Synthesis of zeolite nanocrystals with intercrystal mesopores using an organosilane as the structure directing agent
Jiqiang Daiab,
Xiya Zhoua,
Zhihang Chen*b,
Dingsheng Chenb,
Gai Zengb and
Chaoping Cenb
aSchool of Materials Science and Engineering, South China University of Technology, Guangzhou 510641, P. R. China. E-mail: chenzhihang@scies.org; Tel: +86 2085648556
bGuangdong Key Lab of Water & Air Pollution Control, South China Institute of Environmental Science, Ministry of Environmental Protection, Guangzhou 510655, P. R. China
First published on 31st October 2016
Abstract
Nanocrystalline ZSM-5 with mesoporosity was synthesized within a short reaction time using triethoxyphenylsilane as the mesopore structure directing agent via a direct one-step hydrothermal method. With the addition of the organosilane, the growth of the zeolite grain was restrained, which led to the formation of intercrystal mesoporosity. These zeolite nanocrystals are designed to enhance the adsorption properties by increasing porosity. Their adsorption performance was measured via the static adsorption method with toluene as a model gas on an intelligent gravimetric analyzer.
Zeolites are a family of microporous materials with many promising advantages, such as large surface areas, and high thermal and hydrothermal stabilities. They have shown potential application value in the environmental field due to their high adsorption capacity for volatile organic compounds (VOCs).1,2 However, the use of zeolites is limited by the diffusion limitations of reactants in their intricate pore and channel systems.3 To solve this problem, many efforts have been devoted to the synthesis of ordered mesoporous aluminosilicates.4–6 Unfortunately, compared with zeolites, these mesoporous materials are more akin to amorphous materials and do not show a short range order, which lead to insufficient hydrothermal stability.7
Some research efforts have been concentrated on the synthesis of mesoporous zeolites,8–11 which not only retain the thermal and hydrothermal stability of zeolites, but also have enhanced surface area, pore volume and pore diameter. In the successful cases, the common approach to develop mesopores are template methods using nanosized carbon particles as a hard template or using rationally designed supramolecules as soft templates. However, nanosized hard templates are typically difficult and costly to obtain, and novel soft templates require extra design, all of which make the synthetic process more complicated. Generally, intercrystal mesopores can be formed by the aggregation of zeolite nanocrystals, and mesoporous zeolites are thus obtained, which have a larger pore volume and pore size than conventional zeolites.12 Herein, we report a direct synthetic route to obtain ZSM-5 nanocrystals with intercrystal mesopores using triethoxyphenylsilane as a mesopore structure directing agent. As compared with previous works,2–4 we aim to simplify the synthetic process using a one-step hydrothermal method, and triethoxyphenylsilane can be easily obtained commercially. Moreover, the adsorption property of the synthetic ZSM-5 to toluene is studied.
Nanocrystalline ZSM-5 with mesoporosity was synthesized as follows: 0.30 g of NaOH and 12.18 g of tetrapropylammonium hydroxide (TPAOH, 25 wt% in water) were dissolved in 54 mL of distilled water. After stirring for 10 min, 0.61 g of aluminium isopropoxide (AIP) was added with vigorous stirring. Then 15.60 g of tetraethyl orthosilicate (TEOS) was added to the above solution dropwise. The molar composition of the original reagents was 1 SiO2: 0.02 Al2O3: 0.2 TPAOH: 0.1 NaOH: 40 H2O. After stirring for 1 h at room temperature, 0.45 g of triethoxyphenylsilane (molar composition was 1 SiO2: 0.025 triethoxyphenylsilane) was added. The final mixture was stirred for 2 h at 70 °C, and then transferred to an autoclave for further hydrothermal treatment at 170 °C for 1 d. The final product was separated by centrifugation, washed, dried, and calcined at 550 °C in air for 8 h. The conventional microporous ZSM-5 zeolite was synthesized without triethoxyphenylsilane for comparison with the nanocrystalline ZSM-5.
Powder X-ray diffraction (XRD) patterns were measured using a PANalytical X'Pert PRO diffractometer with CuKα radiation and the scan rate of 2° per min. Transmission electron microscopy (TEM) images were obtained on a Tecnai G2 F20 microscope, working at 200 kV. Scanning electron microscopy (SEM) images were recorded using a Hitachi S-4800 instrument. Solid-state 13C NMR measurements were preformed on a Bruker AVANCE 500 NMR spectrometer. The Si/Al ratios were determined on an Agilent 700 ICP-OES spectrometer. Before measurement, the sample was dissolved in HCl solution with a very small amount of HF, and then, the mixture was transferred to an autoclave and heated at 120 °C for 8 h. Infrared spectroscopy was conducted in transmission mode on a Bruker VERTEX70 spectrometer with a resolution of 4 cm−1 using the KBr method. Nitrogen adsorption and desorption isotherms at 77 K were obtained on a Micromeritics ASAP 2020, and the analyses were carried out after outgassing the samples at 180 °C under vacuum for 6 h. Total surface area (SBET) was calculated using the BET method. Mesopore volume (Vmeso) was obtained from the adsorption branch using the BJH algorithm, and total pore volume (Vp) was obtained from the adsorption at P/P0 = 0.98. Micropore (Smic) and external surface area (SEXT) were determined via the t-plot method. The toluene adsorption capacity of the calcined sample was measured on a high precision intelligent gravimetric analyzer (IGA-002, Hiden). The adsorption measurement was carried out at 35 °C, after the samples were outgassed overnight at 200 °C under vacuum, which ensured that the phenyl groups anchored on the outermost surface of the ZSM-5 zeolites were completely removed.
The XRD patterns of the nanocrystalline ZSM-5 mesoporous zeolites prepared with triethoxyphenylsilane are given in Fig. 1, which are characteristic for the MFI structure and indicate that the crystalline ZSM-5 was synthesized successfully even with the addition of the organic triethoxyphenylsilane to the precursor solution. However, compared with the patterns of conventional ZSM-5 and standard database of JCPDS no. 42-0024 provided by the International Center for Diffraction Data, the diffraction line at 23.2° is most intense, whereas the diffraction peaks at 7.9° and 8.8° are lower. Moreover, the intensities of the first two lines of the uncalcined sample are much lower, which indicate that this difference is caused by the presence of the triethoxyphenylsilane organic material in the intracrystals and is technically associated with the zeolite micropores.13 It is important to note that the peak height of the ZSM-5 nanocrystals is slightly weaker compared with conventional ZSM-5, which indicates that the degree of crystallinity decreased. The crystallinity of the samples was determined using the X-ray diffraction method,14 and it was found that the crystallinity of the conventional ZSM-5 was 88.47%, whereas the crystallinity of the ZSM-5 nanocrystals after and before removal of the triethoxyphenylsilane template was 83.75% and 72.66%, respectively. The broadening of the diffraction lines is associated with crystal size reduction, and the Scherrer equation was employed determine the crystallite size effects:15,16
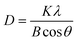 | (1) |
 | ||
| Fig. 1 XRD patterns of the ZSM-5 zeolites: ZSM-5 nanocrystals before (a) and after (b) removal of the triethoxyphenylsilane template, and conventional ZSM-5 (c). | ||
Two factors affecting the synthesis of the ZSM-5 nanocrystals are studied. As shown in Fig. 2, with an increase in the amount of triethoxyphenylsilane added (molar composition was 1 SiO2: S triethoxyphenylsilane, S = 0.025, 0.05, 0.1), the intensity of the peaks tended to be lower slightly. The average crystallite size of the samples calculated by the Scherrer equation was found to be 47 nm, 39 nm and 32 nm, and their crystallinity was 83.75%, 79.47% and 78.09% respectively, which indicate that organosilanes have the effect of inhibiting zeolite crystal growth and are beneficial to the synthesis of ZSM-5 nanocrystals. However, with an increase in the amount of additive, this influence decreased since the formation of smaller nanosized grains became more difficult. Notably, with an extension in the crystallization time (hydrothermal treatment time, D = 1, 2, 3 days), the results show that the crystallinity of all the samples were about 83% and they have the same diffraction peak intensity, which indicate that crystallinity is less susceptible to crystallization time, which is advantageous to reduce the synthetic cost.
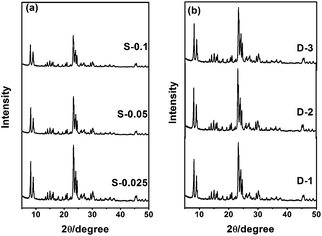 | ||
| Fig. 2 XRD patterns of ZSM-5: synthesized with varied quantities of triethoxyphenylsilane (a) and different crystallization times (b). | ||
The FTIR spectra of the as-synthesized ZSM-5 nanocrystals obtained by the addition of different amounts of triethoxyphenylsilane and the conventional ZSM-5 zeolite are shown in Fig. 3a. The bands clearly observed at 450 cm−1, 790 cm−1, 1095 cm−1 and 1220 cm−1 are due to the bending vibrations, external asymmetric stretch, internal asymmetric stretch, and external asymmetric stretch, respectively, which are typical of silicate materials.17 The band at 550 cm−1 is attributed to the presence of double-rings of tetrahedra in the framework. Since silica does not show any bands at 550 cm−1, this band can be a good probe to characterize the MFI zeolite topology. Moreover, the intensity of the bands located at 550 cm−1 and 1220 cm−1 could be used to compare the crystalline degree of the samples.12 This result shows that the crystallinity of ZSM-5 decreased with an increasing in the quantity of triethoxyphenylsilane, as confirmed by the XRD results. The band in the region of 3400–3700 cm−1 is assigned to the silanol groups and the band at 1630 cm−1 is due to the hydrogen bond bending vibration of deformed water molecules. The peaks at 1480 cm−1 (benzene skeleton vibration) and 2980 cm−1 (aromatic C–H stretching vibration) correspond to the phenyl moiety for the grafted organosilane, which indicate that triethoxyphenylsilane was hydrolyzed and bonded with the surface of the ZSM-5 zeolite. The intensity of the characteristic peaks increased with an increase in the quantity of triethoxyphenylsilane, which means a corresponding increase in the density of the phenyl moiety. The 13C NMR result provided further clear evidence for this. Fig. 3b shows the solid-state 13C NMR spectra for the same as-synthesized sample. The 13C signals at 10, 15, and 63 ppm are due to the TPA+ propyl chains with distinct carbon moieties. The signals at 113, 130, and 151 ppm are attributed to the phenyl moiety, and the 13C signals at 23 and 47 ppm are from the carbon moieties of triethoxyphenylsilane. These peaks are not observed in the conventional ZSM-5, which indicates the absence of the organosilane. Moreover, relatively higher signal intensities are also observed with an increase in the quantity of triethoxyphenylsilane.
 | ||
| Fig. 3 FTIR (a) and 13C NMR (b) patterns of the as-synthesized ZSM-5: synthesized with varied quantities of triethoxyphenylsilane S-0.1, S-0.05, S-0.025, and conventional ZSM-5. | ||
Nitrogen adsorption was employed to characterize the pore structure of the ZSM-5 nanocrystals, which revealed that various hierarchical pore distributions had been formed. As shown in Fig. 4, the isotherm of the mesoporous ZSM-5 nanocrystals exhibits a sharp uptake at low relative pressure because of the characteristics of the MFI microporous structure. Moreover, the isotherm shows capillary condensation at high relative pressure between 0.4 and 0.9, which is indicative of the presence of mesopores. It should be noted that the step observed at a relative pressure of p/p0 > 8.0, suggests that the mesopores in ZSM-5 is formed by nanocrystal aggregation. A wide mesopore diameter distribution in the range of 5–20 nm could be observed. The average diameters were calculated by the BJH method, and the used adsorption branch was centered at 11 nm (Fig. 4, inset). In comparison, the conventional microporous ZSM-5 exhibits a type I isotherm curve with a plateau at higher relative pressures and has no obvious hysteresis loop, which reveal the absence of mesoporosity. Table 1 lists the detailed textural parameters, in which a reduction in the micropore area of the ZSM-5 nanocrystals is observed, which leads to the change in diffraction peaks, as discussed in the previous section. It is considered that the BET and BJH algorithms are not strictly valid for microporous materials, however they are often used as effective methods to estimate and compare the surface area and pore property of different samples in mesoporous zeolite science.11,18 The BET area of the ZSM-5 nanocrystals is 425 m2 g−1 and their pore volume is 0.32 cm3 g−1, which are obviously increased compared to the conventional ZSM-5, which is a result of the presence of a mesoporous structure. Notably, the mesopore volume is significantly increased from a negligible level of 0.07 cm3 g−1 to 0.19 cm3 g−1. The ZSM-5 nanocrystals have a much higher external surface area (233 m2 g−1) than conventional ZSM-5 (89 m2 g−1), which supports that the ZSM-5 nanocrystals have smaller crystal sizes.
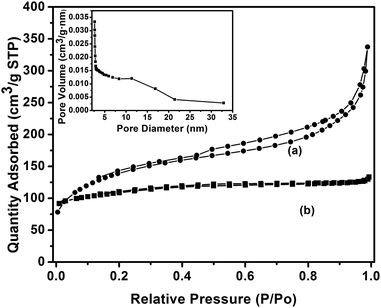 | ||
| Fig. 4 N2 adsorption/desorption isotherms of ZSM-5 nanocrystals (a) and conventional ZSM-5 (b). Inset: Mesopore diameter distribution of the ZSM-5 nanocrystals. | ||
| Si/Al ratio | SBET (m2 g−1) | Smic (m2 g−1) | SEXT (m2 g−1) | Vp (cm3 g−1) | Vmeso (cm3 g−1) | Maximum adsorption capacity (mg g−1) | |
|---|---|---|---|---|---|---|---|
| ZSM-5 nanocrystals | 28.1 | 425 | 192 | 233 | 0.32 | 0.19 | 116 |
| Conventional ZSM-5 | 25.6 | 370 | 281 | 89 | 0.17 | 0.07 | 56 |
Fig. 5a shows the SEM image of the ZSM-5 nanocrystals with the organosilane used as an additive, in which the spherical morphology of many aggregated small particles in the range of 40–60 nm is observed, which is consistent with the value calculated from the Scherrer equation and further confirms that the ZSM-5 zeolite nanocrystals were synthesized successfully as a result of the inhibitory effect on grain growth. Notably, intercrystal mesopores formed by crystal aggregation with a diameter of 10–20 nm could be observed clearly in the SEM image, which fits the result of the BJH calculation. The TEM image of the ZSM-5 nanocrystals (Fig. 5b) indicates that the sample has good crystallinity, and the outline of the nanocrystals is fairly clear. Moreover, it proves direct evidence for the presence of intercrystal mesoporous, in agreement with the results of the N2 adsorption isotherm and SEM image. Comparatively, the conventional ZSM-5 particles show a spherical morphology with a smoother surface and larger diameter, and it was hard to find any ZSM-5 nanocrystals in the SEM or TEM survey. Also, no aggregation phenomenon was observed (Fig. 5c and d).
The mesoporous structure of the ZSM-5 nanocrystals is formed from the aggregation of zeolite nanocrystals using the organosilane as the mesopore structure directing agent. As a result, the pore volume and pore size are significantly increased, which could lead to a greater amount of active sites and effective mass diffusion, which benefit the adsorption performance of VOCs on zeolites. In the present work, the toluene adsorption capacity of ZSM-5 zeolites was studied by the static adsorption method on an IGA-002, which has high stability and measurement accuracy with a sensitivity of 0.1 μg, and the increase in weight was recorded continuously in real time by the fully computerized system. The amount of toluene introduced in the system was regulated by Baratron pressure transducers. The adsorption capacity for each pressure increment was recorded when the adsorption of the sample became saturated, and then next pressure was measured automatically. As seen in Table 1, the maximum adsorption capacity of conventional microporous ZSM-5 for toluene is 56 mg g−1. However, with the introduction of mesoporosity, the adsorption performance of the zeolite nanocrystals is significantly improved (116 mg g−1). Considering that the ZSM-5 nanocrystals and conventional ZSM-5 have approximately the same chemical compositions according to the ICP-OES result, in which the Si/Al ratio is 28.1 and 25.6, respectively, the improvement in adsorption capacity is due to the mesoporous structure. Moreover, the time taken by the ZSM-5 nanocrystals to reach saturation was shorter. The saturated adsorption amount of each pressure was achieved at the average time of 50 min, whereas conventional ZSM-5 needed 120 min, which indicates that toluene transport in the channel system of the ZSM-5 nanocrystals is easier than the conventional microporous ZSM-5.
Fig. 6 illustrates the conceptual approach to the synthesis of the ZSM-5 nanocrystals, in which triethoxyphenylsilane is used. This type of organosilane can strongly interact with the silica–alumina reaction precursor. Firstly, the MFI structure nucleates in the presence of TPAOH. Then, the ethoxy moiety on the organosilane is hydrolyzed to –SiO3 units and grafted to the surface of the zeolite by covalent Si–O–Si linkages, and the hydrophobic phenyl moiety can restrain the grain growth of the ZSM-5 zeolite and form nanoparticles. Finally the nanoparticles aggregate at a high hydrothermal temperature, and the organosilane agent is distributed in the intercrystals of the nanocrystals. After the sample is separated, dried and calcined, the organic compounds are removed and ZSM-5 nanocrystals with intercrystal mesopores are obtained.
In this work, ZSM-5 nanocrystals with mesoporosity have been synthesized successfully using triethoxyphenylsilane as the structure directing agent via a one-step hydrothermal method, which has a short reaction time, operational simplicity and low cost compared with previous works. Organosilanes could be beneficial for preventing zeolite crystal growth, thus leading to the development of an intercrystal mesoporous structure. The increase in pore volume and pore diameter provides more active sites and offers a valid method to overcome the blocking problem of bulky reactants, and as a result, the ZSM-5 nanocrystals exhibit an excellent adsorption performance toward toluene.
Acknowledgements
This work was financially supported by the Science and Technology Planning Project of Guangdong Province, China (2016A020221017), the Natural Science Foundation of Guangdong Province, China (S2013010011772), and Pearl River S&T Nova Program of Guangzhou, China (2014J2200036).References
- C. Y. Yin, Y. J. Wei, F. W. Wang and Y. H. Chen, Mater. Lett., 2013, 98, 194–196 CrossRef CAS.
- K. Na, M. Choi and R. Ryoo, Microporous Mesoporous Mater., 2013, 166, 3–19 CrossRef CAS.
- X. Meng, F. Nawaz and F. S. Xiao, Nano Today, 2009, 4, 292–301 CrossRef CAS.
- C. T. Kresge and W. J. Roth, Chem. Soc. Rev., 2013, 42, 3663–3670 RSC.
- J. S. Beck, J. C. Vartuli, W. J Roth, M. E. Leonowicz, C. T. Kresge and K. D. Schmit, J. Am. Chem. Soc., 1992, 114, 10834–10843 CrossRef CAS.
- C. Salameh, A. Bruma, S. Malo, U. B. Demirci, P. Mielea and S. Bernarda, RSC. Adv., 2015, 5, 58943–58951 RSC.
- D. Trong and S. Kaliaguine, Angew. Chem., 2001, 113, 3348–3351 CrossRef.
- J. J. Zhao, Z. L. Hua, Z. C. Liu, Y. S. Li, L. M. Guo, W. B. Bu, X. Z. Cui, M. L. Ruan, H. R. Chen and J. L. Shi, Chem. Commun., 2009, 48, 7578–7580 RSC.
- D. Verboekend and J. Pérez-Ramírez, Catal. Sci. Technol., 2011, 6, 879–890 Search PubMed.
- Z. X. Yang, Y. D. Xia and R. Mokaya, Adv. Mater., 2004, 16, 727–732 CrossRef CAS.
- M. Choi, H. S. Cho, R. Srivastava, C. Venkatesan, A. H. Choi and R. Ryoo, Nat. Mater., 2006, 5, 718–723 CrossRef CAS PubMed.
- D. P. Serrano, J. Aguado, G. Morales, J. M. Rodrıíguez, A. Peral and M. Thommes, Chem. Mater., 2009, 21, 641–654 CrossRef CAS.
- E. L. Wu, S. L. Lawton, D. H. Olson, A. C. Rohrman and G. T. Kokotailo, J. Phys. Chem., 1979, 83, 2777–2781 CrossRef CAS.
- Y. P. Guo, H. J. Wang, Y. J. Guo, L. H. Guo, L. F. Chu and C. X. Guo, Chem. Eng. J., 2011, 166, 391–400 CrossRef CAS.
- A. W. Burton, K. Ong, T. Rea and I. Y. Chan, Microporous Mesoporous Mater., 2009, 117, 75–90 CrossRef CAS.
- K. Venkatesan, D. R. Babu, M. P. K. Bai, R. Supriya, R. Vidya, S. Madeswaran, P. Anandan, M. Arivanandhan and Y. Hayakawa, Int. J. Nanomed., 2015, 10, 189–198 CAS.
- J. L. Jiang, Y. Yang, C. S. Duanmu, Y. Xu, L. D. Feng, X. Gu and J. Chen, Microporous Mesoporous Mater., 2012, 163, 11–20 CrossRef CAS.
- L. Zhao, B. J. Shen, J. S. Gao and C. M. Xu, J. Catal., 2008, 258, 228–234 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |


