, Mexico
cInstituto de Física, Universidad Nacional Autónoma de México, A.P. 20-364, Coyoacán 01000, Ciudad de México, Mexico
dCentro Conjunto de Investigación en Química Sustentable UAEM-UNAM, Carr. Toluca Atlacomulco km. 14.5, Toluca 50200, Estado de México, Mexico
First published on 4th November 2016
Abstract
Eu2+-Doped ZnS (ZnS:Eu2+) thin (∼550 nm) films with strong and stable blue photoluminescence have been successfully synthesized by a simple and fast ultrasonic spray pyrolysis method. According to X-ray diffraction (XRD) and high resolution scanning electron microscopy (SEM) analysis, the as grown ZnS:Eu2+ films are composed of hexagonal wurtzite nanocrystals with average size of ∼25 nm, which are preferentially oriented along the (002) direction and agglomerate to form hexagonal facet nanobars with diameters from 50 to 200 nm. These films show a strong blue emission centered at 454 nm, which can be observed with the naked eye under ambient illumination. The intensity of this peak is ∼185 times higher than the maximum photoluminescence peak observed from the undoped ZnS films. The presence of the Eu dopant in the valence state Eu2+ was confirmed by electron spin resonance (ESR) measurements. The comparison of the high-resolution UV-vis absorption spectra and the PL characteristics of the ZnS:Eu2+ films with the pure ZnS films, indicate that the strong blue emission of the ZnS:Eu2+ films comes from Eu2+ 4f65d → 4f7 intra-ion transitions.
1. Introduction
Rare earth doped phosphors have been very important for the development of a wide variety of modern luminescent devices such as: fluorescent lamps, solid state lasers, flat panel displays, etc. Nowadays, with the combination of rare earth dopants such as Eu2+ and Ce3+, it is possible to make activated inorganic phosphors emitting in the blue, green and red colors, for the fabrication of white light emitting diodes (WLEDs) with a good control in the light color balance.1 Recently the Eu2+ ion has been intensively investigated as a blue and blue-green-emitting activator in a wide variety of host materials in the form of powders, nanoparticles or nanocrystals, such as: graphene,2 Ba1−x(PO3)2 (x = 0.005–0.040),3 BaAlBO3F2,4 Sr4OCl6,5 Ba3P4O13,6 zeolite derivative,7 ZnS,8 and also in thin films of compounds such as: 12CaO·7Al2O3,9 or Ta2O5.10 More recently, due to the high quantum efficiency of the dipole allowed 5d → 4f transitions of divalent europium, it has proven to be an excellent luminescent activator for the development of new KSr2I5:Eu crystalline scintillators, with X-ray-excited blue emission.11The luminescent characteristics of Eu2+ incorporated in more than 300 different inorganic compounds have been compiled, and it has been shown that the energy of the first 4f7 → 4f65d transitions of free Eu2+ (4.2 eV or 295 nm) is red shifted by effect of the host crystal,12 and consequently that the excitation and emission energies can be modulated and improved through changing the composition and structure of the host crystal.1,12 As a motivation for the present work, it is noteworthy that although in this compilation were included around 35 Eu-doped sulfide compounds, having emission wavelengths in the range from blue (459 nm) to red (680 nm), the Eu-doped ZnS compound was not included in this compilation.12 This is intriguing since ZnS has been widely used as an excellent host material for a variety of luminescent centers based on transition and rare earth metals such as: Mn, Cu, Co, Ni, Sm,13–24 and efficient thin film electroluminescent (TFEL) devices have been fabricated with some of these transition metal-rare earth-doped ZnS systems.14,15 On the other hand, in the literature there are many reports of studies on the structure and photoluminescence (PL) of the ZnS:Eu2+ system, in the form of bulk crystals, powders, nanocrystals, nanoparticles and nanowires.8,25–37 At the beginning the technological efforts were made to obtain stable Eu3+ red emission in the ZnS host, however on the basis of electron spin resonance experiments, it was demonstrated that Eu often tends to be stable in the 2+ charge state due to its half filled 4f shell, and that Eu naturally incorporates in single crystals with hexagonal wurtzite lattice of ZnS, as Eu2+, substituting Zn2+, without any problem of charge compensation.25–27 Although it was also found that the limit of solubility of Eu in the ZnS lattice is rather low (does not exceed 0.1%), these works motivated the investigation of the PL characteristics of a wide variety of ZnS:Eu2+ nanocrystals, nanoparticles and nanowires. Depending on the method of synthesis, precursors, thermal treatments, size of crystals, crystalline phase (cubic zinc blende or hexagonal wurtzite), etc., these nanostructures may present green,28–31,37 or blue emission,8,32–37 or combined blue and green emission.8,36,37 Most of the methods used for the synthesis of the above mentioned ZnS:Eu2+ nanomaterials involve long and complicated processes, and/or thermal treatments. For example, several works use EuCl2, but it is highly sensitive to moisture, and thus hydrothermal or N2 treatments at 80–120 °C for 15–24 h or sinterization at 800 °C for 3 h are required to obtain the final ZnS:Eu2+ nanomaterial.28–32,34,36 Other methods use Eu2O3, which is converted to Eu(NO3)3 and mixed with Zn(NO3)2 and thiourea, and then the mixture is kept at 160 °C for 15 h to get the final luminescent product.34,35 The Eu(NO3)3 precursor is directly used and mixed with zinc acetate and thioacetamide in a co-precipitation method, but the ZnS:Eu2+ phosphors require annealing at 1100 °C to get intense luminescence.8 Eu(NO3)3, ZnS and NaCl were used in a simple vapor deposition method, but the furnace where the ZnS:Eu2+ nanowires were prepared was heated for different times to 1100 °C in a mixture of Ar and H2 gases to ensure that all the Eu3+ ions were reduced to Eu2+.37 The EuCl3·6H2O precursor along with ZnS powder have been used to grow ZnS:Eu2+ nanowires by chemical vapor deposition, heating the source materials and the substrate at 800 °C during 10 min under a constant flow of Ar (97%) and H2 (3%), and then cooling down to room temperature.33
On the other hand, in spite of the previously mentioned studies, very little work has been done on ZnS:Eu2+ phosphors in the form of thin-film, and the scare works have reported other emission features and not the blue or green emission characteristics of Eu2+ ions.38,39 In one of these works ZnS:Eu,Cl thin films were evaporated by electron gun and incorporated in TFEL devices, with emission bands assigned to transitions within the Eu3+ ions.38 In another work ZnS:Eu,F thin films were prepared by r.f. magnetron sputtering, and incorporated in TFEL devices whose luminous characteristics corresponded also to the emission of Eu3+ ions.39 In the latter case, even for the optimum europium concentration (0.94%) in the sputtering target, for which the highest luminance was obtained, the intensity of the electroluminescence of the ZnS:Eu TFEL devices was much lower than that of the ZnS:Mn devices.39
In this work we report a strong blue photoluminescence from ZnS:Eu2+ thin films synthesized by a simple, fast and cheap ultrasonic spray pyrolysis method. These films have potential applications for the development of high intensity miniature electroluminescent displays.14,15
2. Experimental section
The ZnS:Eu2+ films were deposited on glass substrates by the ultrasonic spray pyrolysis technique at atmospheric pressure, using a home-made spray system (see Fig. S1 in the ESI†). To prepare 100 ml of the starting solution, zinc acetate dihydrate [Zn(CH3COO)2·2H2O] (0.724 g, 3.30 mmol), 1,3-dimethyl-2-thiourea [CH3NHCSNHCH3] (0.521 g, 5.00 mmol) and europium chloride hexahydrate [EuCl3 6H2O] (0.037 g, 0.10 mmol, 3 molar% of zinc) were mixed as solids. Subsequently, anhydrous methanol (71.2 ml), deionized water (23.8 ml) and acetic acid (5 ml) were added in this exact order. The final concentration of Zn2+, thiourea and Eu3+ in this solution is 0.033 M, 0.05 M and 0.001 M, respectively. Based on the thermogravimetric analysis (TGA) of the precursor powders, and the possible reactions carried out from the thermal decomposition of the precursors (see Section 3.1.), the substrate temperature was 450 °C for all the deposits, and air was used as the carrier and director gas with constant flow rates fixed at 1.5 l min−1 and 0.3 l min−1, respectively. Under these deposition conditions ∼550 nm thick ZnS:Eu2+ films were obtained using 10 ml of the precursors solution during 15 min of deposition. All reagents were purchased from Sigma Aldrich and used as received without further purification. The air used for the spray pyrolysis deposition process was provided from an oil free air compressor. Un-doped ZnS films were deposited under the same conditions but using a solution without europium chloride.The TGAs of the precursor powders, were obtained using a TGA Q50000 V3.15 equipment from TA Instruments. The crystalline structure of the films was characterized by X-ray diffraction (XRD). The apparatus used for the XRD measurements was a Bragg–Brentano Rigaku ULTIMA IV diffractometer with an X-ray source of Cu Kα line (0.15406 nm), at a grazing beam configuration (incidence angle of 1°). The surface morphology of the films was investigated by atomic force microscopy (AFM) and scanning electron microscopy (SEM), using a JEOL JSPM-4210 scanning probe microscope and a JEOL 7600F field emission scanning electron microscope (FESEM), respectively. The chemical composition of the films was analyzed by energy dispersive spectroscopy (EDS) using an EDX INCA X-act by OXFORD spectrometer coupled to the SEM equipment. The operating voltage was 10 kV for the FESEM images. EDX major operating parameters were: working current 4.5 × 10−10 A, voltage 10 kV, working distance 6 mm, and the atomic number-absorption–fluorescence method was used for quantitative analysis.
The room temperature photoluminescence characteristics of the films, excitation and emission spectra, were recorded using a Spex Fluoromax spectrofluorometer sensitive in the range from 200 to 850 nm, and provided with a xenon lamp for the excitation light. Some PL spectra were also obtained while exciting the sample with an unfocused beam of 25 mW from a Kimmon He–Cd laser operating at 325 nm (3.81 eV), and collecting the emitted light with an optical fiber connected to the spectrofluorometer. Electron spin resonance (ESR) measurements for the detection of Eu2+ in the ZnS:Eu2+ films, were made using a JEOL (JES-RE3X) ESR-X-spectrometer, equipped with rectangular cavity and a gas flow cryostat working in the temperature range from 110 to 300 K. The Eu2+ ESR spectrum was obtained at 113 K (−160 °C) using a resonance frequency of 9.1 GHz and a magnetic field centered at 330 mT. For the ESR measurements the ZnS:Eu2+ films were deposited on KCl single crystalline substrates, in order to avoid the presence of spurious ESR signals due to the paramagnetic impurities (mainly Mn2+ and Fe2+) in the glass substrates.
3. Results and discussion
3.1. Film growth
The precursors in the starting solution were zinc acetate dihydrate, 1,3-dimethyl-2-thiourea and europium chloride hexahydrate, which where dissolved in anhydrous methanol (three parts), deionized water (one part) and acetic acid (5%). Based on related studies, the 1,3-dimethyl-2-thiourea was proposed as the source of hydrogen sulfide as it is released gradually by its acidic hydrolysis at elevated temperature and thus it does not cause precipitation of zinc and europium sulfides in the solution.40 The driving force for the hydrolysis is the difference between the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S (
S ( = 573 kJ mol−1) and C
= 573 kJ mol−1) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (
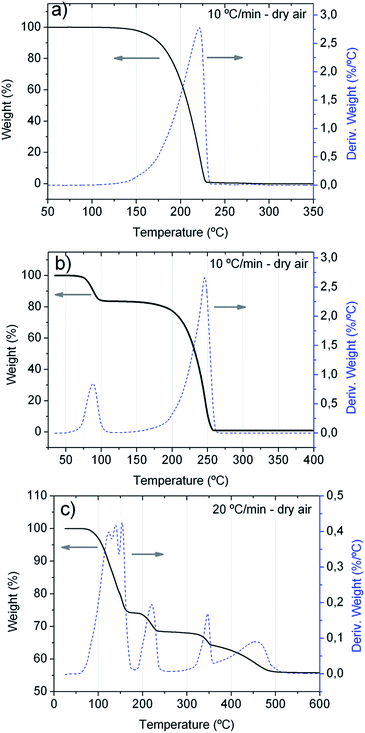
O ( = 789 kJ mol−1) bond enthalpies.41 To determine the optimal temperature for the substrate, TGA analyses of the precursors were performed and the obtained TGA curves are shown in Fig. 1. The TGA curve in Fig. 1a is similar to that previously reported,40 and shows that the 1,3-dimethyl-2-thiourea starts its one-step thermal decomposition, above 100 °C and it decomposes completely at ∼235 °C, while Fig. 1b shows that the zinc acetate dihydrate decomposes in two steps. The first mass loss step corresponds to the loss of the two water molecules, and the other step corresponds to the decomposition of the zinc acetate, which decomposes completely at ∼250 °C. Although a similar behavior has been reported for the thermal decomposition of this precursor, depending on the rate of heating and gas used, its thermal decomposition may be carried out in three steps and the complete decomposition can be shifted to higher temperatures. For example in helium, the complete decomposition occurs at 300–325 °C.42 The TGA curve shown in Fig. 1c indicate that europium chloride hexahydrate does not simply decompose, but undergoes first a stepwise dehydration to EuCl3·H2O (25% mass loss), and that rather complex hydrolytic reactions occur resulting in the formation of different mixed chloride hydroxide species until, EuCl2OH is formed (further 11% mass loss). The final product at 475 °C is the solid EuOCl, which can be recovered from the crucible after thermal analysis. This is in agreement with previously observed results.43,44
= 789 kJ mol−1) bond enthalpies.41 To determine the optimal temperature for the substrate, TGA analyses of the precursors were performed and the obtained TGA curves are shown in Fig. 1. The TGA curve in Fig. 1a is similar to that previously reported,40 and shows that the 1,3-dimethyl-2-thiourea starts its one-step thermal decomposition, above 100 °C and it decomposes completely at ∼235 °C, while Fig. 1b shows that the zinc acetate dihydrate decomposes in two steps. The first mass loss step corresponds to the loss of the two water molecules, and the other step corresponds to the decomposition of the zinc acetate, which decomposes completely at ∼250 °C. Although a similar behavior has been reported for the thermal decomposition of this precursor, depending on the rate of heating and gas used, its thermal decomposition may be carried out in three steps and the complete decomposition can be shifted to higher temperatures. For example in helium, the complete decomposition occurs at 300–325 °C.42 The TGA curve shown in Fig. 1c indicate that europium chloride hexahydrate does not simply decompose, but undergoes first a stepwise dehydration to EuCl3·H2O (25% mass loss), and that rather complex hydrolytic reactions occur resulting in the formation of different mixed chloride hydroxide species until, EuCl2OH is formed (further 11% mass loss). The final product at 475 °C is the solid EuOCl, which can be recovered from the crucible after thermal analysis. This is in agreement with previously observed results.43,44
Based on the TGA characteristics of the precursors and in order to assure the incorporation of Eu in the ZnS films, the substrate temperature of 450 °C was chosen.
When the aerosol droplets of the solution containing all precursors arrive to the heated substrate, several complex hydrolytic and pyrolytic processes and reactions occur that produce many different Zn, S and Eu species and give rise to the film growth. Although we do not have elements to determine the detailed chemical reaction pathways and/or the heterogeneous reactions at the substrate surface that develop the adherent ZnS:Eu2+ film, based on related literature we propose the following steps:
 | (1) |
 | (2) |
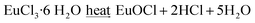 | (3) |
 | (4) |
In the first step, the hydrolysis of the 1,3-dimethyl-2-thiourea produces H2S which reacts with Zn[CH3CO2]2 and EuOCl forming the resulting ZnS:Eu2+ film. The europium atoms are incorporated in the ZnS framework as a substitutional impurity. However the most interesting thing about this synthesis is the fact that although we start from europium in the oxidation state (III) and the preparation of the films is made at high temperature and in the presence of air, the europium gets reduced to Eu2+, which is highly counterintuitive. The mechanism of this reduction is however, unclear. It was recently demonstrated, that from the aqueous solution of praseodymium(III) iodide and thiourea crystallizes PrI3·9H2O·0.5 thiourea. Furthermore, the thiourea has no interaction with the metal ion, but rather forms hydrogen bonds with the coordinated water molecules.45 In acidic aqueous solution of EuCl3, the europium ions form a similar hydrated cation –Eu(H2O)63+ and thus direct interaction of the Eu3+ ions with thiourea in the starting solution can be discarded. On the other hand, reduction of Eu2O3 to EuS under H2S atmosphere can be achieved, however at 1150 °C. In this reaction H2S acts also as the reducing agent and elemental sulfur is formed, but if this reaction is performed at 600 °C, Eu2S3 and water are the only products.46 However, thermal process involving H2S generated from thiourea has been recently used in the reduction of aromatic nitro- to aminocompounds.47 Thus we propose that in this case the Eu3+ is probably in the form of EuOCl and is reduced by H2S to Eu2+ and EuS is formed. Due to the reaction conditions, during the reduction, H2S is probably oxidized directly to SO2. To the best of our knowledge, this is the first example of a direct thermal reduction of Eu3+ to Eu2+ during the synthesis of ZnS under aerobic conditions.
3.2. Structure, morphology and chemical composition
Fig. 2 shows the XRD patterns for the ZnS:Eu2+ film, and that of an un-doped ZnS film. The XRD patterns indicate that both, the Eu-doped and un-doped films are polycrystalline with a preferential orientation at 2θ = 28.54°, and a secondary diffraction peak at 2θ = 51.8°, which correspond to the (002) and (103) planes, respectively, of the hexagonal wurtzite ZnS crystalline structure (card number 00-005-0492 from PDF-2-(2004) data base). All the other smaller XRD peaks also correspond to the hexagonal phase of ZnS. | ||
| Fig. 2 Typical X-ray diffraction patterns for the ZnS and ZnS:Eu2+ films, as deposited by ultrasonic spray pyrolysis, at substrate temperature of 450 °C. | ||
The average crystallite size (D) of each film was assessed using the Debye–Scherrer formula, and the lattice parameters for the hexagonal structure (a and c), and these parameters were: D = 24.0 nm, a = 3.862 Å, c = 6.225 Å and D = 24.8 nm, a = 3.830 Å, c = 6.240 Å, for the ZnS:Eu2+ and ZnS films, respectively. The structure of the ZnS:Eu2+ film is consistent with the results found in some of the earliest investigations which demonstrated that Eu enters in the hexagonal wurtzite lattice of ZnS bulk crystals, as Eu2+, substituting Zn2+.26
The variations in the lattice parameters are expected since the ionic radius of Eu2+ (1.14, 1.17 Å)48,49 is larger than that of Zn2+ ion (0.74 Å), and when the Eu2+ ions replace the Zn2+ ions, a lattice distortion in the structure is produced.35 However the small variations in these parameters indicate that the amount of Eu2+ ions incorporated in the ZnS matrix is very small.
Fig. 3 depicts the FESEM micrographs for the ZnS and ZnS:Eu2+ films. The image in Fig. 3a appears darker because the probe current was lower than in the others (Fig. 3b–d). The micrographs in Fig. 3b–d were obtained at higher probe current in order to get deeper topographic information. The micrographs in Fig. 3a and b are planar surface and inclined cross section views, respectively, for the un-doped ZnS film. As these micrographs show this film consist of well packed hexagonal shaped bars with diameters in the range from 100 to 300 nm, which grew mainly oriented in the z-axis perpendicular to the substrate. The micrographs in Fig. 3c and d, correspond to the ZnS:Eu2+ film, and show that the Eu2+ doped films had a similar growth, although in this case the diameters of the hexagonal shaped bars are smaller, in the range from 50 to 200 nm, approximately. The regular and highly oriented hexagonal structures observed in the FESEM images of the films are in good agreement with the hexagonal wurtzite phase and preferential orientation observed in the XRD spectra. This consistency indicates that during the growth of the films, the crystalline grains aggregate to form the hexagonal bars. The same morphology of agglomerates of small grains was observed from the AFM analysis of the ZnS:Eu2+ films (see the AFM images with different zooms in Fig. S2 in the ESI†). The size of the grains was evaluated in different zones of the AFM images (see Fig. S3 in the ESI†), and the average size was ∼26.5 nm, which is very close to the average size of the crystallites calculated from the XRD analysis.
An attempt was made to detect the Eu atoms incorporated the ZnS:Eu2+ films, by techniques such as X-ray photoelectron spectroscopy, and energy dispersive spectroscopy. However the absence of any signal related to Eu in the spectra obtained for the analyzed films, indicated that the amount of Eu incorporated in the ZnS matrix of the films was below the limit of detection (∼1 at%) of the equipment utilized for these analyses. The low concentration of Eu in our ZnS:Eu2+ films is also consistent with the low limit of solubility reported for Eu in ZnS single crystals.26 In order to confirm the existence of trace amounts of Eu2+ in the ZnS:Eu2+ films, ESR measurements were carried out.
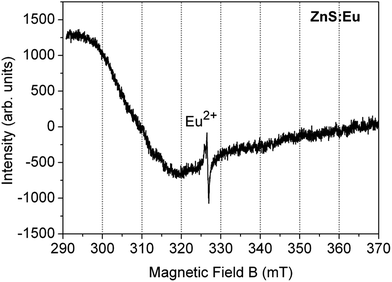
Fig. 4 shows the ESR spectrum of the ZnS:Eu2+ film. The observed spectrum is similar to that reported in previous works for Eu2+ substituting Zn2+ sites in the hexagonal wurtzite-phase ZnS lattice,26,33 with the principal line at 325.2 mT and a g-value of 2.0041. As it is known, any ESR signal associated with europium is originated from Eu2+ ions in the 4f7 [7S7/2] ground state configuration.50
3.3. Optical properties
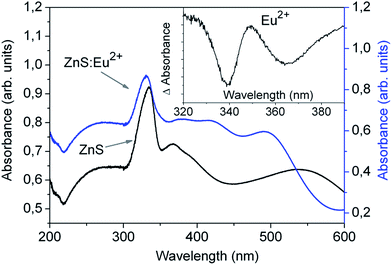
The PL spectra of both films, collected with an optical fiber under excitation with the 325 nm beam of the He-Cd laser are shown in Fig. 7. From this figure and the inset, it is clearly seen that the PL of the ZnS:Eu2+ film is much higher than that of the ZnS film, and the main peaks are located at 454 and 527 nm, respectively. The ratio of the intensity of the main PL peaks is around 185. The green emission band of the un-doped ZnS film, centered at 527 nm can be assigned to the radiative electron transfer from sulfur vacancies to interstitial sulfur states.8,56 The strong blue PL from the ZnS:Eu2+ films, consists of a broad emission centered at 454 nm, with a full-width at half-maximum intensity of (FWHM) of approximately 94 nm.
 | ||
| Fig. 7 Room temperature PL spectra the ZnS and ZnS:Eu2+ films excited at 325 nm with a He-Cd laser. The inset exhibits a zoom of the PL spectrum of the ZnS film. | ||
Fig. 8 shows the PL emission spectra obtained for different excitation wavelengths. As can be seen from this figure the PL intensity increases as the excitation wavelength increases from 250 to 332 nm, and the position of the PL peak is maintained around 454 nm (2.73 eV). The excitation spectrum of the main PL peak at 454 nm is also shown in the inset of Fig. 8. The excitation spectrum indicates that the maximum intensity of the 454 nm peak is obtained for the excitation wavelength of λexc = 331 nm. Since this excitation wavelength corresponds to photons with energy equal to 3.75 eV, and this energy correspond to the band gap of the ZnS:Eu2+ films we can assume that the PL is efficiently excited when the excitation energy is absorbed by the host lattice ZnS, and then it is transferred to the Eu2+ activator, as generally occurs in semiconductor phosphors with luminescent centers.58
According to the compilation made by P. Dorenbos on the emission spectra of Eu2+ in inorganic compounds, there are three types of Eu2+ related emissions, which are, (1) normal broad band dipole and spin allowed d–f emission, (2) ff narrow and (3) “anomalous” Eu2+ emission.12 The most common type of emission observed in the majority of the compounds is the normal broad d–f emission, in which the radiative transition starts from the 4f6 [7F0] 5d excited state and ends in the 4f7 [8S7/2] ground state of Eu2+.12 The peak wavelength (λem) and the full width at half maximum intensity (FWHM) at room temperature of this type of emission depend on the composition, crystalline structure and morphology of the host, because the shift and splitting of the outermost 5d levels of the Eu2+ ions are very sensitive to the local crystal field, and the phonon energies also depend on the crystal lattice.7,12,59 For example, for Eu2+ ions incorporated in microporous zeolite derivatives, the PL spectra have λem = 450 nm and FWHMs in the range from 85 nm to 150 nm, depending on the synthesis conditions.7 For 12CaO·7Al2O3 thin films doped with Eu2+ ions λem = 444 nm and FWHM ≈ 60 nm.9 For Eu2+ ions in BaAlBO3F2, λem ≈ 450 nm and the blue luminescence is very narrow FWHM ≈ 38 nm.4
Based on all these previous and related works, the strong and broad blue PL centered at 454 nm emitted from our ZnS:Eu2+ films, can be ascribed to intra-ion electronic transitions of Eu2+ from the 4f65d excited state to the 4f7 ground state.
Given the absorption and PL excitation and emission characteristics of our ZnS:Eu2+ films, we can propose the following excitation–emission mechanisms. (1) First the incoming radiation with energy equal or higher than 3.75 eV (λexc = 331 nm), excite electrons from the valence band to the conduction band of the ZnS host, and also from the 4f7 ground state of Eu2+ to the conduction band of ZnS.8 (2) Then the photogenerated electrons lose energy and decay to the 4f65d levels of the luminescent Eu2+ ions. (3) Finally the electrons suffer radiative transitions to the 4f7 Eu2+ levels. In order to have more evidence of the energy transfer from the ZnS host to the 4f65d levels of the Eu2+ ions, it is necessary to make further studies such as lifetime analysis of the PL.
4. Conclusions
ZnS:Eu2+ thin films that display intense blue PL at room temperature have been successfully synthesized by a simple and fast ultrasonic spray pyrolysis method. The as deposited ZnS:Eu2+ films are composed of hexagonal wurtzite nanocrystals with an average size of ∼25 nm, which agglomerate to form hexagonal facet nanobars. The incorporation of the Eu dopant atoms in the valence state Eu2+ was confirmed by ESR measurements. These measurements along with the absorption and PL characteristics of the films indicate that the efficient blue luminescent comes from intra-ion transitions of Eu2+ ions incorporated in the wurtzite ZnS matrix. The high intensity and the emitting color of these ZnS:Eu2+ films deposited by this relatively simple and cheap technique ensures their potential application as ideal candidates for modern flat panel electroluminescent displays.Acknowledgements
The authors want to acknowledge the technical assistance of M. Sc. A. Tejeda, D. Cabrero, C. Flores, Dr O. Novelo, M. Sc. J. Romero-Ibarra, J. M. García-León and Fernando Silvar of IIM-UNAM, M. Sc. V. Gómez-Vidales of I. Q.-UNAM, and M. Sc. Angelica Gutierrez Franco of MICRONA-UV. This research work was partially supported under project PAPIIT-UNAM number IG100614-2.References
- G. Li, Y. Tian, Y. Zhao and J. Lin, Chem. Soc. Rev., 2015, 44, 8688–8713 RSC.
- B. Park, S. J. Kim, J. Lim, S. Some, J. Park, S. Kim, C. Kim, T. J. Lee and S. C. Jun, J. Mater. Chem. C, 2015, 3, 4030–4038 RSC.
- Z.-C. Wu, H.-H. Fu, J. Liu, S.-P. Kuang, M.-M. Wu, J.-G. Xu and X.-J. Kuang, RSC Adv., 2015, 5, 42714–42720 RSC.
- Z. Tao, Y. Huang, P. Cai, S. Il Kim and H. J. Seo, Opt. Mater., 2014, 37, 287–292 CrossRef CAS.
- S. Gwak, P. Arunkumar and W. Im, J. Phys. Chem. C, 2014, 118, 2686–2692 CAS.
- X. Zhang, F. Meng, W. Li and H. J. Seo, Ceram. Int., 2013, 39, 8975–8978 CrossRef CAS.
- X. Yang, T. S. Tiam, X. Yu, H. V. Demir and X. W. Sun, ACS Appl. Mater. Interfaces, 2011, 3, 4431–4436 CAS.
- X. Zhang and L. Wang, Chalcogenide Lett., 2015, 12, 435–440 Search PubMed.
- H. Zhu, Y. Liu, D. Yan, H. Bian, S. Li, C. Liu, C. Xu and X. Wang, Opt. Mater., 2014, 36, 1771–1775 CrossRef CAS.
- K. Miura, Y. Arai and O. Hanaizumi, Mater. Sci. Appl., 2015, 6, 676–680 CAS.
- L. Stand, M. Zhuravleva, G. Camarda, A. Lindsey, J. Johnson, C. Hobbs and C. L. Melcher, J. Cryst. Growth, 2016, 439, 93–98 CrossRef CAS.
- P. Dorenbos, J. Lumin., 2003, 104, 239–260 CrossRef CAS.
- B. Bodo, D. Prakash and P. K. Kalita, Int. J. Appl. Phys. Math., 2012, 2, 181–183 CrossRef CAS.
- E. B. Ramírez, M. Bizarro and J. C. Alonso, Thin Solid Films, 2013, 548, 255–258 CrossRef.
- G. Boutaud, W. M. Cranton, D. C. Koutsogeorgis, R. M. Ranson, C. Tsakonas and C. B. Thomas, Mater. Sci. Eng., B, 2009, 165, 202–206 CrossRef CAS.
- P. Thiyagarajan, M. Kottaisamy, K. Sethupathi and M. S. R. Rao, Displays, 2009, 4, 1–5 Search PubMed.
- C. Falcony, M. Garcia, A. Ortiz and J. C. Alonso, J. Appl. Phys., 1992, 72, 1525–1528 CrossRef CAS.
- X. Lu, C. Chen, S. Husurianto and M. D. Koretsky, J. Appl. Phys., 1999, 85, 4154–4159 CrossRef CAS.
- M. Kuppayee, G. K. V. Nachiyar and V. Ramasamy, Mater. Sci. Semicond. Process., 2012, 15, 136–144 CrossRef CAS.
- R. Sarkar, C. S. Tiwary, P. Kumbhakar and A. K. Mitra, Phys. B, 2009, 404, 3855–3858 CrossRef CAS.
- H. Hu and W. Zhang, Opt. Mater., 2006, 28, 536–550 CrossRef CAS.
- W. Chen, J. Z. Zhang and A. G. Joly, J. Nanosci. Nanotechnol., 2004, 4, 919–947 CrossRef CAS PubMed.
- P. Yang, M. Lu, D. Xu, D. Yuan, C. Song, S. Liu and X. Cheng, Opt. Mater., 2003, 24, 497–502 CrossRef CAS.
- P. Yang, M. Lu, D. Xu, D. Yuan and G. Zhou, J. Lumin., 2001, 93, 101–105 CrossRef CAS.
- M. Godlewski and D. Hommel, Phys. Status Solidi, 1986, 95, 261–268 CrossRef CAS.
- K. Swiatek, M. Godlewski, D. Hommel and H. Hartmann, Phys. Status Solidi, 1989, 114, 127–133 CrossRef CAS.
- K. Swiatek, M. Godlewski and D. Hommel, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 42, 3628–3633 CrossRef CAS.
- S. J. Xu, S. J. Chua, B. Liu, L. M. Gan, C. H. Chew and G. Q. Xu, Appl. Phys. Lett., 1998, 73, 478–480 CrossRef CAS.
- S. Liu, H. Q. Guo, Z. H. Zhang, F. Q. Liu and Z. G. Wang, Chin. Phys. Lett., 2000, 17, 609–611 CrossRef CAS.
- W. Chen, J.-O. Malm, V. Zwiller, Y. Huang, S. Liu, R. Wallenberg, J.-O. Bovin and L. Samuelson, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 11021–11024 CrossRef CAS.
- W. Chen, J.-O. Malm, V. Zwiller, R. Wallenberg and J.-O. Bovin, J. Appl. Phys., 2001, 89, 2671 CrossRef CAS.
- G. Sharma, S. Do Han, S. P. Khatkar, V. B. Taxak and Y. W. Rhee, ECS Trans., 2006, 1, 7–12 CAS.
- S. Y. Lee, Y. H. Shin, Y. Kim, S. Kim and S. Ju, J. Lumin., 2011, 131, 1336–1339 CrossRef CAS.
- K. Ashwini, C. Pandurangappa and B. M. Nagabhushana, Phys. Scr., 2012, 85, 65706 CrossRef.
- K. Ashwini, Yashaswini and C. Pandurangappa, Opt. Mater., 2014, 37, 537–542 CrossRef CAS.
- L. Ma, K. Jiang, X. T. Liu and W. Chen, J. Appl. Phys., 2014, 115, 103104 CrossRef.
- B. Cheng and Z. Wang, Adv. Funct. Mater., 2005, 15, 1883–1890 CrossRef CAS.
- M. K. Jayaraj and C. P. G. Vallabhan, J. Electrochem. Soc., 1991, 138, 1512–1516 CrossRef CAS.
- M. Aozasa, H. Chen and A. Keiichi, Thin Solid Films, 1991, 199, 129–138 CrossRef CAS.
- L. Abate, A. Chisari, R. Maggiore and G. Siracusa, Thermochim. Acta, 1988, 136, 153–161 CrossRef CAS.
- J. E. Huheey, E. A. Keiter and R. L. Keiter, Inorganic Chemistry: Principles of Structure and Reactivity, Pearson, 1997 Search PubMed.
- T. Arii and A. Kishi, Thermochim. Acta, 2003, 400, 175–185 CrossRef CAS.
- P. A. Lawson and N. A. Stump, Spectrosc. Lett., 2011, 44, 412–417 CrossRef CAS.
- S. J. Lyle and W. A. Westall, Thermochim. Acta, 1983, 68, 51–58 CrossRef CAS.
- T. A. Antonenko, L. Y. Alikberova and D. V. Albov, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2012, 68, m110 CAS.
- R. D. Archer and W. N. Mitchel, in Inorganic Syntheses, ed. E. I. Muetterties, McGraw-Hill International, 1967, pp. 77–79 Search PubMed.
- M. Lv, G. Lu and C. Cai, Asian J. Org. Chem., 2015, 4, 141–144 CrossRef CAS.
- Y. Q. Jia, J. Solid State Chem., 1991, 187, 184–187 CrossRef.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- M. P. Thi, N. Ruelle, E. Tronc, D. Simons and D. Vivien, Jpn. J. Appl. Phys., 1994, 33, 1876–1884 CrossRef.
- H. C. Ong and R. P. H. Chang, Appl. Phys. Lett., 2001, 79, 3612–3614 CrossRef CAS.
- T. Yamamoto, S. Kishimoto and S. Iida, Phys. B, 2001, 308–310, 916–919 CrossRef CAS.
- G. S. Harish and P. Sreedhara Reddy, Phys. B, 2015, 473, 48–53 CrossRef CAS.
- B. Bodo, D. Prakash, P. K. Kalita and A. X. R. D. Study, Int. J. Appl. Phys. Math., 2012, 2, 181–183 CrossRef CAS.
- D. Denzler, M. Olschewski and K. Sattler, J. Appl. Phys., 1998, 84, 2841 CrossRef CAS.
- X. Wang, J. Shi, Z. Feng, M. Li and C. Li, Phys. Chem. Chem. Phys., 2011, 13, 4715–4723 RSC.
- P. Dorenbos, J. Lumin., 2008, 128, 578–582 CrossRef CAS.
- G. Blasse, J. Alloys Compd., 1995, 225, 529–533 CrossRef CAS.
- X. Piao, K. I. Machida, T. Horikawa and B. Yun, J. Lumin., 2010, 130, 8–12 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra24300b |
| This journal is © The Royal Society of Chemistry 2016 |