Electrochemical supercapacitor based on double perovskite Y2NiMnO6 nanowires†
Mahebub Alam*,
Keshab Karmakar,
Monalisa Pal and
Kalyan Mandal
S. N. Bose National Centre for Basic Sciences, JD Block, Sector-III, Salt Lake, Kolkata-700106, India. E-mail: alammahebub@gmail.com; mahebubalam@bose.res.in
First published on 30th November 2016
Abstract
The present work unveils the electrochemical properties of a newly emerging multiferroic material, double perovskite Y2NiMnO6, as an active material for the positive electrode of electrochemical supercapacitors. We have designed a facile, low temperature hydrothermal route for the fabrication of Y2NiMnO6 nanowires, to achieve the beneficial effects of a large active surface area at the nanoscale on the electrochemical properties of the material. A comparative study reveals that the Y2NiMnO6 nanowire-based electrode is superior than its bulk counterpart, exhibiting higher specific capacitance (77.76 F g−1 at 30 mA g−1), energy density (0.89 W h kg−1 at 30 mA g−1), power density (19.27 W kg−1 at 150 mA g−1) and cyclability (>1800 cycles), in addition to a good retention of 70.17%.
Introduction
Energy storage and conversion have attracted the immense attention of researchers who hope to solve society's major problems, such as the energy crisis and environmental pollution, caused by the use of fossil fuels. In the modern world, the ever-increasing use of portable electronic devices, such as smart mobile phones, notebooks, computers, laptops and hybrid electric vehicles, demands energy storage systems with simultaneous high energy density and power density. Batteries and fuel cells have high energy density but low power density, whereas capacitors have high power density but low energy density. The supercapacitor (SC), which can bridge the gap between capacitors and batteries, is a promising candidate for the next generation energy storage device, because of its simultaneous high energy and power density, quick charge/discharge process, and outstanding cycling stability.1–6 Nanomaterials of various shapes, sizes and morphologies, such as hollow nano-architectures and one dimensional (1D) nanostructures, can greatly optimize electrode properties due to increased effective surface area and short ion transfer pathways.7–12In general, redox active transition-metal oxides (TMOs) such as RuO2, Fe2O3, ZnO, TiO2, NiO, MnO2, SnO2, CuO, Co3O4, WO4 and V2O5 etc.,13–25 demonstrate high specific capacitance, and fast and excellent reversible faradic reactions. Multiferroic oxide materials play a crucial role in digital electronic materials, such as memory devices, actuators, transducers, spintronic devices, magnetic data storage media, sensors and logic devices etc.26–29 The remarkable supercapacitance properties of Mn, Ni and Y based oxides have driven our interest to study their complex oxide, double perovskite Y2NiMnO6, a newly emerging multiferroic material.29 In the present era of multifunctional material development, we are inspired by the recent findings about multiferroics, such as BiFeO3 thin films, nanorods and nanoflakes, and BiMn2O5 nanoparticles as potential candidates for SC electrodes.30–34 The specific capacitance of BiFeO3 nanoflakes is 72.2 F g−1 at a current density of 1 A g−1 and this value sharply falls to 36.3 F g−1 at a current density of 5 A g−1. This indicates that only 50% of the capacitance is retained as the current density increases from 1 A g−1 to 5 A g−1.31 An electrodeposited BiFeO3 thin film showed a comparable specific capacitance of 81 F g−1.30 However, until now there has been no report in the literature on the electrochemical properties of Y2NiMnO6.
Here, we report the electrochemical properties of the bulk structure of double perovskite Y2NiMnO6, which exhibits a specific capacitance of 17.4 F g−1, energy density of 0.197 W h kg−1, power density of 7.81 W kg−1 and 85% retention efficiency after 1800 cycles. Moreover, to improve the supercapacitance properties by enhancing the surface area and improving charge transportation at the nanoscale, we demonstrate a facile, low temperature hydrothermal synthesis route for the fabrication of Y2NiMnO6 nanowires (NWs). Detailed characterization through XRD, HRTEM, EFTEM, EDAX and FESEM reveals the successful formation of Y2NiMnO6 NWs. As anticipated, the NWs show superior electrochemical properties, having a specific capacitance of 77.76 F g−1, energy density of 0.98 W h kg−1, power density of 19.27 W kg−1 and retention of 70.17% efficiency after 1800 cycles. We hope that our newly developed synthesis strategy and the supercapacitance of the Y2NiMnO6 NWs will open up the applicability of this material in the area of energy storage devices.
Experimental
Electrode preparation procedure
Polycrystalline perovskite Y2NiMnO6 NWs were fabricated via a hydrothermal method.29 Y(NO3)3·6H2O (Sigma), Ni(NO3)3·6H2O (Loba Chemie) and C4H6MnO4·4H2O (Loba Chemie) were dissolved into 40 ml of double distilled water in the appropriate stoichiometric ratio. A 5 M 10 ml solution of NaOH was added drop by drop to the above mixture to adjust the pH of the solution to 12. Due to the addition of NaOH, Y(OH)3, Ni(OH)2 and Mn(OH)2 were precipitated. The mixture was stirred for 30 minutes and then transferred into a Teflon-lined autoclave. The autoclave was tightly sealed and then heated at 200 °C for 24 h. It was then cooled naturally to room temperature. The product thus obtained was washed successively in double distilled water, acetone and ethanol (EtOH) and dried at 70 °C for 24 h. Finally, the Y2NiMnO6 nanowires (NWs) were obtained by heating the as-prepared samples at 1000 °C for 1 h in air. The bulk polycrystalline Y2NiMnO6 powder was prepared via the sol–gel method.35 For electrochemical measurements, the working electrodes were constructed by mixing the active material, acetylene black (AB) and polyvinylidene fluoride (PVDF) binder in a weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which were mixed in N-methyl-2-pyrrolidinone (NMP) solvent and dropped uniformly into Ni foam. Then this was dried under an IR lamp for 10 minutes and immersed in a vacuum. The mass of the deposited product was calculated by carefully weighing the piece of Ni foam before and after the growth of the Y2NiMnO6 bulk and nanowires.
1, which were mixed in N-methyl-2-pyrrolidinone (NMP) solvent and dropped uniformly into Ni foam. Then this was dried under an IR lamp for 10 minutes and immersed in a vacuum. The mass of the deposited product was calculated by carefully weighing the piece of Ni foam before and after the growth of the Y2NiMnO6 bulk and nanowires.
Characterization techniques
The phase and structure of the samples were determined by X-ray diffraction (XRD) using CuKα radiation (Panalytical, λ = 1.5418 Å). The morphologies of the Y2NiMnO6 NWs and fresh Ni foam were investigated on a field emission scanning electron microscope (FESEM, FEIQUANTA FEG-250) operating at 5–10 kV. For transmission electron microscopy (TEM) studies, samples were prepared by drop casting the prepared Y2NiMnO6 NWs dispersed in EtOH on a 300 mesh carbon coated copper grid and drying overnight under vacuum. Wire size was determined from the TEM micrographs and elemental analysis was performed from the energy dispersive X-ray (EDAX) spectra and the energy filtered TEM (EFTEM) operating at 200 kV.Electrochemical measurements
The electrochemical properties of the Y2NiMnO6 NWs and its bulk counterpart were investigated using cyclic voltammetry (CV) tests by employing a software controlled conventional three-electrode electrochemical cell (potentiostat Autolab-30) consisting of Y2NiMnO6 NWs over Ni foam as the working electrode, saturated Ag/AgCl as the reference electrode and a highly pure Pt wire as the counter electrode in a 0.5 M KOH electrolyte at room temperature. The CV measurements were obtained at different scan rates of 2, 5, 10, 25, 50 and 100 mV s−1, within a potential range of 0.2 to 0.5 V at room temperature. The specific capacitance (Csp), energy density (E) and power density (P) were measured using galvanostatic charging/discharging (GCD) experiments at different current densities, such as 30, 45, 60, 90, 120 and 150 mA g−1. The capacitances were determined from the charging/discharging curves by using the following formula:where I is the discharge current, Δtd is the discharging time, ΔV is the potential window excluding the IR drop region, m is the active mass of the material, i.e. the mass of the NWs and powder (for bulk) over the Ni foam substrate.
Furthermore, the energy and power densities of the electrodes were determined by using the following formulae, respectively:
where E (W h kg−1), P (kW kg−1), Csp (F g−1), ΔV (V), and Δtd (s) are the energy density, power density, specific capacitance, potential window of discharge, and discharging time, respectively. The alternating current (AC) complex impedance was measured in the frequency range of 10 mHz to 10 kHz at an amplitude of the AC signal of 5 mV. The average mass loading densities of Y2NiMnO6 bulk and NWs on the Ni foam were 3 and 2.5 mg cm−2, respectively.
Results and discussion
Fig. 1(a) and (b) respectively show the XRD patterns of the Y2NiMnO6 NWs and its bulk counterpart at room temperature. The XRD patterns for both of the samples are consistent with the literature, indicating the formation of Y2NiMnO6.35 An FESEM image of the Y2NiMnO6 NWs with uniform diameter (200 nm) is shown in Fig. 1(c) and that of its bulk counterpart is shown in the ESI (Fig. S1†). The EDAX spectra of the Y2NiMnO6 NWs (Fig. 1(d)) and bulk (Fig. 1(e)), analysed to study their chemical compositions, clearly confirm the presence of only Y, Ni, Mn, and O in the NWs, where the atomic ratio of Y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni
Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn
Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O is close to 2
O is close to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6. The TEM and energy filtered TEM (EFTEM) micrographs also clearly show the formation of Y2NiMnO6 NWs with uniform diameter and chemical composition, as shown in Fig. 2(a). The HRTEM image (Fig. 2(b)) and selected area electron diffraction pattern (inset of Fig. 2(b)) also confirm the high crystallinity of the NWs. The calculated interplanar distance (Fig. 2(b)) between the lattice fringes are about 0.26, 0.27 and 0.33 nm, corresponding to the distance between the (112), (020) and (111) planes of the Y2NiMnO6 NWs, respectively.
6. The TEM and energy filtered TEM (EFTEM) micrographs also clearly show the formation of Y2NiMnO6 NWs with uniform diameter and chemical composition, as shown in Fig. 2(a). The HRTEM image (Fig. 2(b)) and selected area electron diffraction pattern (inset of Fig. 2(b)) also confirm the high crystallinity of the NWs. The calculated interplanar distance (Fig. 2(b)) between the lattice fringes are about 0.26, 0.27 and 0.33 nm, corresponding to the distance between the (112), (020) and (111) planes of the Y2NiMnO6 NWs, respectively.
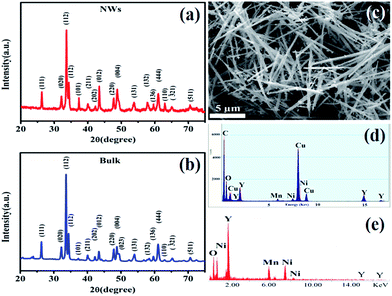 | ||
| Fig. 1 XRD pattern of Y2NiMnO6 NWs (a) and Y2NiMnO6 bulk (b); FESEM micrograph of Y2NiMnO6 NWs (c); EDX spectrum of the Y2NiMnO6 NWs (d) and bulk (e), indicating the presence of only Y, Ni, Mn and O. | ||
To investigate the oxidation states of the different elements in the Y2NiMnO6 NWs, we performed an XPS study. Fig. 2(c)–(f) show the XPS spectra of Y, Ni, Mn and O ions for investigating their oxidation states in the Y2NiMnO6 NWs. Fig. 2(c) shows the XPS spectrum for Y, where the peaks of Y 3d5/2 and Y 3d3/2 are observed at 156.3 and 158.3 eV, respectively, indicating the +3 oxidation state of Y in the Y2NiMnO6 NWs.36 The characteristic peaks of Ni 2p1/2 and Ni 2p3/2 appear at 855.35 and 873 eV (Fig. 2(d)), respectively, representing the divalent (+2) state for Ni in the Y2NiMnO6 NWs.37 The peaks at around 861.5 and 878.9 eV are the satellite peaks related to Ni 2p3/2 and Ni 2p1/2, confirming the +2 oxidation state of Ni.35 Fig. 2(e) shows the XPS spectrum for Mn, where the peaks of Mn 2p3/2 and Mn 2p1/2 are found at 642.1 and 653.5 eV, respectively, corresponding to the +4 oxidation state of Mn in the Y2NiMnO6 NWs. Fig. 2(f) shows the XPS spectrum of the O 1s core level, which appears at 529.7 eV and can be ascribed to the −2 oxidation state of oxygen in the Y2NiMnO6 NWs.37
Fig. 3(a) and (b) respectively show the cyclic voltammetry (CV) curves of the Y2NiMnO6 bulk/Ni foam and Y2NiMnO6 NWs/Ni foam electrodes measured at different scan rates of 2, 5, 10, 25, 50 and 100 mV s−1, within the same voltage window of 0.2–0.5 V in 0.5 M aqueous KOH electrolyte. Distinct oxidation and reduction peaks in each of the curves demonstrate the pseudocapacitive behavior of the Y2NiMnO6 bulk as well as the Y2NiMnO6 NWs, which is quite different from the nearly rectangular CV loops for conventional electric double layer capacitors (EDLC). It is evident that with increasing scan rates, the current response of the electrode increases and the shape of the CV curves remains the same throughout the whole range of scan rates (2 to 100 mV s−1). At low scan rates a thick diffusion layer grows over the electrode, which limits electrolyte flux towards the electrode, resulting in lower current. However, at higher scan rates, the diffusion layer cannot grow wide over the electrolyte surface, which enhances electrolyte flux towards the electrode, leading to the increment of current. Again, from the CV curves of both the bulk and NWs, it is found that with increasing scan rates, the upper and lower redox peaks shift towards the positive and negative side respectively, due the development of overpotential, which limits the faradic reactions. Our comparative investigations of bare Ni foam and Y2NiMnO6 NWs grown over Ni foam at a scan rate of 100 mV s−1 reveal that Ni foam alone has a negligible contribution to the total capacitance, as demonstrated in Fig. S2.†
To further investigate the electrochemical performances of the Y2NiMnO6 bulk and NWs grown over Ni foam as the electrodes for supercapacitors, we performed galvanostatic charging/discharging (GCD) experiments at various current densities in the potential window of 0.2–0.5 V, as shown in Fig. 3(c) and (d). The specific capacitance values of the two electrodes at various current densities were determined based on their corresponding GCD curves (see Fig. 3(e) and (f)). Table 1 shows a basic comparison of the specific capacitance (Csp) for the two electrodes (Y2NiMnO6 bulk/Ni and NWs/Ni foam). This clearly shows that the specific capacitance significantly increases in the case of Y2NiMnO6 NWs/Ni foam, due to the increase in active surface area. Moreover, the Csp of the NWs remains at 63.6 F g−1 even when the current density is increased to be as high as 150 mA g−1, implying 81.8% retention of its initial value when the current density is increased 5-fold. However, in the case of the bulk, only 56.33% capacitance is retained upon a 7.27-fold increase in the current density. This high specific capacitance with impressive rate capability of the NWs can be attributed to the higher surface area of the electrode due to its nano-architectural design.
| Y2NiMnO6 bulk/Ni foam | Y2NiMnO6 NWs/Ni foam | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Current density (mA g−1) | 10 | 29 | 43.6 | 58 | 72.7 | 30 | 60 | 90 | 120 | 150 |
| Specific capacitance (F g−1) | 17.4 | 14.5 | 12.3 | 10.6 | 9.8 | 77.7 | 70.8 | 69.6 | 66.3 | 63.6 |
Fig. 4(a) shows that the energy density of the Y2NiMnO6 bulk electrode decreases from 0.197 W h kg−1 to 0.0629 W h kg−1 and the power density increases from 1.43 to 7.81 W kg−1 with the increment of current density from 10 mA g−1 to 72.7 mA g−1. For the NW electrode, shown in Fig. 4(b), the energy density decreases from 0.98 to 0.58 W h kg−1 and the power density increases from 4.32 to 19.27 W kg−1, as the current density increases from 30 mA g−1 to 150 mA g−1. The Y2NiMnO6 NW electrode has superior supercapacitance compared to its bulk counterpart, exhibiting higher power density as well as energy density. Fig. 4(c) and (d) show the cycling stability of the Y2NiMnO6 bulk and NW electrodes, where charge/discharge tests have been conducted at a current density of 43.6 mA g−1 and 30 mA g−1, respectively, for 1800 cycles. The specific capacitance of the Y2NiMnO6 bulk and NW electrodes reduces to 85% and 70.17%, respectively, after 1800 cycles, confirming the good stability of the electrodes. The insets of Fig. 4(c) and (d) show the last 10 charge/discharge cycles of the bulk and NW electrodes, respectively. Here, it can be seen that the symmetric triangular shape of the charging/discharging profile remains the same during the long cycling test, implying that the electrodes exhibit stable electrochemical performance and facile charge transfer during the reaction without any significant structural change.
Electrochemical impedance spectroscopy (EIS) measurements of the Y2NiMnO6 bulk and NWs were carried out at an open circuit potential within a frequency range of 10 mHz to 104 Hz with a voltage amplitude of 5 mV, as shown in Fig. 4(e) and (f). The frequency response of the capacitance reflects the amount of the surface area accessible to the electrolyte. Capacitance at the high-frequency region shows the outer surface, which may depend on grain boundaries and other inter-particle phenomena. The plot clearly shows that at the high frequency region, the imaginary part of the impedance is parallel to the x-axis, whereas at the low frequency range, it is inclined to the x-axis. This suggests that the charge transfer resistance rates at the high frequency side are faster than those at the low frequency region.31 The intercept of the curve in the high frequency region on the x-axis represents the equivalent series resistance (Rs), whereas the semicircle at the high frequency region signifies the charge transfer resistance (Rct) of the electrode. Using equivalent circuits38,39 (inset of Fig. 4(e) and (f)) the calculated values of Rs (2.44 Ω) and Rct (56.65 Ω) are found to be low in the case of the Y2NiMnO6 NW electrode compared to the Y2NiMnO6 bulk electrode (Rs = 3.2 Ω, Rct = 210.86 Ω), indicating the higher electrical conductivity of the Y2NiMnO6 NW electrode and the quick ion-charge transfer at the electrode–electrolyte interface during the redox reaction. The straight line in the low frequency range represents the Warburg resistance (RW), which exhibits a steep slope due to the high diffusion rate of ions during the redox reactions.21,40
Conclusions
In summary, we have successfully synthesized double perovskite multiferroic Y2NiMnO6 NWs through an easy hydrothermal route. The comparative study demonstrates that as a new pseudocapacitor material, Y2NiMnO6 NWs exhibit higher specific capacitance, power density and energy density compared with their bulk counterpart. The remarkable electrochemical performance arises due to the large surface area of the Y2NiMnO6 NWs, which leads to a better interaction of the electrolyte with the nanostructure, resulting in shorter ion transfer pathways. It is evident that along with multiferroic behavior, Y2NiMnO6 NWs will be a promising candidate in the field of electrochemical supercapacitors.Acknowledgements
Author Mahebub Alam would like to thank the department of Science and Technology, Govt. of India for providing research support through the ‘INSPIRE fellow Award’ [IF150219].References
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845 CrossRef CAS PubMed.
- W. Wei, X. Cui, W. Chen and D. G. Ivey, Chem. Soc. Rev., 2011, 40, 1697 RSC.
- J. R. Miller and P. Simon, Mater. Sci., 2008, 321, 651 CAS.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797 RSC.
- G. Zhang and X. W. Lou, Sci. Rep., 2013, 3, 1470 Search PubMed.
- G. Guo, L. Huang, Q. Chang, L. Ji, Y. Liu, Y. Xie, W. Shi and N. Jia, Appl. Phys. Lett., 2011, 99, 083111 CrossRef.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 2000, 407, 496 CrossRef CAS PubMed.
- X. W. Lou, L. A. Archer and Z. C. Yang, Adv. Mater., 2008, 20, 3987 CrossRef CAS.
- X. Y. Lai, J. E. Halpert and D. Wang, Energy Environ. Sci., 2012, 5, 5604 CAS.
- H. –W. Lee, P. Muralidharan, R. Ruffo, C. M. Mari, Y. Cui and D. k. Kim, Nano Lett., 2010, 10, 3852 CrossRef CAS PubMed.
- K. T. Lee and J. Cho, Nano Today, 2011, 6, 28 CrossRef CAS.
- A. S. Arico, P. Bruce, B. Scrosati, J. M. Tarascon and W. Van Schalkwijk, Nat. Mater., 2005, 4, 366 CrossRef CAS PubMed.
- X. Lang, A. Hirata, T. Fujita and M. W. Chen, Nat. Nanotechnol., 2011, 6, 232 CrossRef CAS PubMed.
- J. Kang, A. Hirata, L. Kang, X. Zhang, Y. Hou, L. Chen, C. Li, T. Fujita, K. Akagi and M. Chen, Adv. Mater., 2013, 52, 1664 CAS.
- L. Wang, H. Ji, S. Wang, L. Kong, X. Jiang and G. Yang, Nanoscale, 2013, 5, 3793 RSC.
- T. Brezesinski, J. Wang, J. Polleux, B. Dunn and S. H. Tolbert, J. Am. Chem. Soc., 2009, 131, 1802 CrossRef CAS PubMed.
- A. David, C. Tompsett, S. C. Parker and M. S. Islam, J. Am. Chem. Soc., 2014, 136, 1418 CrossRef PubMed.
- M. Liu, L. Gan, W. Xiong, Z. Xu, D. Zhu and L. Chen, J. Mater. Chem. A, 2014, 2, 2555 CAS.
- J. Yan, E. Khoo, A. Sumboja and R. S. Lee, ACS Nano, 2010, 4, 4247 CrossRef CAS PubMed.
- L. Athoue, F. Moser, R. Dugas, O. Crosnier, D. Belanger and T. Brousse, J. Phys. Chem. C, 2008, 112, 7270 Search PubMed.
- A. K. Singh, D. Sarkar, G. G. Khan and K. Mandal, ACS Appl. Mater. Interfaces, 2014, 6, 4684 CAS.
- Y. Luo, D. Kong, J. Luo, Y. Wang, D. Zhang, K. Qiu, C. Cheng, C. M. Li and T. Yu, RSC Adv., 2014, 4, 13241 RSC.
- Z. Chen, V. Augustyn, X. Jia, Q. Xiao, B. Dunn and Y. Lu, ACS Nano, 2012, 6, 4319 CrossRef CAS PubMed.
- M. Sathiya, A. S. Prakash, K. Ramesha, J. M. Tarascon and A. K. Shukla, J. Am. Chem. Soc., 2011, 133, 16291 CrossRef CAS PubMed.
- M. Pal, R. Rakshit, A. K. Singh and K. Mandal, Energy, 2016, 103, 481 CrossRef CAS.
- J. Wang, J. B. Neaton, H. Zheng, V. Nagarajan, S. B. Ogale, B. Liu, D. Viehland, V. Vaithyanathan, D. G. Schlom, U. V. Waghmare, N. A. Spaldin, K. M. Rabe, M. Wuttig and R. Ramesh, Science, 2003, 299, 1719 CrossRef CAS PubMed.
- F. D. Martini, V. Buzek, F. Sciarrino and C. Sias, Nature, 2002, 419, 815 CrossRef PubMed.
- W. Eerenstein, N. D. Mathur and J. F. Scott, Nature, 2006, 442, 759 CrossRef CAS PubMed.
- M. Alam, K. Mandal and G. G. Khan, RSC Adv., 2016, 6, 62545 RSC.
- C. D. Lokhande, T. P. Gujar, V. R. Shinde, R. S. Mane and S. H. Han, Electrochem. Commun., 2007, 9, 1805 CrossRef CAS.
- V. V. Jadhav, M. K. Zate, S. Liu, Mu. Naushad, R. S. Mane, K. N. Hui and S.-H. Han, Appl. Nanosci., 2015, 6, 511 CrossRef.
- N. Dutta, S. K. Bandyopadhyay, S. Rana, P. Sen and A. K. Himanshu, Remarkably high value of capacitance in BiFeO3 Nanorod, Cornell University Library, 2013, vol. 1309, p. 5690 Search PubMed.
- Y. Liu and I. Zhitomirsky, J. Power Sources, 2015, 284, 377 CrossRef CAS.
- A. Sarkar, A. K. Singh, D. Sarkar, G. G. Khan and K. Mandal, ACS Sustainable Chem. Eng., 2015, 3, 2254 CrossRef CAS.
- J. Su, Z. Z. Yang, X. M. Lu, J. T. Zhang, L. Gu, C. J. Lu, Q. C. Li, J. M. Liu and J. S. Zhu, ACS Appl. Mater. Interfaces, 2015, 7, 13260 CAS.
- J. R. Manders, S. W. Tsang, M. J. Hartel, T. H. Lai, S. Chen, C. M. Amb and J. R. Reynolds, Adv. Funct. Mater., 2013, 23, 2993 CrossRef CAS.
- P. D. Rouffignac, J.-S. Park and R. G. Gordon, Chem. Mater., 2005, 17, 4808 CrossRef.
- X. L. Guo, X. Y. Liu, X. D. Hao, S. J. Zhu, F. Dong, Z. Q. Wen and Y. X. Zhang, Electrochim. Acta, 2016, 194, 179 CrossRef CAS.
- X. Li, J. Rong and B. Wei, ACS Nano, 2010, 4, 6039 CrossRef CAS PubMed.
- J. Liu, J. Jiang, C. Cheng, H. Li, J. Zhang, H. Gong and H. J. Fan, Adv. Mater., 2011, 23, 2076 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra23318j |
| This journal is © The Royal Society of Chemistry 2016 |






