Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Multicomponent synthesis of hydrazino depsipeptides†
Josipa
Suć
a,
Danijela
Barić
b and
Ivanka
Jerić
*a
aDivision of Organic Chemistry and Biochemistry, Ruđer Bošković Institute, Bijenička cesta 54, 10000 Zagreb, Croatia. E-mail: ijeric@irb.hr
bDivision of Physical Chemistry, Ruđer Bošković Institute, Bijenička cesta 54, 10 000 Zagreb, Croatia
First published on 12th October 2016
Abstract
The Passerini reaction of α-hydrazino acids, carbonyl compounds and isocyanides yielded hydrazino depsipeptides, a new class of backbone extended peptidomimetics comprising amide bond isostere. A wide range of carbonyl and isocyano components were used along the α-hydrazino acids carrying three different Nα protecting groups. The kinetics and thermodynamic equilibrium of the rate-determining step of the reaction with different α-hydrazino acids were studied by DFT approach.
Introduction
Screening of small-molecule libraries has been the most commonly used tool in drug discovery over the past two decades.1 However, most libraries lack diversity in terms of biological and chemical properties, i.e. “chemical space” covered is infinitely small. Contrary to that, natural products with complex molecular architectures and numerous stereogenic centers represent valuable pool of drug candidates.2 Therefore, access to libraries of natural product-like compounds with high degree of structural diversity is important for development of novel leads needed to tackle new generations of biological targets.3Natural products that contain an N–N bond compose of a fascinating group of compounds with a tremendous variety of structures and biological activities.4 Among them are compounds that contain α-hydrazino acid motif, like antibiotic negamycine,5 the B6 vitamin antagonist linatine6 and numerous peptides with embedded cyclic hydrazine skeleton – piperazic acid.7 Synthetic hydrazino peptidomimetics show enhanced stability toward proteolytic enzymes and extended pharmacokinetic properties,8 exhibit antimicrobial9 and proteasome inhibition activity.10 Also, hydrazino peptidomimetics show high propensity toward specific secondary structure known as hydrazino-turn, an eight-membered hydrogen-bonded pseudo-cycles.8 Ability to adopt well-defined folded structures is an important feature for various biomedical applications, but also construction of functional materials. Thus, incorporation of only one hydrazino-derivative of trans-2-aminocyclobutanecarboxylic acid caused stabilization of 8-helix over 12-helix conformation for an oligomer length up to 6 residues.11 Also, heterochiral cyclic oligomers composed of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of α-amino acids and α-hydrazino acids self-assemble into nanotubular structures in solution and solid phase.12 We have recently designed a small series of hydrazino peptidomimetics and showed that their interaction with DNA and RNA can be finely modulated with the number and relative position of α-hydrazino acid(s) within the peptide chain.13
1 mixture of α-amino acids and α-hydrazino acids self-assemble into nanotubular structures in solution and solid phase.12 We have recently designed a small series of hydrazino peptidomimetics and showed that their interaction with DNA and RNA can be finely modulated with the number and relative position of α-hydrazino acid(s) within the peptide chain.13
Since wider utilization of hydrazino peptidomimetics depends on easy access to the libraries of compounds, we are interested in developing reliable strategies for fast construction of structurally distinct compounds based on α-hydrazino acid motif. Multicomponent reactions (MCRs) offer numerous advantages over traditional sequential reactions for the synthesis of diverse structurally demanding compounds.14 MCRs were applied in the synthesis of small drug-like molecule libraries,15 but also large and more complex molecules.16 Isocyanide-based MCRs, particularly Passerini and Ugi reactions, are of special importance, because they provide peptide-like compounds.14b,17 The utility of the Passerini reaction relays on the mild reaction conditions required for the condensation of a carbonyl compound, a carboxylic acid and an isocyanide to afford α-acyloxycarboxamides – depsipeptides.14b Many naturally occurring depsipeptides have a wide range of biological activity,18 therefore, depsipetides are considered a lead compounds in the drug development process,19 but also promising polymers for biomedical applications.20 Since backbone structure of the Passerini product is influenced mainly by the carboxylic component,21 introduction of α-hydrazino acid as a carboxylic component could yield a novel class of peptidomimetics – hydrazino depsipeptides. These peptidomimetics bear two distinct structural features: backbone extension steaming from the hydrazino acid component and amide bond isostere as a result of the Passerini reaction (Scheme 1).
Results and discussion
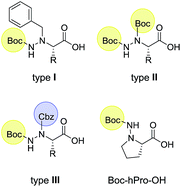
N-Terminal position of α-hydrazino acid-derived part in the Passerini product (Scheme 1) allows further modification through some other MCR or a standard peptide coupling procedure, therefore three types of α-hydrazino acids were used in this study. Nα-Benzyl, Nβ-Boc protected hydrazino acids (type I, Scheme 2) were obtained by electrophilic amination of the corresponding N-benzyl-L-amino acid with N-Boc oxaziridine.22 Nucleophilic substitution of D-amino acid-derived α-bromo acid with Boc-hydrazine yielded Nβ-Boc protected α-hydrazino acids,23 where Nα position was further protected with Boc (type II) or Cbz group (type III, Scheme 2).24 Also, hydrazino proline derivative, Boc-hPro-OH (Scheme 2) was used to explore the utility of cyclic acid components in the Passerini reaction.In order to introduce α-hydrazino acid in the Passerini reaction, we performed a model reaction with Nα-benzyl, Nβ-Boc protected hydrazino-L-leucine, p-nitro benzaldehyde and methyl 2-isocyano-3-phenylpropanoate in order to optimize reaction conditions. First reaction performed in dry dichloromethane under argon for 24 h gave the corresponding Passerini product 1 in 40% yield (Table 1, Entry 1). Encouraged by this result, we optimized reaction conditions by testing different solvents and found tetrahydrofurane as the most appropriate solvent for the Passerini reaction (54%, Entry 3). Performing reaction at 50 °C in THF failed to improve the yield of 1 (Entry 5). Bousquet et al. described a convenient and efficient Passerini multicomponent reaction under solvent-free conditions at high temperature,25 therefore, we examined the effect of solvents on the course of the reaction. The solvent-free reaction carried out at 80 °C gave Passerini product 1 in 31% yield (Entry 6). Therefore, optimal conditions for the Passerini reaction with α-hydrazino acids were THF as a solvent at room temperature for 24 h. With optimized conditions in hand, we set out to test the scope of the protocol with different oxo-compounds, isocyanides and α-hydrazino acids.
The Passerini reactions performed with Nα-benzyl, Nβ-Boc protected hydrazino acids (I) furnished corresponding Passerini products in 22–74% yields (Table 2, products 1–12). It is known that the nature of an oxo-component (aldehyde/ketone) have significant impact on the outcome of the Passerini reactions. Comparison of the Passerini reactions performed with p-NO2 benzaldehyde (products 1, 2, 5 and 9), benzaldehyde (product 6) and p-Cl benzaldehyde (10) revealed that aromatic aldehydes bearing electron withdrawing substituent furnished corresponding products in moderate to very good yields (36–63%), compared to unsubstituted aldehyde 6 (22%), while no product was obtained with aldehyde bearing electron donating substituents. Reactions performed with aliphatic aldehyde 2-methylpentanal (compounds 3, 4, 11 and 12) gave Passerini products in 21–74% yields, indicating that reaction is also strongly influenced by the nature of acid and isocyano-component. Finally, two ketones were used in the Passerini reaction; product 7 was obtained with acetone in fair yield (42%), while acetophenone failed to give the expected product 8. Generally, lower yields were obtained with commercially available isocyanides (compounds 4, 9, 12) than with amino acid-derived ones. Also, amino acid side-chain group seems to have low impact on the reactivity in the Passerini reaction.
| a Isolated yields are given in parentheses. Compounds were isolated as mixture of diastereoisomers. n.o. – not obtained. |
|---|

|
Next, we tested reactions with type II α-hydrazino acids, namely leucine- and phenylalanine-related Nα-Boc, Nβ-Boc protected derivatives. Reactions were performed with p-NO2 benzaldehyde, and 2-methylpentanal or acetone as oxo-components in the presence of amino acid-derived isocyanides. Regardless oxo- and isocyano-components used, products 13–16 were obtained in low yields (10–24%, Table 2). The utility of Nα-Cbz, Nβ-Boc protected hydrazino acids (type III) in the Passerini reaction was tested with the same set of oxo-compounds and various isocyanides, yielding hydrazino depsipeptides 17–24 (Table 2). Passerini products with type III hydrazino acids are obtained in generally higher yields than with type II hydrazino acids (11–55%). It is interesting to note, that two products isolated in highest yield, 18 and 21, are assembled of all three different components.
Finally, the success of the protocol prompted us to test its scope with Nβ-Boc protected hydrazino proline. Because of the low reactivity of ketones observed in previous reactions, Passerini reactions were performed only with aldehydes. The corresponding products were isolated in low yields (25–28, Table 3), with the exception of p-NO2 benzaldehyde/cyclohexyl isocyanide combination which yielded the Passerini product 26 in 55% yield.
| a Isolated yields are given in parentheses. Compounds were isolated as mixture of diastereoisomers. |
|---|

|
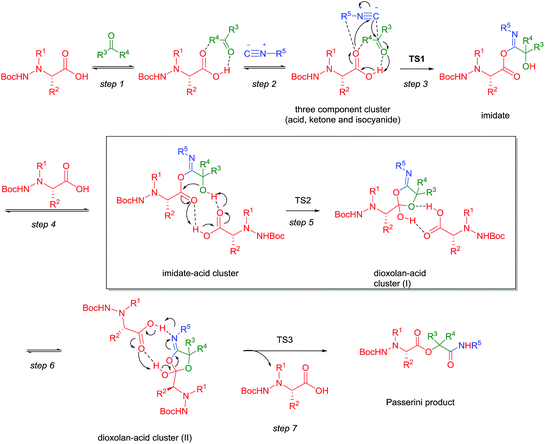
Comparison of obtained results revealed that efficiency of hydrazino acids in the Passerini reaction decreases in line: type I > type III > type II. The best examples are Passerini products 11 (74%) – 21 (54%) – 14 (24%) and also 1 (54%) – 17 (11%) – 13 (17%), where type II hydrazino acid afforded Passerini product is slightly better yield than type III hydrazino acid, but type I hydrazino acid-related Passerini product is obtained in by far better yield. Possible explanation for such outcome can be find in steric hindrance of Cbz and especially Boc group at the Nα atom, but also different nature of substituent; N-benzyl vs. urethane type of bond. To investigate the disparity in a yield of products obtained by different hydrazino acids, we decided to get insight into reaction mechanism. An extensive research has been devoted to elucidate the mechanism of the Passerini reaction,14c,17b,26 including the theoretical approach by DFT methods in a paper of Maeda et al.27 Using the simplest set of reactants (formaldehyde, methyl isocyanide and formic acid) and calculating energies of all possible stationary points for the reaction at M0628/6-31G(d,p) + ZPE level of theory,28 it was found that the most productive mechanism in gas phase consists of seven steps: formation of H-bonded cluster between acid and aldehyde (1), followed by reaction with isocyanide (2) that results in occurrence of imidate (3). Imidate then forms H-bonded cluster with additional molecule of acid that serves as a catalyst (4), enabling rearrangement of this cluster with imidate to dioxolane intermediate (5) stabilized by H-bonds with additional acid molecule. Conformational change of H-bonded dioxolane-acid cluster (6) is necessary to allow the last step – Mumm rearrangement which results in the final product. The described mechanism is shown in Scheme 3, with acid counterpart represented as α-hydrazino acid.
Maeda et al. found that the rate-determining step is the rearrangement of the H-bonded imidate-acid cluster into the dioxolane-acid cluster (step 5, framed on Scheme 3).27 The calculated activation energy needed for this process was 51.8 kJ mol−1 (at M06/6-31+G(d,p) + ZPE level of theory) with formic acid, formaldehyde and methyl isocyanide as reactants. Implicit model of solvation (PCM-M06/6-31+G(d,p)) in dichloromethane was used to estimate the influence of solvent to the energy profile of the reaction. The solvent effect was very mild, lowering energy barrier of the rate-determining step to 49.8 kJ mol−1. In more recent work by Ramozzi and Morokuma, the influence of solvent to the mechanism is additionally studied by DFT.29 It was found that in aprotic solvents, the stable nitrilium intermediate could be formed before the occurrence of imidate intermediate. The formation of nitrilium is also catalysed by additional molecule of acid, which means that in aprotic solvent the second (catalytic) molecule of acid enters the mechanism earlier than in the gas phase. The reaction then proceeds into the formation of imidate and rearrangements that lead to the final product.28 It is important to note that in this revised mechanism in aprotic solvents, the rate-determining step still remains the same: the transformation of imidate into dioxalane intermediate.
We used DFT approach to calculate the kinetics and thermodynamic equilibrium of the rate-determining step of the Passerini reaction, with some of the reactants used in our work (Scheme 4). The results of computational modeling of the rate-determining step of the Passerini reaction were compared for two products with profound difference in yield, 11 (74%), and 14 (24%), Table 2. Two products differentiate only in α-hydrazino acid used in the synthesis, Nα-benzyl, Nβ-Boc protected hydrazino phenylanine (type I) for 11 and Nα-Boc, Nβ-Boc protected hydrazino phenylanine (type II) for 14. Since the only difference between these two sets of reactants was the protective group on Nα atom of hydrazino phenylalanine (R1, Schemes 3 and 4), we estimated that it is safe to employ smaller model molecules as carbonyl species and isocyanide, for the sake of computational feasibility. Specifically, instead of 2-methylpentanal the formaldehyde was used, and methyl isocyanide was utilized in place of 2-isocyano-3-methyl-methylbutanoate. One additional simplification was the replacement of the protective group on Nβ atom of hydrazino acid; tert-butyloxycarbonyl (Boc) by methoxycarbonyl group (Moc), as shown in Scheme 4.
Geometries of all stationary points (two minima and transition state) were optimized at the M06-2X28/6-31+G(d,p) level of theory. Vibrational analysis was performed to confirm the nature of each stationary point (NImag = 0 for minima and NImag = 1 for transition structure) and, in case of transition state, the single imaginary mode was confirmed to correspond to the reaction path that connects two minima. All energies were corrected for zero point vibrational energy (ZPE). Additionally, to be able to compare our results with those obtained by Maeda et al.27 we had to calculate the same process using Maeda's reactants at the same level of theory applied in our work. Results were presented in Table 4. Calculated activation energies for the modelled reaction step are almost the same for all three analysed systems, as well as the thermodynamical equilibria between the two minima. Obviously, the disparity in product yield is not consequence of a different kinetics for this critical step of a reaction.
| Imidate-acid cluster | TS | Dioxolan-acid cluster | |
|---|---|---|---|
| R1 = Bn | 0 | 35.2 | −13.1 |
| R1 = Boc | 0 | 33.0 | −18.2 |
| Maeda's reactants27 | 0 | 37.3 | −17.6 |
However, if hydrazino acids are used instead of formic acid, imidate-acid cluster (Scheme 4) may undergo conformational changes that induce formation of alternative H-bonds between additional acid molecule (which serves as a catalyst for this reaction step) and the cluster. Alternative H-bonds stabilize these non-productive conformations of cluster with imidate, so the reaction step from Scheme 4 cannot occur, and reaction does not proceed. Structures of productive conformations of imidate-acid cluster are shown in Fig. 1, for Nα-benzyl, Nβ-Moc protected hydrazino phenylanine (left) and Nα-Boc, Nβ-Moc protected hydrazino phenylanine (right).
Non-productive conformers of imidate-acid cluster are schematically also presented in Fig. 1. Two types of non-productive conformers of imidate cluster are identified when hydrazino acid with R1 = benzyl is used, while three non-productive conformations of imidate cluster are possible for the hydrazino acid with R1 = Boc. This third conformer is result of H-bond formation between the carbonyl oxygen on Boc and hydrogen from carboxylic group of acid. Obviously, in system when benzyl group is present instead of Boc at Nα atom, this additional H-bond cannot occur.
To rationalize a difference in yield for Passerini reaction when using hydrazino acid with R1 = benzyl and R1 = Boc, respectively, we compared thermodynamical stability of non-productive conformers with productive imidate cluster, for systems containing former and latter acid. Results are shown in Table 5. It turned out that non-productive minima for hydrazino acid where R1 = benzyl are only slightly more stable when compared to the productive conformation of imidate cluster (∼10 kJ mol−1). Given that the whole reaction is relatively fast, that means that only small part of reactants finish in non-productive conformations. On the other side, two of three existing non-productive minima for system with R1 = Boc show significant stability when compared with productive conformation of imidate cluster (∼50–60 kJ mol−1). The occurrence of very stable non-productive conformations of imidate cluster implies that the rearrangement of imidate to dioxolan which leads to the final product is then impeded. The data from Table 2 indicate that all products with R1 = Boc come with relatively low yield, in a range from 10 to 24% (compounds 13–16), which may be rationalized in a same way as in case of the compound 14.
| Productive | Non-productive 1 | Non-productive 2 | Non-productive 3 | |
|---|---|---|---|---|
| R1 = Bn | 0 | −11.9 | −10.2 | Non-existent |
| R1 = Boc | 0 | −6.4 | −50.5 | −61.3 |
Presence of additional functionalities in MCR products offers diverse post-condensation modifications affording new, structurally advanced scaffolds. Passerini-amine deprotection-acyl migration (PADAM) is the most exploited post-Passerini protocol used for conversion of depsipeptides into α-oxoamides, structural motifs found in natural products and synthetic anticancer compounds and protease inhibitors.15b,17b,26,30,31 Next, diverse protocols were developed toward medium-sized cyclic and macrocyclic compounds, including ring-closing metathesis (RCM), macrolactonizations, click chemistry approaches and MCR-based cyclization.15b In our particular case, transformation of linear hydrazino depsipeptides into their cyclic analogous would be attractive additional benefit of new peptidomimetic structures. As a proof-of-principle, we chose Nα-benzyl, Nβ-Boc-protected derivative 1 bearing methyl ester group at C-terminal position. Two-step deprotection procedure, base conditions for the removal of ester group followed by acid-promoted Boc cleavage, yielded unprotected hydrazino depsipeptide that was subjected to intramolecular cyclization in the presence of HATU coupling reagent, under pseudo-diluted conditions (Scheme 5). After HPLC purification cyclic hydrazino depsipeptide 29 was isolated in 15% yield.
Recently, a decarboxylative multicomponent reactions of α-amino acid (proline) with various aldehydes and isocyanides yielding N-substituted proline amides were performed under metal-free conditions.32 Reaction is based on thermal decarboxylation of in situ formed imine to form azomethine ylide, which undergoes nucleophilic insertion of isocyanide, followed by hydrolysis to furnish N-substituted proline amides. We speculated that in a similar manner, Nα-unsubstituted hydrazino acids could yield Nα-substituted amides. Therefore, a test reaction was performed under conditions found optimal for the decarboxylative multicomponent coupling with proline.32 A reaction of Nβ-Boc-protected hydrazino-L-phenylalanine, p-nitro benzaldehyde and cyclohexyl isocyanide gave cyclohexyl amide of the Nα-substituted hydrazino acid 30 (Scheme 6) in 20% yield.
Therefore, this procedure could be used for the synthesis of various Nα-substituted derivatives of hydrazino acids, but further development requires optimization of decarboxylative multicomponent reaction for hydrazino acids. Finally, Nα-unsubstituted hydrazino acids can be exploited as bifunctional reagents in MCRs. While reaction performed under reaction conditions optimized for Passerini reaction gave no product, the same reactants in methanol after 24 h yielded Ugi-type of highly branched Nα-substituted hydrazino derivative 31 in 25% yield (Scheme 6). So, our current efforts are directed toward further exploring the utility of hydrazino acids in other MCRs. Also, based on here proved methodology, we plan to develop a focused library of hydrazino depsipeptides, to probe interactions with nucleic acids and proteins.
Conclusions
We have developed methodology for the synthesis of new class of peptidomimetics − hydrazino depsipeptides, by multicomponent reaction of an α-hydrazino acid, an aldehyde or ketone and an isocyanide component. α-Hydrazino acids bearing different protecting groups at Nα were used to allow further specific modification of Passerini products. Nα-Benzyl, Nβ-Boc protected hydrazino acids gave hydrazino depsipeptides in considerably better yields then their Nα-Boc and Nα-Cbz-protecting analogues. This finding was rationalized by DFT calculations showing that significant stability of two non-productive conformations of imidate intermediate in the rate-determining step of the reaction hinders rearrangement into dioxolan intermediate which leads to the Passerini product.Experimental section
General method
All experiments were monitored by analytical thin layer chromatography (TLC) performed on Merck Kieselgel 60 F254 0.25 mm precoated aluminium plates. After elution, plate was visualized under UV illumination at 254 nm for UV active materials. Further visualization was achieved by staining with ammonium molybdate and charring on a hot plate. Flash column chromatography was performed on silica gel (Merck, 40–63 μm particle size) by standard techniques eluting with solvents as indicated. 1H NMR and 13C NMR spectra were recorded on Bruker Avance 600 MHz and 300 MHz spectrometers, operating at 150.92 or 75.47 MHz for 13C and 600.13 or 300.13 MHz for 1H nuclei. Chemical shifts are quoted in ppm, and tetramethylsilane (TMS) was used as internal standard. Spectra were acquired at 298 K. Mass spectrometry measurements were performed on a triple quadrupole mass spectrometer, operating in a positive electrospray ionization (ESI) mode. High resolution mass spectrometry (HRMS) was performed on a nanoUPLC-ESI-qTOF in positive and negative mode. Racemic amino acid-derived isocyanides were prepared according to procedure published by Zhu and Danishesky.33General procedure
To a glass vial containing 1 M solution of oxo-compound (0.11 mmol) in THF under nitrogen were added Nα, Nβ-protected hydrazino acid (0.11 mmol) and the isocyanide (0.11 mmol). With all reactants added, the solution was allowed to stir for either 24 h in reactions with aldehydes or 48 h in reactions with ketones. The reactions were concentrated under reduced pressure and reaction mixtures were purified by flash column chromatography using petrol ether/ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Products were isolated and characterized as mixture of diastereoisomers.
1. Products were isolated and characterized as mixture of diastereoisomers.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v); 1H NMR (CDCl3): δ 0.71–0.91 (m, 6H), 1.26–1.58 (m, 12H), 3.15–3.25 (m, 3H), 3.72–3.81 (m, 2H), 3.83 (s, 3H), 4.75–4.79 (m, 1H), 6.05–6.16 (m, 1H), 7.27–7.40 (m, 10H), 8.07 (d, J = 8.7 Hz, 2H), 8.18–8.25 (m, 2H), 8.39 (d, J = 8.6 Hz, 2H). 13C NMR (CDCl3): δ 20.4, 21.4, 21.5, 22.0, 22.30, 23.9, 24.0, 24.3, 27.5, 27.7, 38.4, 38.5, 40.8, 41.1, 41.3, 50.2, 51.8, 52.7, 54.0, 54.6, 60.1, 75.7, 76.3, 81.2, 127.0, 127.1, 127.4, 127.9, 128.2, 128.3, 128.5, 128.7, 128.9, 129.1, 129.3, 129.4, 136.5, 159.8, 167.1, 171.7. MS (ESI+): m/z = 677.6. HRMS: calcd for C36H45N4O9 [M + H]+ 677.3187; found 677.3184.
1, v/v); 1H NMR (CDCl3): δ 0.71–0.91 (m, 6H), 1.26–1.58 (m, 12H), 3.15–3.25 (m, 3H), 3.72–3.81 (m, 2H), 3.83 (s, 3H), 4.75–4.79 (m, 1H), 6.05–6.16 (m, 1H), 7.27–7.40 (m, 10H), 8.07 (d, J = 8.7 Hz, 2H), 8.18–8.25 (m, 2H), 8.39 (d, J = 8.6 Hz, 2H). 13C NMR (CDCl3): δ 20.4, 21.4, 21.5, 22.0, 22.30, 23.9, 24.0, 24.3, 27.5, 27.7, 38.4, 38.5, 40.8, 41.1, 41.3, 50.2, 51.8, 52.7, 54.0, 54.6, 60.1, 75.7, 76.3, 81.2, 127.0, 127.1, 127.4, 127.9, 128.2, 128.3, 128.5, 128.7, 128.9, 129.1, 129.3, 129.4, 136.5, 159.8, 167.1, 171.7. MS (ESI+): m/z = 677.6. HRMS: calcd for C36H45N4O9 [M + H]+ 677.3187; found 677.3184.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (CDCl3): δ 0.71–0.89 (m, 3H), 1.01–1.13 (m, 3H), 1.32–1.46 (m, 9H), 1.98–2.14 (m, 1H), 3.00–3.27 (m, 3H), 3.72–4.10 (m, 5H), 4.88–4.98 (m, 1H), 6.04–6.19 (m, 1H), 6.69–6.80 (m, 2H), 7.05–768 (m, 12H), 8.16–8.25 (m, 2H). 13C NMR (CDCl3): δ 18.4, 18.8, 19.1, 27.1, 27.7, 28.1, 29.2, 34.9, 36.0, 52.0, 52.1, 52.3, 52.5, 52.6, 52.8, 66.8, 70.7, 73.8, 74.0, 80.0, 123.4, 123.8, 126.8, 127.1, 127.4, 127.8, 128.2, 128.4, 128.5, 128.6, 128.9, 129.2, 129.3, 129.4, 130.0, 134.7, 136.0, 136.3, 141.4, 147.8, 166.0, 170.8, 170.9. MS (ESI+): m/z = 663.5. HRMS: calcd for C35H43N4O9 [M + H]+ 663.3030; found 663.3036.
1, v/v). 1H NMR (CDCl3): δ 0.71–0.89 (m, 3H), 1.01–1.13 (m, 3H), 1.32–1.46 (m, 9H), 1.98–2.14 (m, 1H), 3.00–3.27 (m, 3H), 3.72–4.10 (m, 5H), 4.88–4.98 (m, 1H), 6.04–6.19 (m, 1H), 6.69–6.80 (m, 2H), 7.05–768 (m, 12H), 8.16–8.25 (m, 2H). 13C NMR (CDCl3): δ 18.4, 18.8, 19.1, 27.1, 27.7, 28.1, 29.2, 34.9, 36.0, 52.0, 52.1, 52.3, 52.5, 52.6, 52.8, 66.8, 70.7, 73.8, 74.0, 80.0, 123.4, 123.8, 126.8, 127.1, 127.4, 127.8, 128.2, 128.4, 128.5, 128.6, 128.9, 129.2, 129.3, 129.4, 130.0, 134.7, 136.0, 136.3, 141.4, 147.8, 166.0, 170.8, 170.9. MS (ESI+): m/z = 663.5. HRMS: calcd for C35H43N4O9 [M + H]+ 663.3030; found 663.3036.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (CDCl3): δ 0.91–1.05 (m, 18H), 1.13–1.40 (m, 13H), 2.05–2.35 (m, 3H), 3.10–3.45 (m, 1H), 3.58–4.05 (m, 5H), 4.55–4.70 (m, 1H), 4.80–5.20 (m, 1H), 7.27–7.68 (m, 7H). 13C NMR (CDCl3): δ 13.4, 13.5, 13.6, 13.9, 14.1, 14.5, 14.6, 16.2, 16.6, 17.3, 17.5, 17.8, 19.0, 19.2, 19.7, 19.9, 27.6, 27.8, 28.1, 28.2, 30.8, 31.4, 32.6, 36.0, 52.2, 52.4, 57.1, 57.3, 65.7, 78.3, 78.9, 87.2, 127.0, 127.6, 128.5, 128.9, 129.2, 129.4, 137.0, 154.9, 156.6, 171.9, 177.0, 178.2. MS (ESI+): m/z = 564.4. HRMS: calcd for C30H50N3O7 [M + H]+ 564.3649; found 564,3649.
1, v/v). 1H NMR (CDCl3): δ 0.91–1.05 (m, 18H), 1.13–1.40 (m, 13H), 2.05–2.35 (m, 3H), 3.10–3.45 (m, 1H), 3.58–4.05 (m, 5H), 4.55–4.70 (m, 1H), 4.80–5.20 (m, 1H), 7.27–7.68 (m, 7H). 13C NMR (CDCl3): δ 13.4, 13.5, 13.6, 13.9, 14.1, 14.5, 14.6, 16.2, 16.6, 17.3, 17.5, 17.8, 19.0, 19.2, 19.7, 19.9, 27.6, 27.8, 28.1, 28.2, 30.8, 31.4, 32.6, 36.0, 52.2, 52.4, 57.1, 57.3, 65.7, 78.3, 78.9, 87.2, 127.0, 127.6, 128.5, 128.9, 129.2, 129.4, 137.0, 154.9, 156.6, 171.9, 177.0, 178.2. MS (ESI+): m/z = 564.4. HRMS: calcd for C30H50N3O7 [M + H]+ 564.3649; found 564,3649.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (CDCl3): δ 0.88–0.94 (m, 15H), 1.30–1.37 (m, 19H), 2.05–2.10 (m, 1H), 2.48–2.52 (m, 1H), 3.15–3.35 (m, 1H), 3.75–4.15 (m, 2H), 4.75–5.10 (m, 1H), 5.65–5.75 (br s, 1H), 6.75–7.75 (m, 7H). 13C NMR (CDCl3): δ 13.6, 16.3, 19.3, 19.9, 27.7, 28.16, 28.22, 33.6, 34.9, 35.5, 38.1, 65.7, 66.2, 68.3, 78.3, 78.9, 87.4, 127.0, 127.7, 131.4, 147.7, 161.9, 170.7, 172.2. MS (ESI+): m/z = 506.4. ESI-HRMS: calcd for C28H48N3O5 [M + H]+ 506.3594; found 506.3594.
1, v/v). 1H NMR (CDCl3): δ 0.88–0.94 (m, 15H), 1.30–1.37 (m, 19H), 2.05–2.10 (m, 1H), 2.48–2.52 (m, 1H), 3.15–3.35 (m, 1H), 3.75–4.15 (m, 2H), 4.75–5.10 (m, 1H), 5.65–5.75 (br s, 1H), 6.75–7.75 (m, 7H). 13C NMR (CDCl3): δ 13.6, 16.3, 19.3, 19.9, 27.7, 28.16, 28.22, 33.6, 34.9, 35.5, 38.1, 65.7, 66.2, 68.3, 78.3, 78.9, 87.4, 127.0, 127.7, 131.4, 147.7, 161.9, 170.7, 172.2. MS (ESI+): m/z = 506.4. ESI-HRMS: calcd for C28H48N3O5 [M + H]+ 506.3594; found 506.3594.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v); 1H NMR (CDCl3): δ 1.32–1.41 (m, 12H), 3.03–3.30 (m, 2H), 3.68–3.80 (m, 4H), 3.85–4.05 (m, 2H), 4.79–4.94 (m, 1H), 6.13–6.20 (m, 1H), 6.25–6.40 (m, 1H), 6.80–7.60 (m, 13H), 8.14–8.23 (m, 2H). 13C NMR (CDCl3): δ 18.4, 18.8, 19.1, 19.4, 27.1, 27.7, 28.2, 29.2, 34.9, 36.7, 37.0, 38.4, 52.0, 52.1, 52.3, 52.5, 52.6, 52.8, 66.8, 70.7, 73.8, 74.0, 79.4, 123.4, 123.8, 126.8, 127.1, 127.2, 127.8, 128.1, 128.3, 128.4, 128.6, 128.9, 129.3, 129.2, 129.4, 134.7, 136.0, 136.3, 141.3, 147.8, 166.0, 171.6, 171.9. MS (ESI+): m/z = 635.4. HRMS: calcd for C33H39N4O9 [M + H]+ 635.2717; found 635.2716.
1, v/v); 1H NMR (CDCl3): δ 1.32–1.41 (m, 12H), 3.03–3.30 (m, 2H), 3.68–3.80 (m, 4H), 3.85–4.05 (m, 2H), 4.79–4.94 (m, 1H), 6.13–6.20 (m, 1H), 6.25–6.40 (m, 1H), 6.80–7.60 (m, 13H), 8.14–8.23 (m, 2H). 13C NMR (CDCl3): δ 18.4, 18.8, 19.1, 19.4, 27.1, 27.7, 28.2, 29.2, 34.9, 36.7, 37.0, 38.4, 52.0, 52.1, 52.3, 52.5, 52.6, 52.8, 66.8, 70.7, 73.8, 74.0, 79.4, 123.4, 123.8, 126.8, 127.1, 127.2, 127.8, 128.1, 128.3, 128.4, 128.6, 128.9, 129.3, 129.2, 129.4, 134.7, 136.0, 136.3, 141.3, 147.8, 166.0, 171.6, 171.9. MS (ESI+): m/z = 635.4. HRMS: calcd for C33H39N4O9 [M + H]+ 635.2717; found 635.2716.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (CDCl3): δ 0.85–0.98 (m, 12H), 1.49–1.57 (m, 11H), 1.66–1.78 (m, 4H), 3.58–3.80 (m, 5H), 3.84–4.20 (m, 2H), 4.55–4.67 (m, 1H), 5.25–5.55 (m, 1H), 6.10–6.40 (m, 1H), 7.30–8.20 (m, 12H). 13C NMR (CDCl3): δ 21.1, 22.2, 24.4, 27.5, 27.7, 27.8, 29.2, 36.1, 38.2, 41.1, 41.3, 50.2, 50.3, 51.9, 52.2, 53.0, 54.0, 58.6, 60.1, 62.6, 75.0, 75.36, 75.41, 79.2, 80.9, 126.8, 127.0, 127.2, 127.7, 128.1, 128.2, 128.4, 128.5, 128.8, 129.1, 129.3, 129.8, 133.1, 133.2, 137.9, 140.7, 164.3, 164.4, 167.3. MS (ESI+): m/z = 598.5. HRMS: calcd for C33H48N3O7 [M + H]+ 598.3492; found 598.3486.
1, v/v). 1H NMR (CDCl3): δ 0.85–0.98 (m, 12H), 1.49–1.57 (m, 11H), 1.66–1.78 (m, 4H), 3.58–3.80 (m, 5H), 3.84–4.20 (m, 2H), 4.55–4.67 (m, 1H), 5.25–5.55 (m, 1H), 6.10–6.40 (m, 1H), 7.30–8.20 (m, 12H). 13C NMR (CDCl3): δ 21.1, 22.2, 24.4, 27.5, 27.7, 27.8, 29.2, 36.1, 38.2, 41.1, 41.3, 50.2, 50.3, 51.9, 52.2, 53.0, 54.0, 58.6, 60.1, 62.6, 75.0, 75.36, 75.41, 79.2, 80.9, 126.8, 127.0, 127.2, 127.7, 128.1, 128.2, 128.4, 128.5, 128.8, 129.1, 129.3, 129.8, 133.1, 133.2, 137.9, 140.7, 164.3, 164.4, 167.3. MS (ESI+): m/z = 598.5. HRMS: calcd for C33H48N3O7 [M + H]+ 598.3492; found 598.3486.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (CDCl3): δ 0.96–1.01 (m, 12H), 1.32–1.43 (m, 5H), 1.50–1.63 (m, 12H), 1.67–1.77 (m, 2H), 1.80–1.95 (m, 2H), 3.74 (s, 3H), 3.78 (s, 2H), 3.85–4.10 (m, 1H), 4.20–4.32, (m, 1H), 4.55–4.80 (m, 1H), 7.30–7.45 (m, 7H). 13C NMR (CDCl3): δ 20.4, 21.4, 21.5, 22.0, 22.4, 24.0, 24.3, 27.5, 27.7, 27.8, 38.4, 38.5, 40.8, 41.1, 41.3, 48.8, 50.2, 50.3, 51.8, 52.3, 52.7, 54.1, 54.6, 58.6, 60.1, 67.1, 75.7, 81.3, 127.0, 127.4, 127.7, 127.8, 128.0, 128.5, 129.0, 129.2, 131.1, 136.6, 160.0, 168.8, 171.8. MS (ESI+): m/z = 550.6. HRMS: calcd for C29H48N3O7 [M + H]+ 550.3492, found 550.3491.
1, v/v). 1H NMR (CDCl3): δ 0.96–1.01 (m, 12H), 1.32–1.43 (m, 5H), 1.50–1.63 (m, 12H), 1.67–1.77 (m, 2H), 1.80–1.95 (m, 2H), 3.74 (s, 3H), 3.78 (s, 2H), 3.85–4.10 (m, 1H), 4.20–4.32, (m, 1H), 4.55–4.80 (m, 1H), 7.30–7.45 (m, 7H). 13C NMR (CDCl3): δ 20.4, 21.4, 21.5, 22.0, 22.4, 24.0, 24.3, 27.5, 27.7, 27.8, 38.4, 38.5, 40.8, 41.1, 41.3, 48.8, 50.2, 50.3, 51.8, 52.3, 52.7, 54.1, 54.6, 58.6, 60.1, 67.1, 75.7, 81.3, 127.0, 127.4, 127.7, 127.8, 128.0, 128.5, 129.0, 129.2, 131.1, 136.6, 160.0, 168.8, 171.8. MS (ESI+): m/z = 550.6. HRMS: calcd for C29H48N3O7 [M + H]+ 550.3492, found 550.3491.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (CDCl3): δ 1.27–1.60 (m, 19H), 2.95–3.25 (m, 2H), 3.55–4.15 (m, 4H), 4.80–5.20 (m, 1H), 5.85–6.18 (m, 1H), 7.20–7.35 (m, 6H), 8.05–8.11 (m, 4H), 8.36–8.44 (m, 5H). 13C NMR (75 MHz, CDCl3) δ 24.3, 24.8, 24.9, 27.5, 27.7, 29.2, 32.2, 32.3, 35.4, 48.3, 66.9, 74.2, 74.3, 74.6, 83.0, 123.3, 123.8, 126.3, 127.3, 127.7, 127.8, 127.9, 128.1, 128.3, 128.5, 128.6, 128.8, 129.3, 130.0, 133.6, 139.4, 170.1, 170.2. MS (ESI+): m/z = 631.3. HRMS: calcd for C35H43N4O7 [M + H]+ 631.3132; found 631.3134.
1, v/v). 1H NMR (CDCl3): δ 1.27–1.60 (m, 19H), 2.95–3.25 (m, 2H), 3.55–4.15 (m, 4H), 4.80–5.20 (m, 1H), 5.85–6.18 (m, 1H), 7.20–7.35 (m, 6H), 8.05–8.11 (m, 4H), 8.36–8.44 (m, 5H). 13C NMR (75 MHz, CDCl3) δ 24.3, 24.8, 24.9, 27.5, 27.7, 29.2, 32.2, 32.3, 35.4, 48.3, 66.9, 74.2, 74.3, 74.6, 83.0, 123.3, 123.8, 126.3, 127.3, 127.7, 127.8, 127.9, 128.1, 128.3, 128.5, 128.6, 128.8, 129.3, 130.0, 133.6, 139.4, 170.1, 170.2. MS (ESI+): m/z = 631.3. HRMS: calcd for C35H43N4O7 [M + H]+ 631.3132; found 631.3134.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (CDCl3): δ 0.92 (d, J = 6.9 Hz, 6H), 0.96 (d, J = 6.9 Hz, 6H), 1.40–1.46 (m, 4H), 1.52 (s, 9H), 2.20–2.25 (m, 2H), 2.30–2.35 (m, 3H), 3.70–3.73 (m, 5H), 4.23 (s, 3H), 4.53–4.61 (m, 1H), 4.63–4.70 (m, 1H), 7.17–7.38 (m, 10H), 8.30 (br s, 1H), 8.92 (d, 1H). 13C NMR (CDCl3): δ 16.8, 17.8, 17.9, 19.1, 19.4, 20.3, 28.1, 28.3, 31.4, 31.5, 33.7, 34.0, 52.4, 53.3, 55.8, 56.7, 63.0, 63.1, 78.2, 78.6, 83.9, 126.7, 127.6, 128.2, 128.3, 128.5, 128.9, 129.3, 129.5, 129.8, 160.7, 167.0, 172.2. MS (ESI+): m/z = 612.6. ESI-HRMS: calcd for C34H50N3O7 [M + H]+ 612.3649; found 612.3646.
1, v/v). 1H NMR (CDCl3): δ 0.92 (d, J = 6.9 Hz, 6H), 0.96 (d, J = 6.9 Hz, 6H), 1.40–1.46 (m, 4H), 1.52 (s, 9H), 2.20–2.25 (m, 2H), 2.30–2.35 (m, 3H), 3.70–3.73 (m, 5H), 4.23 (s, 3H), 4.53–4.61 (m, 1H), 4.63–4.70 (m, 1H), 7.17–7.38 (m, 10H), 8.30 (br s, 1H), 8.92 (d, 1H). 13C NMR (CDCl3): δ 16.8, 17.8, 17.9, 19.1, 19.4, 20.3, 28.1, 28.3, 31.4, 31.5, 33.7, 34.0, 52.4, 53.3, 55.8, 56.7, 63.0, 63.1, 78.2, 78.6, 83.9, 126.7, 127.6, 128.2, 128.3, 128.5, 128.9, 129.3, 129.5, 129.8, 160.7, 167.0, 172.2. MS (ESI+): m/z = 612.6. ESI-HRMS: calcd for C34H50N3O7 [M + H]+ 612.3649; found 612.3646.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (CDCl3): δ 0.84–0.91 (m, 6H), 1.30–1.50 (m, 13H), 3.10–3.18 (m, 1H), 3.58–3.80 (m, 4H), 3.85–4.20 (m, 3H), 4.35–4.60 (br s, 1H), 5.11–5.28 (m, 1H), 6.45–6.75 (m, 1H), 7.05–7.42 (m, 8H), 7.45–8.15 (m, 3H). 13C NMR (CDCl3): δ 13.6, 13.8, 19.7, 27.7, 28.2, 32.8, 35.1, 35.8, 40.4, 46.3, 52.3, 60.8, 78.1, 79.8, 83.7, 127.8, 128.2, 128.4, 128.5, 128.8, 130.5, 133.2, 148.1, 176.6, 178.5, 180.9. MS (ESI+): m/z = 570.3. ESI-HRMS: calcd for C31H44N3O7 [M + H]+ 570.3179; found 570.3173.
1, v/v). 1H NMR (CDCl3): δ 0.84–0.91 (m, 6H), 1.30–1.50 (m, 13H), 3.10–3.18 (m, 1H), 3.58–3.80 (m, 4H), 3.85–4.20 (m, 3H), 4.35–4.60 (br s, 1H), 5.11–5.28 (m, 1H), 6.45–6.75 (m, 1H), 7.05–7.42 (m, 8H), 7.45–8.15 (m, 3H). 13C NMR (CDCl3): δ 13.6, 13.8, 19.7, 27.7, 28.2, 32.8, 35.1, 35.8, 40.4, 46.3, 52.3, 60.8, 78.1, 79.8, 83.7, 127.8, 128.2, 128.4, 128.5, 128.8, 130.5, 133.2, 148.1, 176.6, 178.5, 180.9. MS (ESI+): m/z = 570.3. ESI-HRMS: calcd for C31H44N3O7 [M + H]+ 570.3179; found 570.3173.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (CDCl3): δ 0.90–0.95 (m, 6H), 1.48–1.52 (m, 20H), 1.73–1.85 (m, 2H), 3.02–3.19 (m, 3H), 3.71–3.79 (m, 3H), 4.75–4.96 (m, 2H), 6.09–6.15 (br s, 1H), 7.15–7.27 (m, 7H), 7.55–7.63 (br s, 1H), 8.12–8.25 (m, 2H). 13C NMR (CDCl3) δ 21.9, 23.1, 24.7, 28.2, 28.3, 29.8, 37.3, 37.5, 39.1, 52.6, 53.4, 53.6, 75.3, 82.5, 82.6, 123.9, 124.3, 127.3, 127.8, 128.1, 128.6, 129.2, 129.7, 130.6, 148.2, 166.9, 171.4. MS (ESI+): m/z = 687.4. ESI-HRMS: calcd for C34H47N4O11 [M + H]+ 687.3241; found 687.3237.
1, v/v). 1H NMR (CDCl3): δ 0.90–0.95 (m, 6H), 1.48–1.52 (m, 20H), 1.73–1.85 (m, 2H), 3.02–3.19 (m, 3H), 3.71–3.79 (m, 3H), 4.75–4.96 (m, 2H), 6.09–6.15 (br s, 1H), 7.15–7.27 (m, 7H), 7.55–7.63 (br s, 1H), 8.12–8.25 (m, 2H). 13C NMR (CDCl3) δ 21.9, 23.1, 24.7, 28.2, 28.3, 29.8, 37.3, 37.5, 39.1, 52.6, 53.4, 53.6, 75.3, 82.5, 82.6, 123.9, 124.3, 127.3, 127.8, 128.1, 128.6, 129.2, 129.7, 130.6, 148.2, 166.9, 171.4. MS (ESI+): m/z = 687.4. ESI-HRMS: calcd for C34H47N4O11 [M + H]+ 687.3241; found 687.3237.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (CDCl3): δ 0.85–0.98 (m, 8H), 1.01 (d, 3H), 1.12 (d, 3H), 1.29–1.55 (m, 20H), 2.12–2.35 (m, 2H), 3.18–3.32 (m, 2H), 3.82 (s, 3H), 4.17 (m, 1H), 4.35–4.55 (m, 1H), 5.02–5.16 (m, 1H), 7.13–7.44 (m, 6H). 13C NMR (CDCl3): δ 13.6, 14.8, 16.2, 18.6, 19.6, 27.5, 27.6, 29.2, 30.7, 34.2, 34.9, 51.4, 52.6, 56.7, 62.3, 78.7, 126.2, 126.3, 128.0, 128.1, 129.0, 160.1, 166.4, 172.5. MS (ESI+): m/z = 622.5. ESI-HRMS: calcd for C32H52N3O9 [M + H]+ 622,3704; found 622.3694.
1, v/v). 1H NMR (CDCl3): δ 0.85–0.98 (m, 8H), 1.01 (d, 3H), 1.12 (d, 3H), 1.29–1.55 (m, 20H), 2.12–2.35 (m, 2H), 3.18–3.32 (m, 2H), 3.82 (s, 3H), 4.17 (m, 1H), 4.35–4.55 (m, 1H), 5.02–5.16 (m, 1H), 7.13–7.44 (m, 6H). 13C NMR (CDCl3): δ 13.6, 14.8, 16.2, 18.6, 19.6, 27.5, 27.6, 29.2, 30.7, 34.2, 34.9, 51.4, 52.6, 56.7, 62.3, 78.7, 126.2, 126.3, 128.0, 128.1, 129.0, 160.1, 166.4, 172.5. MS (ESI+): m/z = 622.5. ESI-HRMS: calcd for C32H52N3O9 [M + H]+ 622,3704; found 622.3694.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (CDCl3): δ 1.34–1.50 (m, 18H), 3.08–3.27 (m, 2H), 3.46 (s, 3H), 3.69 (s, 2H), 3.97–4.12 (m, 1H), 6.02–6.18 (m, 1H), 7.02–7.42 (m, 6H), 7.55–7.75 (br s, 1H), 8.02–8.45 (m, 4H). 13C NMR (CDCl3): δ 28.1, 29.7, 30.2, 40.7, 50.8, 52.1, 75.2, 75.5, 82.4, 82.6, 123.5, 124.3, 126.6, 127.1, 128.6, 129.0, 129.3, 130.5, 140.1, 142.4, 148.0, 167.8, 169.6, MS (ESI+): m/z = 631.2. ESI-HRMS: calcd for C30H39N4O11 [M + H]+ 631.2615; found 631.2609.
1, v/v). 1H NMR (CDCl3): δ 1.34–1.50 (m, 18H), 3.08–3.27 (m, 2H), 3.46 (s, 3H), 3.69 (s, 2H), 3.97–4.12 (m, 1H), 6.02–6.18 (m, 1H), 7.02–7.42 (m, 6H), 7.55–7.75 (br s, 1H), 8.02–8.45 (m, 4H). 13C NMR (CDCl3): δ 28.1, 29.7, 30.2, 40.7, 50.8, 52.1, 75.2, 75.5, 82.4, 82.6, 123.5, 124.3, 126.6, 127.1, 128.6, 129.0, 129.3, 130.5, 140.1, 142.4, 148.0, 167.8, 169.6, MS (ESI+): m/z = 631.2. ESI-HRMS: calcd for C30H39N4O11 [M + H]+ 631.2615; found 631.2609.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (CDCl3): δ 0.91–0.96 (m, 6H), 1.32–1.48 (m, 20H), 1.57–1.66 (m, 7H), 3.05–3.19 (m, 2H), 3.55–3.69 (br s, 3H), 4.35–4.73 (m, 2H), 7.20–7.35 (m, 7H). 13C NMR (CDCl3): δ 21.2, 22.1, 22.4, 22.9, 24.3, 27.5, 27.6, 37.4, 50.0, 50.3, 51.2, 51.5, 81.4, 82.2, 126.4, 127.3, 127.8, 128.3, 128.6, 129.0, 167.2, 173.0. MS (ESI+): m/z = 594.5. ESI-HRMS: calcd for C30H48N3O9 [M + H]+ 594.3391; found 594.3384.
1, v/v). 1H NMR (CDCl3): δ 0.91–0.96 (m, 6H), 1.32–1.48 (m, 20H), 1.57–1.66 (m, 7H), 3.05–3.19 (m, 2H), 3.55–3.69 (br s, 3H), 4.35–4.73 (m, 2H), 7.20–7.35 (m, 7H). 13C NMR (CDCl3): δ 21.2, 22.1, 22.4, 22.9, 24.3, 27.5, 27.6, 37.4, 50.0, 50.3, 51.2, 51.5, 81.4, 82.2, 126.4, 127.3, 127.8, 128.3, 128.6, 129.0, 167.2, 173.0. MS (ESI+): m/z = 594.5. ESI-HRMS: calcd for C30H48N3O9 [M + H]+ 594.3391; found 594.3384.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (CDCl3): δ 0.80–0.98 (m, 6H), 1.50–1.65 (br s, 20H), 3.01–3.29 (m, 2H), 3.55–3.75 (m, 3H), 4.65–4.79 (m, 2H), 5.07–5.28 (m, 2H), 5.95–6.25 (m, 1H), 7.25–7.40 (m, 12H), 7.75–8.40 (m, 4H). 13C NMR (CDCl3): δ 21.7, 23.0, 24.4, 28.4, 35.3, 39.0, 53.6, 59.2, 65.6, 68.9, 72.3, 124.1, 124.5, 127.1, 127.7, 129.4, 129.5, 129.7, 130.6, 131.1, 141.0, 156.9, 169.1, 170.8, 171.5. MS (ESI+): m/z = 721.6. ESI-HRMS: calcd for C37H45N4O11 [M + H]+ 721.3085; found 721.3076.
1, v/v). 1H NMR (CDCl3): δ 0.80–0.98 (m, 6H), 1.50–1.65 (br s, 20H), 3.01–3.29 (m, 2H), 3.55–3.75 (m, 3H), 4.65–4.79 (m, 2H), 5.07–5.28 (m, 2H), 5.95–6.25 (m, 1H), 7.25–7.40 (m, 12H), 7.75–8.40 (m, 4H). 13C NMR (CDCl3): δ 21.7, 23.0, 24.4, 28.4, 35.3, 39.0, 53.6, 59.2, 65.6, 68.9, 72.3, 124.1, 124.5, 127.1, 127.7, 129.4, 129.5, 129.7, 130.6, 131.1, 141.0, 156.9, 169.1, 170.8, 171.5. MS (ESI+): m/z = 721.6. ESI-HRMS: calcd for C37H45N4O11 [M + H]+ 721.3085; found 721.3076.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (CDCl3) δ 0.75–2.15 (m, 26H), 3.76 (br s, 1H), 5.15–5.25 (m, 1H), 6.95–6.20 (m, 1H), 7.29–7.33 (m, 5H), 7.55–7.63 (m, 2H), 8.10–8.25 (m, 2H). 13C NMR (CDCl3) δ 22.9, 24.8 25.4, 28.0, 29.4, 32.6, 32.8, 35.0, 48.7, 57.9, 65.4, 71.1, 85.3, 123.7, 127.0, 127.7, 128.6, 130.5, 139.3, 140.1, 140.9, 150.2, 151.2, 169.2, 170.6, 171.2 MS (ESI+): m/z = 641.2. ESI-HRMS: calcd for C33H45N4O9 [M + H]+ 641.3187; found 641.3187.
1, v/v). 1H NMR (CDCl3) δ 0.75–2.15 (m, 26H), 3.76 (br s, 1H), 5.15–5.25 (m, 1H), 6.95–6.20 (m, 1H), 7.29–7.33 (m, 5H), 7.55–7.63 (m, 2H), 8.10–8.25 (m, 2H). 13C NMR (CDCl3) δ 22.9, 24.8 25.4, 28.0, 29.4, 32.6, 32.8, 35.0, 48.7, 57.9, 65.4, 71.1, 85.3, 123.7, 127.0, 127.7, 128.6, 130.5, 139.3, 140.1, 140.9, 150.2, 151.2, 169.2, 170.6, 171.2 MS (ESI+): m/z = 641.2. ESI-HRMS: calcd for C33H45N4O9 [M + H]+ 641.3187; found 641.3187.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (CDCl3): δ 0.63–1.15 (m, 12H), 1.16–1.25 (m, 6H), 1.30–1.42 (m, 6H), 1.55–1.62 (m, 3H), 1.65–1.75 (m, 4H), 1.80–1.90 (m, 3H), 3.70–3.85 (m, 2H), 5.05–5.20 (m, 2H), 5.73 (m, 1H), 7.19–7.23 (m, 5H). 13C NMR (CDCl3): δ 13.6, 15.1, 19.6, 20.4, 24.3, 25.0, 27.5, 32.5, 32.6, 33.1, 34.6, 34.7, 47.4, 68.0, 74.1, 76.4, 78.0, 127.8, 128.1, 128.7, 142.1, 167.6, 168.0, 169.1, 169.2. MS (ESI+): m/z = 590.6. HRMS: calcd for C32H52N3O7 [M + H]+ 590.3805; found for 590.3805.
1, v/v). 1H NMR (CDCl3): δ 0.63–1.15 (m, 12H), 1.16–1.25 (m, 6H), 1.30–1.42 (m, 6H), 1.55–1.62 (m, 3H), 1.65–1.75 (m, 4H), 1.80–1.90 (m, 3H), 3.70–3.85 (m, 2H), 5.05–5.20 (m, 2H), 5.73 (m, 1H), 7.19–7.23 (m, 5H). 13C NMR (CDCl3): δ 13.6, 15.1, 19.6, 20.4, 24.3, 25.0, 27.5, 32.5, 32.6, 33.1, 34.6, 34.7, 47.4, 68.0, 74.1, 76.4, 78.0, 127.8, 128.1, 128.7, 142.1, 167.6, 168.0, 169.1, 169.2. MS (ESI+): m/z = 590.6. HRMS: calcd for C32H52N3O7 [M + H]+ 590.3805; found for 590.3805.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (300 MHz, CDCl3) δ 0.79–1.85 (m, 25H), 4.60–4.75 (m, 3H), 5.21 (br s, 1H), 7.25–7.42 (m, 7H). 13C NMR (CDCl3): δ 13.5, 13.6, 13.7, 14.5, 14.6, 19.6, 24.0, 27.6, 28.1, 29.2, 34.6, 42.2, 61.3, 64.9, 67.8, 80.6, 88.6, 127.9, 128.0, 128.1, 137.1, 153.8, 155.4, 169.4, 172.3. MS (ESI+): m/z = 564.4. ESI-HRMS: calcd for C30H50N3O7 [M + H]+ 564.3649; found 564.3647.
1, v/v). 1H NMR (300 MHz, CDCl3) δ 0.79–1.85 (m, 25H), 4.60–4.75 (m, 3H), 5.21 (br s, 1H), 7.25–7.42 (m, 7H). 13C NMR (CDCl3): δ 13.5, 13.6, 13.7, 14.5, 14.6, 19.6, 24.0, 27.6, 28.1, 29.2, 34.6, 42.2, 61.3, 64.9, 67.8, 80.6, 88.6, 127.9, 128.0, 128.1, 137.1, 153.8, 155.4, 169.4, 172.3. MS (ESI+): m/z = 564.4. ESI-HRMS: calcd for C30H50N3O7 [M + H]+ 564.3649; found 564.3647.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (CDCl3) δ 0.66–0.82 (m, 12H), 1.10–1.55 (m, 13H), 1.84 (br s, 1H), 2.09 (br s, 1H), 3.12–3.30 (m, 2H), 3.53–3.65 (m, 3H), 4.43 (br s, 1H), 4.68 (s, 2H), 5.12–5.25 (m, 2H), 7.14–7.24 (m, 11H). 13C NMR (CDCl3) δ 14.0, 14.1, 15.3, 18.1, 19.0, 20.1, 20.2, 28.0, 30.7, 34.9, 52.0, 57.2, 65.3, 68.5, 78.6, 81.6, 127.0, 127.6, 128.0, 128.5, 128.6, 128.7, 135.6, 141.0, 155.7, 156.0, 169.2, 169.8, 172.0. MS (ESI+): m/z = 656.4. ESI-HRMS: calcd for C35H50N3O9 [M + H]+ 656.3547; found 656.3544.
1, v/v). 1H NMR (CDCl3) δ 0.66–0.82 (m, 12H), 1.10–1.55 (m, 13H), 1.84 (br s, 1H), 2.09 (br s, 1H), 3.12–3.30 (m, 2H), 3.53–3.65 (m, 3H), 4.43 (br s, 1H), 4.68 (s, 2H), 5.12–5.25 (m, 2H), 7.14–7.24 (m, 11H). 13C NMR (CDCl3) δ 14.0, 14.1, 15.3, 18.1, 19.0, 20.1, 20.2, 28.0, 30.7, 34.9, 52.0, 57.2, 65.3, 68.5, 78.6, 81.6, 127.0, 127.6, 128.0, 128.5, 128.6, 128.7, 135.6, 141.0, 155.7, 156.0, 169.2, 169.8, 172.0. MS (ESI+): m/z = 656.4. ESI-HRMS: calcd for C35H50N3O9 [M + H]+ 656.3547; found 656.3544.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (CDCl3) δ 0.77–0.88 (m, 4H), 1.20–1.50 (m, 20H), 1.55–1.85 (m, 5H), 3.03–3.30 (m, 2H), 4.55–4.70 (m, 2H), 5.09–5.25 (m, 2H), 7.16–7.43 (m, 12H). 13C NMR (CDCl3) δ 14.0, 14.1, 20.1, 28.5, 28.6, 29.7, 34.9, 35.2, 35.7, 51.4, 65.3, 68.5, 82.1, 127.0, 127.6, 128.7, 128.8, 140.9, 154.4, 155.2, 168.5, 169.2. MS (ESI+): m/z = 598.5. ESI-HRMS: calcd for C33H48N3O7 [M + H]+ 598.3492; found 598.3489.
1, v/v). 1H NMR (CDCl3) δ 0.77–0.88 (m, 4H), 1.20–1.50 (m, 20H), 1.55–1.85 (m, 5H), 3.03–3.30 (m, 2H), 4.55–4.70 (m, 2H), 5.09–5.25 (m, 2H), 7.16–7.43 (m, 12H). 13C NMR (CDCl3) δ 14.0, 14.1, 20.1, 28.5, 28.6, 29.7, 34.9, 35.2, 35.7, 51.4, 65.3, 68.5, 82.1, 127.0, 127.6, 128.7, 128.8, 140.9, 154.4, 155.2, 168.5, 169.2. MS (ESI+): m/z = 598.5. ESI-HRMS: calcd for C33H48N3O7 [M + H]+ 598.3492; found 598.3489.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (CDCl3) δ 1.25–1.45 (s, 9H), 3.15–3.30 (m, 2H), 3.55–4.18 (m, 6H), 5.12–5.25 (m, 2H), 6.04–6.20 (br s, 1H), 7.10–7.60 (m, 10H), 7.50–7.60 (m, 2H), 8.00–8.20 (m, 2H). 13C NMR (75 MHz, CDCl3) δ 28.1, 29.8, 40.9, 52.3, 67.0, 68.6, 73.7, 82.6, 123.6, 123.7, 126.9, 127.0, 127.3, 127.8, 128.1, 128.6, 128.7, 128.8, 129.1, 129.2, 136.3, 136.7, 142.2, 146.8, 148.2, 155.1, 167.7, 169.7, 169.9, 171.2. MS (ESI+): m/z = 665.3. ESI-HRMS: calcd for C33H37N4O11 [M + H]+ 665.2459; found for 665.2458.
1, v/v). 1H NMR (CDCl3) δ 1.25–1.45 (s, 9H), 3.15–3.30 (m, 2H), 3.55–4.18 (m, 6H), 5.12–5.25 (m, 2H), 6.04–6.20 (br s, 1H), 7.10–7.60 (m, 10H), 7.50–7.60 (m, 2H), 8.00–8.20 (m, 2H). 13C NMR (75 MHz, CDCl3) δ 28.1, 29.8, 40.9, 52.3, 67.0, 68.6, 73.7, 82.6, 123.6, 123.7, 126.9, 127.0, 127.3, 127.8, 128.1, 128.6, 128.7, 128.8, 129.1, 129.2, 136.3, 136.7, 142.2, 146.8, 148.2, 155.1, 167.7, 169.7, 169.9, 171.2. MS (ESI+): m/z = 665.3. ESI-HRMS: calcd for C33H37N4O11 [M + H]+ 665.2459; found for 665.2458.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (CDCl3): δ 0.88–0.97 (m, 6H), 1.49 (s, 9H), 1.62–1.78 (m, 4H), 1.82–2.10 (m, 4H), 3.75–2.95 (m, 1H), 3.25–3.80 (m, 1H), 3.67–3.75 (m, 3H), 3.80–3.92 (m, 1H), 4.60–4.71 (m, 1H), 5.35–5.55 (m, 2H), 6.19 (s, 1H),7.20–7.45 (m, 2H), 7.64–7.74 (m, 2H), 8.12–8.45 (m, 3H). 13C NMR (CDCl3): δ 21.6, 21.8, 22.7, 22.8, 22.9, 24.2, 24.7, 24.9, 28.2, 28.4, 40.6, 40.8, 40.9, 45.8, 46.7, 50.7, 52.3, 52.5, 64.0, 74.6, 74.9, 78.0, 78.3, 78.6, 81.3, 81.5, 123.8, 124.3, 127.9, 129.0, 130.5, 142.4, 148.2, 155.6, 167.1, 171.7, 172.6. MS (ESI+): m/z = 422.2. ESI-HRMS: calcd for C20H26N3O7 [M − BocNH − H]− 420.1771; found 420.1778.
1, v/v). 1H NMR (CDCl3): δ 0.88–0.97 (m, 6H), 1.49 (s, 9H), 1.62–1.78 (m, 4H), 1.82–2.10 (m, 4H), 3.75–2.95 (m, 1H), 3.25–3.80 (m, 1H), 3.67–3.75 (m, 3H), 3.80–3.92 (m, 1H), 4.60–4.71 (m, 1H), 5.35–5.55 (m, 2H), 6.19 (s, 1H),7.20–7.45 (m, 2H), 7.64–7.74 (m, 2H), 8.12–8.45 (m, 3H). 13C NMR (CDCl3): δ 21.6, 21.8, 22.7, 22.8, 22.9, 24.2, 24.7, 24.9, 28.2, 28.4, 40.6, 40.8, 40.9, 45.8, 46.7, 50.7, 52.3, 52.5, 64.0, 74.6, 74.9, 78.0, 78.3, 78.6, 81.3, 81.5, 123.8, 124.3, 127.9, 129.0, 130.5, 142.4, 148.2, 155.6, 167.1, 171.7, 172.6. MS (ESI+): m/z = 422.2. ESI-HRMS: calcd for C20H26N3O7 [M − BocNH − H]− 420.1771; found 420.1778.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (CDCl3) δ 1.20–1.40 (m, 5H), 1.52–1.96 (m, 20H), 2.70–2.78 (m, 1H), 3.16–3.29 (m, 1H), 3.74–3.88 (m, 2H), 5.30–5.70 (m, 2H), 6.13 (s, 1H), 6.92–7.05 (m, 1H), 7.66–7.72 (m, 2H), 8.20–8.25 (m, 2H). 13C NMR (CDCl3): δ 24.3, 24.7, 25.1, 25.4, 27.5, 28.2, 28.3, 32.7, 32.8, 45.8, 48.6, 48.7, 63.1, 64.1, 74.8, 75.1, 78.0, 81.4, 81.5, 123.7, 123.8, 124.3, 128.0, 128.7, 130.5, 142.9, 148.1, 155.7, 155.8, 166.0, 166.1, 170.6, 171.4. MS (ESI+): m/z = 376.3. ESI-HRMS: calcd for C24H33N4O7 [M − H]− 489.2349; found 489.2336.
1, v/v). 1H NMR (CDCl3) δ 1.20–1.40 (m, 5H), 1.52–1.96 (m, 20H), 2.70–2.78 (m, 1H), 3.16–3.29 (m, 1H), 3.74–3.88 (m, 2H), 5.30–5.70 (m, 2H), 6.13 (s, 1H), 6.92–7.05 (m, 1H), 7.66–7.72 (m, 2H), 8.20–8.25 (m, 2H). 13C NMR (CDCl3): δ 24.3, 24.7, 25.1, 25.4, 27.5, 28.2, 28.3, 32.7, 32.8, 45.8, 48.6, 48.7, 63.1, 64.1, 74.8, 75.1, 78.0, 81.4, 81.5, 123.7, 123.8, 124.3, 128.0, 128.7, 130.5, 142.9, 148.1, 155.7, 155.8, 166.0, 166.1, 170.6, 171.4. MS (ESI+): m/z = 376.3. ESI-HRMS: calcd for C24H33N4O7 [M − H]− 489.2349; found 489.2336.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (CDCl3): δ 1.39 (s, 9H), 1.49 (s, 9H), 1.96–2.15 (m, 4H), 2.88–2.95 (br s, 1H), 3.20–3.28 (br s, 1H), 3.93–4.05 (m, 1H), 5.45–5.57 (m, 2H), 6.04 (s, 1H), 6.55 (br s, 1H), 7.64 (d, J = 8.7 Hz, 2H), 8.21 (d, J = 8.8 Hz, 2H). 13C NMR (CDCl3) δ 24.7, 24.9, 28.2, 28.5, 28.6, 28.7, 29.7, 46.2, 46.8, 52.0, 64.3, 64.5, 74.7, 75.3, 81.2, 81.3, 123.8, 124.1, 124.3, 127.1, 128.0, 128.4, 130.5, 143.0, 143.1, 148.0, 155.5, 166.1, 166.2, 171.1, 171.5. MS (ESI+): m/z = 350.1. ESI-HRMS: calcd for C17H22N3O5 [M − BocNH − H]− 348.1559; found 348.1559.
1, v/v). 1H NMR (CDCl3): δ 1.39 (s, 9H), 1.49 (s, 9H), 1.96–2.15 (m, 4H), 2.88–2.95 (br s, 1H), 3.20–3.28 (br s, 1H), 3.93–4.05 (m, 1H), 5.45–5.57 (m, 2H), 6.04 (s, 1H), 6.55 (br s, 1H), 7.64 (d, J = 8.7 Hz, 2H), 8.21 (d, J = 8.8 Hz, 2H). 13C NMR (CDCl3) δ 24.7, 24.9, 28.2, 28.5, 28.6, 28.7, 29.7, 46.2, 46.8, 52.0, 64.3, 64.5, 74.7, 75.3, 81.2, 81.3, 123.8, 124.1, 124.3, 127.1, 128.0, 128.4, 130.5, 143.0, 143.1, 148.0, 155.5, 166.1, 166.2, 171.1, 171.5. MS (ESI+): m/z = 350.1. ESI-HRMS: calcd for C17H22N3O5 [M − BocNH − H]− 348.1559; found 348.1559.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (CDCl3): δ 0.87–0.90 (m, 3H), 0.90–0.98 (m, 3H), 1.46–1.50 (m, 11H), 1.8–2.20 (m, 6H), 2.65–2.80 (m, 1H), 3.20–3.30 (m, 1H), 3.80–4.25 (m, 3H), 5.25, 5.33 (m, 1H), 5.75–5.80 (m, 1H), 7.25–7.50 (br s, 1H). 13C NMR (CDCl3) δ 14.1, 14.2, 15.5, 15.8, 20.1, 20.2, 20.3, 23.3, 24.3, 28.1, 28.2, 28.5, 34.9, 35.1, 35.5, 25.6, 40.7, 46.8, 52.0, 52.1, 52.5, 63.5, 63.7, 63.8, 63.9, 67.0, 78.2, 78.3, 78.4, 81.3, 81.4, 81.5, 155.7, 155.8, 156.1, 156.2, 160.9, 169.6, 169.7, 169.9, 170.1, 170.3, 171.6, 171.8, 172.2. ESI-HRMS: calcd for C20H34N3O7 [M − H]− 428.2397; found 428.2389.
1, v/v). 1H NMR (CDCl3): δ 0.87–0.90 (m, 3H), 0.90–0.98 (m, 3H), 1.46–1.50 (m, 11H), 1.8–2.20 (m, 6H), 2.65–2.80 (m, 1H), 3.20–3.30 (m, 1H), 3.80–4.25 (m, 3H), 5.25, 5.33 (m, 1H), 5.75–5.80 (m, 1H), 7.25–7.50 (br s, 1H). 13C NMR (CDCl3) δ 14.1, 14.2, 15.5, 15.8, 20.1, 20.2, 20.3, 23.3, 24.3, 28.1, 28.2, 28.5, 34.9, 35.1, 35.5, 25.6, 40.7, 46.8, 52.0, 52.1, 52.5, 63.5, 63.7, 63.8, 63.9, 67.0, 78.2, 78.3, 78.4, 81.3, 81.4, 81.5, 155.7, 155.8, 156.1, 156.2, 160.9, 169.6, 169.7, 169.9, 170.1, 170.3, 171.6, 171.8, 172.2. ESI-HRMS: calcd for C20H34N3O7 [M − H]− 428.2397; found 428.2389.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). After 1 h, solvent was evaporated, residue dissolved in water, pH adjusted to 3 by citric acid and product extracted with EtOAc. Crude product was dissolved in TFA–H2O (9
1, v/v). After 1 h, solvent was evaporated, residue dissolved in water, pH adjusted to 3 by citric acid and product extracted with EtOAc. Crude product was dissolved in TFA–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, 1 mL), reaction was stirred at room temperature 1 h and then product precipitated with di-isopropyl ether. Crude product (22 mg, 0.042 mmol) and TEA (6 μL; 0.042 mmol) were dissolved in dry DMF (12 mL), and the solution was added dropwise via a syringe pump (flow rate 0.8 mL h−1) into the solution of HATU (36 mg; 0.092 mmol) and TEA (6 μL; 0.042 mmol) in dry DMF (2 mL). The solvent was diluted with water, product extracted with ether, washed with brine and water and purified by HPLC. Yield: 15% (4.5 mg); colourless oil; TR = 26.7 min (C-18 RP HPLC column (150 × 4.5 mm, ID 5 μm), liner gradient from 30% MeOH to 90% MeOH in 60 min; λ 254 nm). 1H NMR (600 MHz, CDCl3): δ 1.30–1.60 (m, 6H), 2.25 (m, 1H), 3.25–3.20 (m, 4H), 3.55–3.65 (m, 2H), 4.15–4.20 (m, 1H), 4.75–4.80 (m, 1H), 4.95–5.05 (m, 1H), 7.15–7.25 (m, 5H), 7.30–7.45 (m, 7H), 7.95–8.10 (m, 1H), 8.25–8.35 (m, 2H), 8.40–8.50 (m, 1H). 13C NMR (151 MHz, CDCl3): δ 13.9, 21.7, 29.6, 37.7, 51.5, 53.1, 57.4, 64.8, 68.6, 86.7, 122.3, 123.4, 123.6, 125.8, 126.7, 127.7, 128.2, 128.7, 128.8, 129.2, 129.3, 130.9, 131.2, 132.2, 135.1, 139.7, 147.7, 169.1, 171.5, 173.9. ESI-HRMS: calcd for C27H27N4O6 [M + H]+ 503.1931; found 503.1928.
1, v/v, 1 mL), reaction was stirred at room temperature 1 h and then product precipitated with di-isopropyl ether. Crude product (22 mg, 0.042 mmol) and TEA (6 μL; 0.042 mmol) were dissolved in dry DMF (12 mL), and the solution was added dropwise via a syringe pump (flow rate 0.8 mL h−1) into the solution of HATU (36 mg; 0.092 mmol) and TEA (6 μL; 0.042 mmol) in dry DMF (2 mL). The solvent was diluted with water, product extracted with ether, washed with brine and water and purified by HPLC. Yield: 15% (4.5 mg); colourless oil; TR = 26.7 min (C-18 RP HPLC column (150 × 4.5 mm, ID 5 μm), liner gradient from 30% MeOH to 90% MeOH in 60 min; λ 254 nm). 1H NMR (600 MHz, CDCl3): δ 1.30–1.60 (m, 6H), 2.25 (m, 1H), 3.25–3.20 (m, 4H), 3.55–3.65 (m, 2H), 4.15–4.20 (m, 1H), 4.75–4.80 (m, 1H), 4.95–5.05 (m, 1H), 7.15–7.25 (m, 5H), 7.30–7.45 (m, 7H), 7.95–8.10 (m, 1H), 8.25–8.35 (m, 2H), 8.40–8.50 (m, 1H). 13C NMR (151 MHz, CDCl3): δ 13.9, 21.7, 29.6, 37.7, 51.5, 53.1, 57.4, 64.8, 68.6, 86.7, 122.3, 123.4, 123.6, 125.8, 126.7, 127.7, 128.2, 128.7, 128.8, 129.2, 129.3, 130.9, 131.2, 132.2, 135.1, 139.7, 147.7, 169.1, 171.5, 173.9. ESI-HRMS: calcd for C27H27N4O6 [M + H]+ 503.1931; found 503.1928.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 20% (18 mg); yellow oil; Rf = 0.32 (petrol ether/ethyl acetate 2
1. Yield: 20% (18 mg); yellow oil; Rf = 0.32 (petrol ether/ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (600 MHz, CDCl3): δ 1.30–1.45 (m, 17H), 2.95–3.20 (m, 2H), 3.30–3.80 (m, 3H), 4.10 (br s, 1H), 4.20–4.40 (br s, 1H), 5.85–6.10 (br s, 1H), 7.15–7.20 (m, 2H), 7.30–7.45 (m, 2H), 7.55–7.80 (m, 2H), 8.10–8.30 (m, 4H). 13C NMR (151 MHz, CDCl3): δ 24.9, 25.4, 25.7, 28.0, 28.1, 28.2, 28.3, 29.7, 32.5, 48.3, 48.7, 55.3, 65.3, 83.5, 123.6, 124.0, 127.6, 128.3, 128.8, 129.0, 129.3, 129.5, 155.7, 165,4, 168.0. MS (ESI+): m/z = 497.1. ESI-HRMS: calcd for C27H35N4O5 [M − H]− 495.2607; found 495.2609.
1, v/v). 1H NMR (600 MHz, CDCl3): δ 1.30–1.45 (m, 17H), 2.95–3.20 (m, 2H), 3.30–3.80 (m, 3H), 4.10 (br s, 1H), 4.20–4.40 (br s, 1H), 5.85–6.10 (br s, 1H), 7.15–7.20 (m, 2H), 7.30–7.45 (m, 2H), 7.55–7.80 (m, 2H), 8.10–8.30 (m, 4H). 13C NMR (151 MHz, CDCl3): δ 24.9, 25.4, 25.7, 28.0, 28.1, 28.2, 28.3, 29.7, 32.5, 48.3, 48.7, 55.3, 65.3, 83.5, 123.6, 124.0, 127.6, 128.3, 128.8, 129.0, 129.3, 129.5, 155.7, 165,4, 168.0. MS (ESI+): m/z = 497.1. ESI-HRMS: calcd for C27H35N4O5 [M − H]− 495.2607; found 495.2609.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield (14 mg; 25%); brown oil; Rf = 0.57 (petrol ether/ethyl acetate 2
1. Yield (14 mg; 25%); brown oil; Rf = 0.57 (petrol ether/ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1H NMR (600 MHz, CDCl3): δ 1.34 (s, 9H), 1.55–1.80 (m, 10H), 2.95–3.05 (m, 2H), 3.55–3.65 (m, 1H), 3.72 (s, 3H), 3.75–3.85 (m, 1H), 4.55–4.70 (br s, 1H), 7.25–7.36 (m, 5H), 7.55–7.70 (m, 2H), 8.8.05–8.15 (br s, 1H), 8.20–8.25 (m, 2H). 13C NMR (151 MHz, CDCl3): δ 24.2, 24.3, 24.4, 24.9, 27.6, 27.9, 31.6, 31.8, 32.4, 36.1, 42.0, 43.0, 47.9, 48.0, 51.5, 51.8, 53.0, 69.0, 72.6, 73.5, 74.3, 80.2, 80.8, 122.8, 123.1, 123.4, 126.5, 126.8, 128.0, 128.3, 129.0, 130.4, 136.9, 141.3, 147.4, 155.6, 167.9, 173.0. MS (ESI+): m/z = 555. ESI-HRMS: calcd for C29H39N4O7 [M + H]+ 555.2819; found 555.2820.
1, v/v). 1H NMR (600 MHz, CDCl3): δ 1.34 (s, 9H), 1.55–1.80 (m, 10H), 2.95–3.05 (m, 2H), 3.55–3.65 (m, 1H), 3.72 (s, 3H), 3.75–3.85 (m, 1H), 4.55–4.70 (br s, 1H), 7.25–7.36 (m, 5H), 7.55–7.70 (m, 2H), 8.8.05–8.15 (br s, 1H), 8.20–8.25 (m, 2H). 13C NMR (151 MHz, CDCl3): δ 24.2, 24.3, 24.4, 24.9, 27.6, 27.9, 31.6, 31.8, 32.4, 36.1, 42.0, 43.0, 47.9, 48.0, 51.5, 51.8, 53.0, 69.0, 72.6, 73.5, 74.3, 80.2, 80.8, 122.8, 123.1, 123.4, 126.5, 126.8, 128.0, 128.3, 129.0, 130.4, 136.9, 141.3, 147.4, 155.6, 167.9, 173.0. MS (ESI+): m/z = 555. ESI-HRMS: calcd for C29H39N4O7 [M + H]+ 555.2819; found 555.2820.
Acknowledgements
We thank the Croatian Science Foundation, Grant number 3102 for financial support and the University of Zagreb Computing Centre (SRCE) for granting computational time on ISABELLA cluster.Notes and references
- A. Barker, J. G. Kettle, T. Nowak and J. E. Pease, Drug Discovery Today, 2013, 18, 298 CrossRef CAS PubMed.
- D. J. Newman and G. M. Cragg, J. Nat. Prod., 2012, 75, 311 CrossRef CAS PubMed.
- M. Dow, M. Fisher, T. James, F. Marchetti and A. Nelson, Org. Chem. Biochem., 2012, 10, 17 RSC.
- L. M. Blair and J. Sperry, J. Nat. Prod., 2013, 76, 794 CrossRef CAS PubMed.
- M. Hamada, T. Takeuchi, S. Kondo, Y. Ikeda, H. Naganawa, K. Maeda, Y. Okami and H. Umezawa, J. Antibiot., 1970, 23, 170 CrossRef CAS PubMed.
- H. J. Klosterman, G. L. Lamoureux and J. L. Parsons, Biochemistry, 1967, 6, 170 CrossRef CAS PubMed.
- A. J. Oelke, D. J. France, T. Hofmann, G. Wuitschik and S. V. Ley, Nat. Prod. Rep., 2011, 28, 1445 RSC.
- I. Avan, C. D. Hallb and A. R. Katritzky, Chem. Soc. Rev., 2014, 43, 3575 RSC.
- M. Laurencin, B. Legrand, E. Duval, J. Henry, M. Baudy-Floc'h, C. Zatylny-Gaudin and A. Bondon, J. Med. Chem., 2012, 55, 2025 CrossRef CAS PubMed.
- (a) A. Bordessa, M. Keita, X. Maréchal, L. Formicola, N. Lagarde, J. Rodrigoa, G. Bernadat, C. Bauvais, J. L. Soulier, L. Dufau, T. Milcent, B. Crousse, M. Reboud-Ravauxb and S. Ongeri, Eur. J. Med. Chem., 2013, 70, 505 CrossRef CAS PubMed; (b) S. Aubin, B. Martin, J.-G. Delcros, Y. Arlot-Bonnemains and M. Baudy-Floc'h, J. Med. Chem., 2005, 48, 330 CrossRef CAS PubMed.
- A. Altmayer-Henzien, V. Declerck, J. Farjon, D. Merlet, R. Guillot and D. J. Aitken, Angew. Chem., Int. Ed., 2015, 54, 10807 CrossRef CAS PubMed.
- R.-O. Moussodia, S. Acherar, E. Romero, C. Didierjean and B. Jamart-Gregoire, J. Org. Chem., 2015, 80, 3022 CrossRef CAS PubMed.
- J. Suć, L.-M. Tumir, L. Glavaš-Obrovac, M. Jukić, I. Piantanida and I. Jerić, Org. Biomol. Chem., 2016, 14, 4865 Search PubMed.
- (a) B. Ganem, Acc. Chem. Res., 2010, 42, 463 CrossRef PubMed; (b) C. de Graaff, E. Ruijter and R. V. A. Orru, Chem. Soc. Rev., 2012, 41, 3969 RSC; (c) S. Brauch, S. S. van Berkel and B. Westermann, Chem. Soc. Rev., 2013, 42, 4948 RSC.
- (a) P. Slobbe, E. Ruijter and R. V. A. Orru, Med. Chem. Commun., 2012, 3, 1189 RSC; (b) A. Dömling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083 CrossRef PubMed.
- (a) C. M. Madsen and M. H. Clausen, Eur. J. Org. Chem., 2011, 3107 CrossRef CAS; (b) D. G. Rivera, F. León, O. Concepción, F. E. Morales and L. A. Wessjohann, Chem.–Eur. J., 2013, 19, 6417 CrossRef CAS PubMed.
- (a) A. D. F. S. Barreto, O. E. Vercillo, L. A. Wessjohann and C. K. Z. Andrade, Beilstein J. Org. Chem., 2014, 10, 1017 CrossRef PubMed; (b) G. Koopmanschap, E. Ruijter and R. V. Orru, Beilstein J. Org. Chem., 2014, 10, 544 CrossRef PubMed.
- M. Pelay-Gimeno, J. Tulla-Puche and F. Albericio, Mar. Drugs, 2013, 11, 1693 CrossRef CAS PubMed.
- R. Lemmens-Gruber, M. R. Kamyar and R. Dornetshuber, Curr. Med. Chem., 2009, 16, 1122 CrossRef CAS PubMed.
- Y. K. Feng, J. A. Lu, M. Behl and A. Lendlein, Macromol. Biosci., 2010, 10, 1008 CrossRef CAS PubMed.
- (a) L. El Kaïm, M. Gizolme and L. Grimaud, Org. Lett., 2006, 8, 5021 CrossRef PubMed; (b) L. El Kaïm, M. Gizolme, L. Grimaud and J. Oble, J. Org. Chem., 2007, 72, 4169 CrossRef PubMed; (c) H. Yanai, T. Sakiyama, T. Oguchi and T. Taguchi, Tetrahedron Lett., 2012, 53, 3161 CrossRef CAS; (d) H. Yanai, T. Oguchi and T. Taguchi, J. Org. Chem., 2009, 74, 3927 CrossRef CAS PubMed; (e) M. Tobisu, A. Kitajima, S. Yoshioka, I. Hyodo, M. Oshita and N. Chatani, J. Am. Chem. Soc., 2007, 129, 11431 CrossRef CAS PubMed; (f) T. Soeta, S. Matsuzaki and Y. Ukaji, J. Org. Chem., 2015, 0, 3688 CrossRef CAS PubMed.
- G. Lelais and D. Seebach, Helv. Chim. Acta, 2003, 86, 4152 CrossRef CAS.
- J. Suć and I. Jerić, SpringerPlus, 2015, 4, 507 CrossRef PubMed.
- D. Bonnet, C. Grandjean, P. Rousselot-Pailley, P. Joly, L. Bourel-Bonnet, V. Santraine, H. Gras-Masse and O. Melnyk, J. Org. Chem., 2003, 68, 7033 CrossRef CAS PubMed.
- T. Bousquet, M. Jida, M. Soueidan, R. Deprez-Poulain, F. Agbossou-Niedercorn and L. Pelinski, Tetrahedron Lett., 2012, 53, 306 CrossRef CAS.
- A. Dömling, Chem. Rev., 2006, 106, 17 CrossRef PubMed.
- S. Maeda, S. Komagawa, M. Uchiyama and K. Morokuma, Angew. Chem., Int. Ed., 2011, 50, 644 CrossRef CAS PubMed.
- Y. Zhao and D. G. Truhlar, Acc. Chem. Res., 2008, 41, 157 CrossRef CAS PubMed.
- R. Ramozzi and K. Morokuma, J. Org. Chem., 2015, 80, 5652 CrossRef CAS PubMed.
- S. Faure, T. Hjelmgaard, S. P. Roche, D. J. Aitken and V. Uni, Org. Lett., 2009, 11, 1167 CrossRef CAS PubMed.
- L. Banfi, R. Riva and A. Basso, Synlett, 2009, 23 Search PubMed.
- S. Dighe, U. K. S. Anil Kumar, S. Srivastava, P. Shukla, S. Singh, M. Dikshit and S. Batra, J. Org. Chem., 2015, 80, 99 CrossRef CAS PubMed.
- J. Zhu, X. Wu and S. J. Danishefsky, Tetrahedron Lett., 2009, 50, 577 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H NMR, 13C NMR and ESI-HRMS spectra of products. Cartesian coordinates of minima and transition state for the rate-determining step of the reaction. Cartesian coordinates of non-productive conformers of imidate cluster. Energies, number of imaginary frequences and zero point vibrational energies for minima and transition state for the rate determining step of the reaction. Energies, number of imaginary frequences and zero point vibrational energies for non-productive conformers of imidate cluster. See DOI: 10.1039/c6ra23317a |
| This journal is © The Royal Society of Chemistry 2016 |